
J Clin Aesthet Dermatol. 2021;14(12):55–63.
by Olga Marushchak, MA; Rose Yakubov, BHSc (Candidate); Rebecca Yakubov, BHSc (Candidate); and Gary Goldenberg, MD
Ms. Marushchak and Dr. Goldenberg are with the Icahn School of Medicine at Mount Sinai in New York, New York. Ms. Rose Yakubov and Ms. Rebecca Yakubov are with McMaster University in Hamilton, Ontario, Canada.
FUNDING: No funding was provided for this study.
DISCLOSURES: Dr. Goldenberg is a consultant for Abbvie, Amgen, Celgene, Janssen, Novartis, Lilly, BMS, and Pfizer, and a speaker for Abbvie, Amgen, Lilly, and Novartis. Ms. Yakubov, Ms. Yakubov, and Ms. Marushchak have no conflicts of interest to disclose.
ABSTRACT: Currently, several classes of oral therapies for psoriasis are in use, in development, or in investigative stages. Standard non-biologic treatments for psoriasis, such as methotrexate, cyclosporine, and acitretin, have generally unfavorable safety profiles and are not ideal for long-term use. This review will address the safety and efficacy of existing and novel oral therapies for psoriasis that target inflammatory pathways via modulation of phosphodiesterase 4 (PDE4), Janus kinases (JAKs), sphingosine 1-phosphate (S1P), A3 adenosine receptors, and rho-associated kinase 2 (ROCK2), with an emphasis on JAK inhibitors.
Key words: Cutaneous lesions, field cancerization, photosensitizing agent, protoporphyrin, lesion count
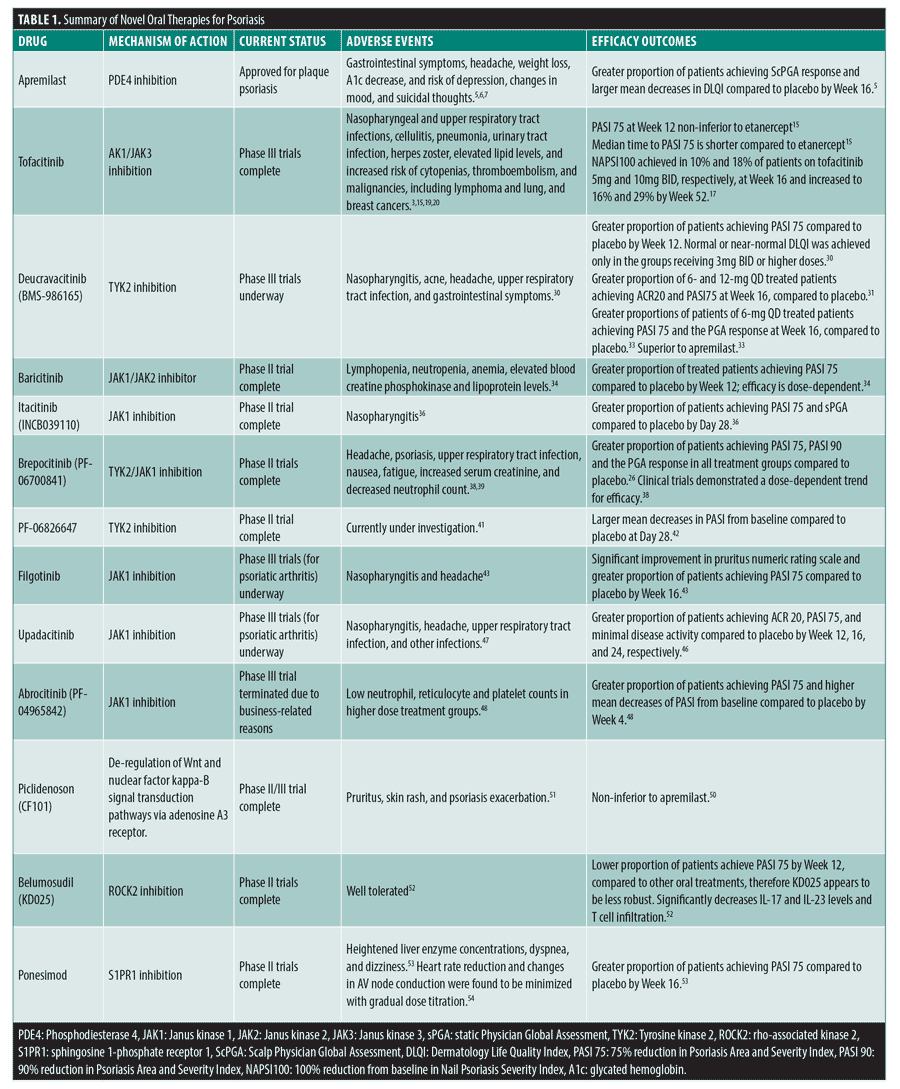
Plaque psoriasis is a chronic, immune-mediated, multifactorial skin disease that is characterized by dysregulated keratinocyte proliferation, manifesting on the skin as erythematous, scaly plaques. Psoriasis is associated with other serious comorbidities, work limitations, productivity loss, and elevated healthcare costs of over $35 billion annually.1 The continued negative impact on quality of life, as well as significant financial burden, necessitates the need for effective long-term management of the disease. While effective biologic medications are available, studies suggest that oral administration is statistically significantly preferred to subcutaneous or intravenous routes by both physicians and patients.2 Commonly used oral therapies for psoriasis, including methotrexate, cyclosporine, and acitretin, have generally unfavorable safety profiles, presenting increased risk for significant short- and long-term adverse effects (AEs), such as hepatotoxicity, nephrotoxicity, dyslipidemia, hypertension, malignancy, and teratogenicity.3 Therefore, classes of new oral therapies for psoriasis are in development or in investigative stages. In this article, we review the safety and efficacy of existing and emerging oral therapies for psoriasis that target inflammatory pathways via modulation of phosphodiesterase 4 (PDE4), Janus kinases (JAKs), A3 adenosine receptors, rho-associated kinase 2 (ROCK2), and sphingosine1-phosphate (S1P) (Table 1). Novel oral therapies might be beneficial to patients with moderate to severe psoriasis, preference for oral route therapies, and history of failed conventional treatment options.
Current Approved Treatment
Apremilast. Apremilast inhibits PDE4 thereby preventing PDE4-mediated degradation of cyclic adenosine monophosphate, a secondary messenger that impedes anti-inflammatory processes.4 Apremilast is approved for treatment of psoriatic arthritis and moderate to severe plaque psoriasis. In the STYLE study, apremilast showed efficacy in patients with moderate to severe plaque psoriasis.5 In the Phase III study, 303 patients with severe plaque psoriasis of the scalp (scalp surface area greater than or equal to 20%) were randomized to receive placebo (n=102) or apremilast 30 mg twice daily (BID) (n=201). At Week 16, significantly more apremilast-treated patients achieved scalp Physician Global Assessment (ScPGA) response (score of “clear” or “almost clear”), with at least a 2-point reduction from baseline, 43.3 percent [95% CI, 36.2–50.5], compared to 13.7% [95% CI, 6.6–20.8] in placebo (p<0.0001). The proportion of patients achieving ScPGA response was higher in the apremilast group as early as Week 2 (p<0.05). Additionally, larger mean decreases in Dermatology Life Quality Index (DLQI) were observed in apremilast-treated patients by Week 16, compared to placebo, -6.7 [95% CI, -7.5 – -5.9] vs. -3.8 [95% CI, -4.9 – -2.7] (p<0.0001), respectively.5
Common AEs reported in the study were diarrhea, nausea, headache, and vomiting, experienced by 30.5 percent, 21.5 percent, 12.0 percent, 5.5 percent of apremilast-treated patients compared to 10.8 percent, 5.9 percent, 4.9 percent, 2.0 percent of placebo-treated patients, respectively. Overall, 11 patients in the apremilast group and 3 patients in the placebo group had at least one AE leading to drug withdrawal.5 However, dose titration during the first week of treatment can help minimize the gastrointestinal symptoms, which generally resolve within one month.6 Apremilast may also cause weight loss in patients with psoriasis and psoriatic arthritis.7 In an analysis of Phase III ESTEEM and PALACE trials, the effect of apremilast on metabolic parameters, such as weight and glycated hemoglobin (A1c) concentrations, was assessed in 2,242 patients receiving placebo or apremilast 30mg BID. Mean weight change in apremilast-treated patients (n=1329) was -1.32 percent by Week 16, compared to 0.11 percent of placebo-treated patients (n=913). Decreases in weight were also observed in apremilast-treated patients undergoing concomitant insulin treatment. The mean weight change in this patient group was -1.83 percent At Week 16, the highest decreases in A1c occurred in apremilast-treated patients who met the American Diabetes Association (ADA) A1c criterion for diabetes (A1c greater than or equal to 6.5%) at baseline, with a mean change of -0.31.7 Additionally, it is important to screen patients for the signs of depression, as apremilast is associated with increased risk of depression, changes in mood, and suicidal thoughts.6
Overall drug survival was analyzed in a retrospective multicenter study of patients with moderate to severe plaque psoriasis or palmoplantar psoriasis. The study found the drug survival rate to be 54.9 percent at 12 months and a mean survival time to be 52 weeks, ranging from 2 to 147 weeks.8 Similar survival rate of 53.4 percent at 12 months was reported in studies by Papadavid et al9 and Kishimoto et al10 The main reasons for drug discontinuation were loss of effectiveness (23.9-46.4%), AEs (15.9-26.9%), patients’ choice (12.5%) and symptom relief (5.4%).8,9
A five year cost-effectiveness and budget impact analysis compared two alternative treatment sequences, one with and one without pre-biologic apremilast use in patients with moderate-to-severe plaque psoriasis. Over the five-year period, the costs of the treatment sequence including apremilast was €57,965, compared to €59,134 for biologics. In the analysis of the total impact on the Italian National Health Services budget, pre-biologic apremilast use showed total savings of €16 million within three years. The savings were mainly related to reduction in pharmaceutical spending, hospital admissions, and drug administration costs.11 Lower total healthcare costs associated with apremilast initiation were also seen in retrospective claims analyses of biologic-naïve psoriasis patients.12,13
Therapeutic Targeting of Janus kinase-signal transducers and activators of transcription (JAK-STAT) Pathway
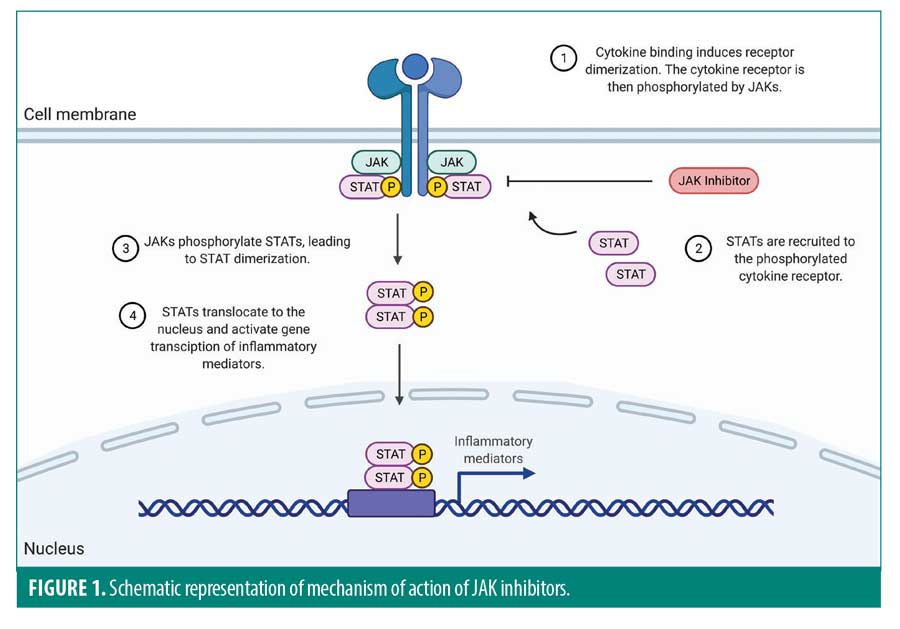
The JAK-STAT pathway is a key cytokine signaling mechanism contributing to inflammation in many autoimmune diseases, which makes this pathway an important drug target. Currently, the United States Food and Drug Administration (FDA) has approved therapeutic use of JAK-STAT inhibitors for rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. The pathway is used by multiple cytokines highly expressed in psoriasis, including interleukin (IL)-2, IL-12, IL-19, IL-20, IL-22, IL-23, and interferon-alpha, -beta, and -gamma.3,14 Due to the critical role of the JAK-STAT pathway in the disease pathogenesis, multiple novel JAK-STAT inhibitors are currently under clinical investigation for their effectiveness in treating psoriasis (Figure 1).
Tofacitinib. Tofacitinib is a JAK1/JAK3 inhibitor that is FDA approved for the treatment of rheumatoid arthritis, psoriatic arthritis and ulcerative colitis.15,16 Tofacitinib’s evident efficacy makes it an attractive candidate for psoriasis treatment. In a dose-ranging, non-inferiority, Phase III trial, the proportion of patients with affected body surface area (BSA) equal to or greater than 10 percent, achieving a 75-percent reduction of psoriasis area and severity index (PASI 75) at Week 12 in the 10-mg tofacitinib BID group (63.6%) was non-inferior to the 50-mg etanercept twice weekly (TW) group (58.8%) and superior to placebo (5.6%), (p<0.0001 vs. placebo). Observations also indicate that the median time to PASI 75 was generally shorter in patients treated with tofacitinib 10mg BID than with etanercept or tofacitinib 5mg BID, suggesting a quicker response time.15 A post-hoc analysis of the Phase III OPT Pivotal 1 and OPT Pivotal 2 studies evaluated the efficacy of tofacitinib in patients with nail psoriasis using the Nail Psoriasis Severity Index (NAPSI).17 Significant improvements were noticed in patients treated with tofacitinib 5mg and tofacitinib 10 mg BID compared to placebo at Week 16 and through Week 28 and maintained up to Week 52. NAPSI100, which signifies complete clearance of nail psoriasis, was achieved in 10 percent and 18 percent of patients on tofacitinib 5mg and 10mg BID, respectively, at Week 16 and increased to 16 percent and 29 percent by Week 52.17
To further investigate the efficacy of tofacitinib, Bissonnette at al18 compared outcomes following tofacitinib withdrawal with outcomes of continuation. Following a 16-week treatment withdrawal period, significantly greater proportion of patients continuing 5- and 10-mg tofacitinib BID, 56.2 percent and 62.3 percent, respectively, maintained a PASI 75 response compared to those who were switched to placebo 5mg and 10mg, 23.3 percent (P=0.008) and 26.1 percent (P<0.0001), respectively. Correspondingly, by 16 weeks, 92.3 percent and 93.0 percent of patients on continued tofacitinib 5- and 10-mg BID had not relapsed, compared to 32.8 percent and 42.9 percent for those who were switched to placebo 5 and 10mg, respectively. Patients who were switched to placebo demonstrated a median time to loss of a PASI 75 response of eight weeks and median time to relapse of 16 weeks.18
Although efficacious, tofacitinib has been associated with several AEs. The most common AEs in the aforementioned trial were nasopharyngeal and upper respiratory tract infections.15 A systematic review of seven randomized control trials assessing the efficacy and safety of tofacitinib in 3,743 patients with chronic plaque psoriasis concluded a higher incidence of AEs in tofacitinib-treated patients compared to placebo-treated patients.16 Overall, 54.8 percent of patients treated with tofacitinib 5mg experienced AEs, compared to 50.2 percent with placebo (p<0.04). Similarly, 59.5 percent of patients treated with tofacitinib 10mg experienced AEs, compared to 49.3 percent with placebo (p<0.0001).16 More serious infections associated with tofacitinib use are cellulitis, pneumonia, urinary tract infection, and herpes zoster (HZ).3,19,20 Winthrop et al19 reviewed data from Phase II, III and open-label long-term extension (LTE) study of tofacitinib in patients with psoriasis (5,204 patient years) to assess HZ risk. HZ incidence rate (per 100 patient years) in tofacitinib-treated patients was 2.55 (95% CI, 2.13–3.03), compared to zero in placebo and 2.68 (95% CI, 0.32–9.68) in etanercept. Risk factors for HZ in patients with psoriasis included Asian descent, prior biologic use, and older age.19
The effect of tofacitinib on lipid levels and lipid-related parameters was investigated in patients with psoriasis from the OPT Pivotal 1 Phase III study.21 Data from this study suggests that tofacitinib results in small increases in total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels, with the ratio of TC/HDL-C remaining unchanged. However, the effects of tofacitinib on various lipid-related parameters do not indicate an increased cardiovascular disease risk.21
Tofacitinib also increases risk of cytopenias, thromboembolism, and malignancies, including lymphoma and lung and breast cancers.3 In an open-label LTE study, the safety profile of tofacitinib was further analyzed in 2,867 patients with moderate to severe plaque psoriasis.22 Patients were treated with either 5 or 10mg tofacitinib BID. Overall, 82.5 percent of patients experienced AEs, 13.7 percent of patients experienced serious adverse events (SAEs), 13.9 percent of patients discontinued treatment due to AEs and nine patient deaths were related to the study treatment. Incidence rates (per 100 patient years) of AEs of special interest were 1.16 (95% CI, 0.93–1.44), 2.51 (95% CI, 2.15–2.91), 0.58 (95% CI, 0.42–0.78) for serious infections, HZ and opportunistic infections, respectively. Incidence rates for major adverse cardiac events and malignancies were 0.26 (95% CI, 0.16–0.41) and 0.67 (95% CI, 0.50–0.88), respectively.22 However, a pooled analysis across six clinical trials by Strober et al20 concluded benefit-risk profile of tofacitinib to be comparable to other systemic psoriasis therapies.
The economic impact of tofacitinib incorporation in treatment sequences for patients with active psoriatic arthritis demonstrated cost-effectiveness. Including tofacitinib in the treatment of patients with inadequate response to conventional disease-modifying antirheumatic drugs or TNF inhibitors was estimated to save up to $8,454,858 over two years based on one million insurants.23
Deucravacitinib. Deucravacitinib is a highly potent and selective TYK2 inhibitor.24 Notably, TYK2 has been identified as a new locus for psoriasis.25 Deactivation of TYK2 coding variants was shown to protect from psoriasis and other immune mediated disorders.26 Unlike other inhibitors of closely related JAK proteins which bind to the active site of the kinase domain, deucravacitinib allosterically binds to the regulatory domain of TYK2, inhibiting pathways in the IL-23/Th17 axis that are central to psoriasis pathogenesis.27–29 Deucravacitinib displayed high selectivity for the TYK2 pseudokinase JH2 domain, stabilizing it in a conformational state and blocking both TYK2 receptor-mediated activation and downstream signaling.29 This high selectivity is a unique feature that may enable deucravacitinib to deliver maximum efficacy while avoiding potential side effects of less selective JAK1-3 inhibitors.27–29
In a Phase II trial, the efficacy and safety of variable deucravacitinib dosing regimens (3mg every other day (QOD), 3mg QD, 3mg BID, 6mg BID, or 12mg QD) were assessed in 267 patients with moderate to severe plaque psoriasis (BSA greater than or equal to 10%).30 By Week 12, a significantly greater proportion of patients in the 3-mg QD (39%), 3-mg BID (69%), 6-mg BID (67%), and 12-mg QD (75%) groups achieved at least PASI 75 compared to the placebo group (7%) (p<0.001). The difference between the 3-mg QOD (9%) and placebo (7%) groups lacked statistical significance (p=0.49). Notably, achievement of normal or near-normal DLQI, was only seen in the groups receiving 3mg BID or higher doses.30 Following these results, deucravacitinib was further studied in patients with psoriatic arthritis. Interim data from a Phase II trial evaluating the safety and efficacy of deucravacitinib in patients with active psoriatic arthritis showed a significantly greater proportion of 6- and 12-mg deucravacitinib QD-treated patients achieved ACR20 and PASI75 at Week 16, compared to placebo.31 Furthermore, emerging Phase III data from the POETYK PSO-1 trial shows significantly greater proportions of patients in the 6-mg deucravacitinib QD group achieving PASI 75 and the sPGA score of clear or almost clear at Week 16, compared to placebo.32, 33 Superiority to apremilast was also observed.33
AEs were observed in 51 percent of patients in the placebo group, and in 55 to 80 percent of patients in deucravacitinib-treated groups.30 The highest incidence of AEs was reported in the 6-mg BID group, occurring in 80 percent of patients. The most common AE in this group was nasopharyngitis, which occurred in 16 percent of patients, compared to 4 percent of placebo-treated patients. Acne developed in nine percent of patients taking 12mg of deucravacitinib but not in placebo patients (0%). Other common AEs included headache, diarrhea, nausea, and upper respiratory tract infection.30
Baricitinib. Baricitinib, an oral JAK1/JAK2 inhibitor, demonstrated efficacy in treating patients with moderate to severe psoriasis.34 A dose-ranging, Phase IIb study of 271 patients (BSA greater than or equal to 12%) compared four baricitinib doses (2, 4, 8, 10mg QD) and placebo treatment, demonstrating efficacy at higher doses.34, 35 At Week 12, 43.8 percent of 8-mg and 55.1 percent of 10-mg baricitinib-treated patients achieved PASI 75, compared to 17.6 percent of placebo-treated patients (p<0.05 and p<0.001, respectively). Similarly, a significantly higher proportion of patients in the 8- and 10-mg baricitinib dose groups achieved PASI 90. Of the patients who achieved PASI 75 by Week 12, over 81 percent maintained PASI 75 through Week 24. Whereas, of the patients who did not achieve PASI 75 by Week 12, 48 percent achieved PASI 75 at Week 24 after receiving additional baricitinib treatment through Weeks 13 to 24. Safety data concluded dose-dependent occurrence of treatment-emergent adverse events (TEAEs), with incidences in 44.1 percent, 50.0 percent, 47.2 percent 57.8 percent and 63.8 percent of the placebo, 2-, 4-, 8- and 10-mg baricitinib groups, respectively. The most common TEAEs were infestations and infections, occurring in 26.5 percent and 21.1 percent of placebo-treated and baricitinib-treated patients, respectively. Blood and lymphatic system disorders, such as lymphopenia, neutropenia, and anemia, were also noted at higher baricitinib doses (0%, 0%, 1.4%, 6.3% and 8.7 percent in the placebo, 2-, 4-, 8- and 10-mg groups, respectively), suggesting potential baricitinib dose intolerances in some patients. At Week 12, baricitinib-treated patients also had a 9.3-percent incidence of other reported AEs relating to laboratory results, most commonly elevation in blood creatine phosphokinase levels, compared to zero percent in the placebo group. Small increases in lipoproteins were also observed.34
Itacitinib. Itacitinib is a JAK1 inhibitor that is being investigated as an oral therapy for psoriasis. A Phase II, dose-escalation study demonstrated efficacy of itacitinib in treating patients with stable, chronic plaque psoriasis.36, 37 Overall, 48 patients (BSA greater than or equal to 5%) were randomized to treatment with itacitinib 100, 200, or 600mg QD, 200mg BID, or placebo for 28 days.36 By Day 28, the proportion of patients achieving PASI 75 was 0 percent, 11.1 percent, 0 percent, 22.2 percent, and 27.3 percent in the placebo, 100-, 200-mg QD, 200-mg BID and 600-mg QD itacitinib treatment groups, respectively. A statistically significant difference for achievement of PASI 75 vs. placebo was observed in the 600-mg QD itacitinib group (p=0.093). Moreover, by Day 28, a significantly greater proportion of patients achieved the static Physician Global Assessment (sPGA) response in the 200-mg BID and 600-mg QD groups, compared to placebo; 33.3 percent (p=0.063) and 45.5 percent (p=0.014), respectively, compared to 0 percent in the placebo group. The most common TEAE was nasopharyngitis, which occurred in 18.4 percent of patients in itacitinib-treated groups and in 8.3 percent of patients in the placebo group. Itacitinib-treated groups exhibited slight decreases in platelets and slight increases in lipid parameters, but these findings were considered clinically insignificant. The results of this study displayed that itacitinib is generally well tolerated.36
Brepocitinib. Brepocitinib is a potent TYK2/JAK1 selective inhibitor that is currently in development for the treatment of psoriasis. A Phase I trial of 30 patients (BSA greater than or equal to 15%) compared placebo and brepocitinib 30 or 100mg QD, demonstrating a dose-dependent trend for efficacy.38 At Day 28, a greater proportion of patients achieved a PGA response in the 30-mg (57.1%) and 100-mg (100%) brepocitinib QD groups, compared to the placebo group (0%). Mean change in PASI from baseline was -67.92 percent and -96.31 percent in the 30- and 100-mg brepocitinib QD groups, respectively. During the treatment period, 11.1 percent, 64.3 percent, and 85.7 percent of patients in the placebo, 30-mg, and 100-mg brepocitinib QD treatment groups, respectively, experienced TEAEs. Six patients in the 30-mg brepocitinib QD group and one patient in the 100-mg brepocitinib QD group discontinued treatment due to increased serum creatinine level and/or decreased neutrophil count. However, all TEAEs were mild in severity and there were no SAEs.38
Following these results, the efficacy of brepocitinib was further validated in a Phase II trial.39,40 Overall, 212 patients with plaque psoriasis (BSA greater than or equal to 10%) were enrolled in the study which included an induction phase (placebo, 30, or 60 mg QD until Week 4) and maintenance phase (placebo, 10mg QD, 30mg QD or 100mg QW until Week 12).39 At Week 12, the proportion of patients achieving PASI 75, PASI 90 and the PGA response was greater in all treatment groups compared to placebo, with statistically significant decreases in PASI in the 60- to 30-mg QD treatment group (p<0.001), 30-mg QD continuous treatment group (p<0.0001), 30- to 10-mg QD treatment group, 30-mg QD to 100-mg QW treatment group and 60-mg QD to 100-mg QW treatment group (p<0.05 all others), compared to placebo. The greatest change from baseline PASI, -17.3 (95% CI, —20.0 to —14.6), was observed in the 30-mg QD continuous treatment group. Correspondingly, the proportion of patients achieving PASI 75 and PASI 90 (86.2% and 51.7%, respectively), and the proportion of patients achieving the PGA response (83.8%), was also greatest in this treatment group. Safety data concluded that the risk difference for TEAEs between the pooled doses and placebo was 15.4 [95% CI, —5.9–37.8]. TEAEs were considered treatment-related in 44 of 189 brepocitinib-treated patients and 4 of 23 placebo-treated patients. In brepocitinib-treated patients, the most common TEAEs were headache (3.2%), psoriasis (2.1%), upper respiratory tract infection (1.6%), nausea (1.6%), and fatigue (1.6%).39
PF-06826647. PF-06826647 is a novel TYK2 inhibitor that has recently completed Phase II trial to evaluate its safety and efficacy in patients with moderate to severe plaque psoriasis.41 In its first in human study, AEs and changes in PASI scores were assessed in 40 patients with plaque psoriasis (BSA greater than or equal to 15%).42 Patients were randomized to receive placebo, 100mg, or 400mg PF-06826647 QD for 28 days. Patients in the 100- and 400-mg PF-06826647 QD groups experienced -14.62 and -24.18 mean changes in PASI from baseline, respectively, compared to -11.13 in the placebo group. TEAEs occurred in 35.7 percent, 27.3 percent and 33.3 percent of patients in the placebo, 100-mg PF-06826647 QD, and 400-mg PF-06826647 QD groups, respectively. No SAEs were observed in the trial.42
Filgotinib. Filgotinib, a selective JAK1 inhibitor, has shown efficacy in improving the pruritic component of psoriasis and decreasing the severity of psoriatic lesions and is currently undergoing a Phase III trial, which includes PASI response, among other outcome measures, to evaluate its effect on cutaneous manifestation in patients with psoriatic arthritis.43,44 In a Phase II trial designed to evaluate the efficacy and safety of filgotinib (200 mg) in patients with psoriatic arthritis, improvements in patients’ plaque psoriasis were observed and assessed by PASI 75 and the pruritus numerical rating scale in 82 patients with psoriasis (BSA greater than or equal to 3%). More patients on filgotinib than placebo achieved PASI 75 by Week 16 (treatment difference 30% [95% CI, 10.4-47.0], p=0·0034). Moreover, 58 percent of filgotinib-treated patients had an improvement in pruritus numeric rating scale of at least three points, compared to 22 percent of placebo-treated patients (p=0.0022). The most common TEAEs were nasopharyngitis and headache, which occurred at similar rates in the treatment and placebo groups. Nasopharyngitis occurred in 12 percent of filgotinib-treated patients and 15 percent of placebo-treated patients. Whereas headache occurred in five percent of filgotinib-treated patients and eight percent of placebo-treated patients. The incidence of infections was similar between the two groups.43
Upadacitinib. Upadacitinib is a selective JAK1 inhibitor that has recently met all primary and secondary endpoints in a Phase III trial designed to evaluate the safety and efficacy of the medication in 641 adult patients with active psoriatic arthritis.45 Patients were randomized to placebo, 15, or 30 mg upadacitinib QD, followed by either 15 or 30 mg upadacitinib QD at Week 24.45 At Week 12, 57 percent and 64 percent of patients treated with 15 and 30 mg upadacitinib QD, respectively, achieved the American College of Rheumatology 20-percent improvement (ACR 20) response, compared to 24 percent in the placebo group (p<0.0001). At Week 16, 16 percent, 52 percent, and 57 percent of the placebo, 15-, and 30-mg upadacitinib QD groups, respectively, achieved PASI 75 (p<0.0001). Moreover, at Week 24, three percent, 25 percent, and 29 percent, of the placebo, 15-, and 30-mg upadacitinib groups, respectively, achieved minimal disease activity (p<0.0001).46 In another study evaluating the safety of upadacitinib in patients with rheumatoid arthritis, the incidence of infection in the 15- and 30-mg upadacitinib-treated groups (29% and 32%, respectively) was higher than in placebo-treated group (21%).47 Other frequently reported adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache. A higher incidence of serious AEs was reported in the 15-mg upadacitinib-treated group (4%), compared to the 30-mg upadacitinib-treated group (3%) and placebo group (2%).47
Abrocitinib. Abrocitinib is a JAK1 inhibitor that was evaluated for the treatment of patients with plaque psoriasis in a Phase II trial.48,49 In this trial, 59 patients with moderate-to-severe chronic plaque psoriasis were randomized to placebo, 200mg, 400mg abrocitinib QD or 200mg abrocitinib BID. At Week 4, the mean change from baseline (90% CI) in PASI was -6.6 (-10.1 to -3.2), -11.7 (-14.9 to -8.6), -13.1 (-16.9 to -9.3) and -13.7 (-17.0 to -10.5) in the placebo, 200mg QD, 400mg QD and 200mg BID groups, respectively.48 Correspondingly, the proportion of patients achieving PASI 75 at Week 4 was 17 percent, 17 percent, 50 percent and 60 percent in the placebo, 200mg QD, 400mg QD and 200mg BID groups, respectively. Notably, abnormal laboratory test results of clinical interest, such as low neutrophil, reticulocyte, and platelet counts, occurred more frequently in the 200mg BID group compared to the QD treatment groups. However, no serious infections or bleeding events related to neutropenia or thrombocytopenia were reported.48 This trial was later terminated due to business-related reasons.49
Other Potential Oral Therapies
Piclidenoson (CF101). Adenosine A3 receptor is overly expressed in inflammatory cells, playing a role in propagation of inflammatory and autoimmune states. Piclidenoson, an adenosine A3 receptor agonist, de-regulates Wnt and the nuclear factor kappa-B signal transduction pathways, therefore inhibiting tumor necrosis factor alpha (TNF-alpha), IL-6 and IL-12.50 In a Phase II/III study, the safety and efficacy of piclidenoson in patients with moderate to severe plaque-type psoriasis (BSA greater than or equal to 10%) was assessed.50 In segment 1 of this trial, 103 patients were randomized to placebo, 1mg piclidenoson BID, or 2mg piclidenoson BID for 12 weeks (1-mg BID group was later eliminated due to futility). In segment 2, an additional 223 patients were randomized to receive placebo or piclidenoson 2mg BID for 16 weeks, followed by a transition to piclidenoson 2 mg BID open-label extension period. The primary endpoint of PASI 75 at Week 12 was not met; only 8.5 percent of piclidenoson-treated patients achieved PASI 75 compared to 6.9 percent of placebo-treated patients (p=0.621). At Week 32, the proportion of 2-mg piclidenoson-treated patients achieving PASI 75 was 35.5 percent (p<0.05). Mean percent improvement in PASI in piclidenoson-treated patients was 57 percent, compared to baseline (p<0.001). Overall, piclidenoson compared favorably to apremilast; 35.3 percent of patients on piclidenoson achieved PASI 75 by Week 32, compared to 26.3 percent of patients on apremilast. The safety profile of all tested piclidenoson dosage groups was similar to that of placebo.50 Another study conducted by David et al51 also suggested the relatively favourable safety profile of piclidenoson.AEs occurred in 58.3 percent of 1-mg piclidenoson-treated patients, 17.6 percent of 2-mg piclidenoson-treated patients and 13.3 percent of 4-mg piclidenoson-treated patients, compared to an incidence of 21.1 percent in the placebo group. Reported AEs were mostly mild to moderate and included pruritus, skin rash, and psoriasis exacerbation.51
Belumosudil (KD025). ROCK2 inhibition downregulates pro-inflammatory T cell response. Belumosudil is a ROCK2 inhibitor that appears to be efficacious in patients with psoriasis vulgaris.52 In a Phase II study, 38 patients with psoriasis vulgaris (BSA greater than or equal to 3%) were randomized to receive 200mg belumosudil BID, 400 mg belumosudil QD or 400 mg belumosudil BID. Notably, at Week 12, 71 percent, 42 percent, and 29 percent of patients achieved PASI 50 in the 200-mg BID, 400-mg QD, and 400-mg BID treatment groups, respectively, suggesting a potential clinical benefit of using a lower dosage regimen. A time-dependent decrease of IL-17 and IL-23 levels was observed in patients that responded clinically to belumosudil. After 12 weeks of belumosudil treatment, histological analysis of skin biopsy revealed a significant decrease of epidermal thickness, K16 expression, and the T cell infiltrate in both epidermis and dermis compartments. However, at Week 12, only 14.2 percent, 16.7 percent, and 14.2 percent patients achieved PASI 75 in the 200-mg BID, 400-mg QD, and 400-mg BID treatment groups, respectively, making belumosudil appear to be less robust than other oral treatments, such as methotrexate, JAK inhibitors, apremilast, and biologics. Safety data concluded that the medication appears to be generally well tolerated with only limited treatment related AEs and no SAEs reported.52
Ponesimod. Ponesimod inhibits sphingosine 1-phosphate receptor 1 (S1PR1), therefore modulating lymphocyte recirculation. A dose-ranging, Phase II trial of ponesimod 20 or 40 mg QD demonstrated efficacy in patients with moderate to severe plaque psoriasis (BSA greater than or equal to 10%).53 Overall, 46.0 percent and 48.1 percent of patients in the 20- and 40-mg QD groups achieved PASI 75 at Week 16, compared to 13.4 percent of placebo-treated patients (p<0.0001). At Week 28, the estimated probability of relapse was higher in patients switched to placebo compared to patients who continued ponesimod treatment.53
Heightened liver enzyme concentrations were observed in ponesimod-treated patients. Increased alanine aminotransferase concentrations occurred in 14.3 percent and 10.5 percent of the 20- and 40-mg ponesimod-treated patients, respectively, compared to 3.0 percent of the placebo-treated patients. Increased aspartate aminotransferase concentrations occurred in 5.6 percent and 6.8 percent of the 20- and 40-mg ponesimod-treated patients, respectively, compared to 3.0 percent of the placebo-treated patients. Higher incidence of dyspnea was also observed in the 20-mg QD (11.1%) and 40-mg QD (26.3%) groups compared to the placebo group (1.5%). Moreover, dizziness was also more common in the 20-mg QD (5.6%) and 40-mg QD (4.5%) groups compared to the placebo group (1.5%).53 In addition, clinical data has shown a consistent dose-dependent heart rate reduction and changes in atrioventricular node conduction following first-dose administration of ponesimod.54 A gradual dose titration regimen was found to minimize the cardiac effects associated with initiation of ponesimod treatment.54
Conclusion
In this article, we reviewed the safety and efficacy of existing and emerging oral treatments for psoriasis. Apremilast, an FDA-approved treatment for psoriasis, has demonstrated efficacy and a relatively favorable safety profile. Notably, apremilast also appears to affect metabolic parameters in patients with psoriasis. Furthermore, the modulation of the JAK-STAT pathway via oral pharmaceuticals for the treatment of psoriasis is undergoing investigation. Tofacitinib has demonstrated non-inferiority to etanercept. Other JAK-STAT inhibitors, such as baricitinib, itacitinib, baricitinib, BMS-986165, PF-06826647, filgotinib and upadacitinib have also demonstrated efficacy in Phase II to III clinical trials. However, long-term safety is still being investigated. Finally, the targeting of pro-inflammatory pathways through ROCK2, S1PR1 and adenosine A3 modulation is also currently in development.
References
- Evans C. Managed care aspects of psoriasis and psoriatic arthritis. Am J Manag Care. 2016;22(8 Suppl):s238-243.
- Alcusky M, Lee S, Lau G, et al. Dermatologist and Patient Preferences in Choosing Treatments for Moderate to Severe Psoriasis. Dermatol Ther (Heidelb). 2017;7(4):463-483.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445-1486.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583-1590.
- Van Voorhees AS, Stein Gold L, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: Results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83(1):96-103.
- Martin G, Young M, Aldredge L. Recommendations for Initiating Systemic Therapy in Patients with Psoriasis. J Clin Aesthet Dermatol. 2019;12(4):13-26.
- Puig L, Korman N, Greggio C, et al. Hemoglobin A1c and weight changes with apremilast in patients with psoriasis and psoriatic arthritis: Pooled laboratory analysis of the phase 3 ESTEEM and PALACE trials. J Am Acad Dermatol. 2018;79(3):AB151.
- Del Alcázar E, Suárez-Pérez JA, Armesto S, et al. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicentre study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol. 2020;34(12):2821-2829.
- Papadavid E, Rompoti N, Theodoropoulos K, Kokkalis G, Rigopoulos D. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatology Venereol 2018;32: 1173–1179.
- Kishimoto M, Komine M, Kamiya K, et al. Drug survival of apremilast in a real-world setting. J Dermatol. 2019;46(7):615–617.
- Barbieri M, Loconsole F, Migliore A, Capri S. A cost-effectiveness and budget impact analysis of apremilast in patients with psoriasis in the Italian setting. J Med Econ. 2020;23(4):362-370.
- Kaplan DL, Ung BL, Pelletier C, Udeze C, Khilfeh I, Tian M. Switch Rates and Total Cost of Care Associated with Apremilast and Biologic Therapies in Biologic-Naive Patients with Plaque Psoriasis. Clinicoecon Outcomes Res. 2020;12:369-377.
- Wu JJ, Pelletier C, Ung B, Tian M, Khilfeh I, Curtis JR. Real-world switch patterns and healthcare costs in biologic-naive psoriasis patients initiating apremilast or biologics. J Comp Eff Res. 2020;9(11):767-779.
- Welsch K, Holstein J, Laurence A, Ghoreschi K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol. 2017;47(7):1096-1107.
- Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552-561.
- Tian F, Chen Z, Xu T. Efficacy and safety of tofacitinib for the treatment of chronic plaque psoriasis: a systematic review and meta-analysis. J Int Med Res. 2019;47(6): 2342-2350.
- Merola JF, Elewski B, Tatulych S, et al. Efficacy of tofacitinib for the treatment of nail psoriasis: Two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2017;77(1):79-87.e1.
- Bissonnette R, Iversen L, Sofen H, et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol. 2015;172(5):1395-1406.
- Winthrop KL, Lebwohl M, Cohen AD, et al. Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol. 2017;77(2):302-309.
- Strober B, Gottlieb AB, van de Kerkhof PCM, et al. Benefit–risk profile of tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: pooled analysis across six clinical trials. Br J Dermatol. 2019;180(1):67-75.
- Wolk R, Armstrong EJ, Hansen PR, et al. Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J Clin Lipidol. 2017;11(5):1243-1256.
- Valenzuela F, Korman NJ, Bissonnette R, et al. Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br J Dermatol. 2018;179(4):853-862.
- Bungey G, Chang-Douglass S, Hsu MA, Cappelleri JC, Young P, Woolcott J. Costs and Health Outcomes Associated with Tofacitinib Treatment for Active Psoriatic Arthritis in the United States. J Manag Care Spec Pharm. 2020;26(8):1027-1038.
- Gillooly K, Zhang Y, Yang X, et al. BMS-986165 Is a Highly Potent and Selective Allosteric Inhibitor of Tyk2, Blocks IL-12, IL-23 and Type I Interferon Signaling and Provides for Robust Efficacy in Preclinical Models of Systemic Lupus Erythematosus and Inflammatory Bowel Disease. Presented at: ACR/ARHP Annual Meeting; November 15, 2016; Washington.
- Patrick MT, Stuart PE, Raja K, et al. Genetic signature to provide robust risk assessment of psoriatic arthritis development in psoriasis patients. Nat Commun. 2018;9(1):4178.
- Dendrou CA, Cortes A, Shipman L, et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med. 2016;8(363):363ra149.
- O’Shea JJ et al. In: Rich RR et al. eds. Clinical Immunology: Principles and Practices. 5th ed. Cambridge, MA: Elsevier Inc; 2019.
- Kim, L.S., Wu, J.J. & Han, G. Deucravacitinib for Psoriasis. Curr Derm Rep. 2021;10:1-5.
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019 Jul 24;11(502):eaaw1736.
- Papp K, Gordon K, Thaçi D, et al. Phase 2 Trial of Selective Tyrosine Kinase 2 Inhibition in Psoriasis. N Engl J Med. 2018;379:1313-1321.
- Mease P, Deodhar A, van der Heijde D, et al. Efficacy and Safety of Deucravacitinib (BMS-986165), an Oral, Selective Tyrosine Kinase 2 Inhibitor, in Patients with Active Psoriatic Arthritis: Results from a Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial. Presented at: American College of Rheumatology Convergence 2020; October 23, 2020; Virtual.
- Bristol Myers Squibb Announces Deucravacitinib (BMS-986165) Demonstrated Superiority to Placebo and Otezla® (apremilast) in Pivotal Phase 3 Psoriasis Study [press release]. New York, NY: Bristol-Myers Squibb; November 3, 2020. Available at: https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Announces-Deucravacitinib-BMS-986165-Demonstrated-Superiority-to-Placebo-and-Otezla-apremilast-in-Pivotal-Phase-3-Psoriasis-Study/default.aspx. Accessed January 30, 2021.
- Terry, M., 2021. Bristol Myers’ Experimental Psoriasis Drug Beats Out Amgen’s Otezla In Phase III [online]. BioSpace. Available at: https://www.biospace.com/article/bristol-myers-squibb-s-deucravacitinib-beats-out-amgen-s-otezla-for-psoriasis/ Accessed 24 January 2021.
- Papp KA, Menter MA, Raman M, et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2016;174(6):1266-1276.
- Eli Lilly and Company. A Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging, Phase 2b Study of Baricitinib in Patients With Moderate-to-Severe Plaque Psoriasis. Available at: https://clinicaltrials.gov/ct2/show/study/NCT01490632. NLM identifier: NCT01490632. Accessed January 30, 2020.
- Bissonnette R, Luchi M, Fidelus-Gort R, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of the safety and efficacy of INCB039110, an oral janus kinase 1 inhibitor, in patients with stable, chronic plaque psoriasis. J Dermatolog Treat. 2016;27(4):332-338.
- Incyte Corporation. A Double-Blind, Placebo-Controlled Study Exploring the Safety, Tolerability, and Efficacy of a 28-Day Course of Escalating Doses of Oral Itacitinib in Subjects With Stable, Chronic Plaque Psoriasis. Available at: https://clinicaltrials.gov/ct2/show/study/NCT01634087. NLM identifier: NCT01634087. Accessed January 30, 2020.
- Banfield C, Scaramozza M, Zhang W, et al. The Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of a TYK2/JAK1 Inhibitor (PF-06700841) in Healthy Subjects and Patients with Plaque Psoriasis. J Clin Pharmacol. 2018;58(4):434–447.
- Forman SB, Pariser DM, Poulin Y, et al. TYK2/JAK1 Inhibitor PF-06700841 in Patients with Plaque Psoriasis: Phase IIa, Randomized, Double-Blind, Placebo-Controlled Trial. J Invest Dermatol. 2020;140(12):2359-2370.e5.
- Pfizer. A Phase 2A, Randomized, Double-Blind Placebo-Controlled Study to Evaluate Safety and Efficacy of PF-06700841 in Subjects With Moderate to Severe Plaque Psoriasis. Available at: https://clinicaltrials.gov/ct2/show/study/NCT02969018. NLM identifier: NCT02969018. Accessed January 30, 2020.
- Pfizer. A Phase 2, Randomized, Double Blind, Placebo-Controlled, Study to Evaluate the Safety and Efficacy of PF-06826647 in Participants with Moderate to Severe Plaque Psoriasis. Available at: https://clinicaltrials.gov/ct2/show/NCT03895372. NLM identifier: NCT03895372. Accessed December 25, 2020.
- Pfizer. A Phase 1, Within Cohort, Randomized, Double Blind, Third-party Open, Placebo-controlled, Single- and Multiple Dose escalation, Parallel Group Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of PF-06826647 in Healthy Subjects and Subjects with Plaque Psoriasis. Available at: clinicaltrials.gov/show/NCT03210961. NLM identifier: NCT03210961. Accessed November 15, 2020.
- Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10162):2367-2377.
- Gilead Sciences. A Phase 3, Randomized, Double-blind, Placebo and Adalimumab-controlled Study to Evaluate the Efficacy and Safety of Filgotinib in Subjects With Active Psoriatic Arthritis Who Are Naive to Biologic DMARD Therapy. Available at: https://clinicaltrials.gov/ct2/show/study/NCT04115748. NLM identifier: NCT04115748. Accessed January 30, 2020.
- AbbVie. A Phase 3, Randomized, Double-Blind, Study Comparing Upadacitinib (ABT-494) to Placebo in Subjects With Active Psoriatic Arthritis Who Have a History of Inadequate Response to at Least One Biologic Disease Modifying Anti-Rheumatic Drug (bDMARD). Available at: https://clinicaltrials.gov/ct2/show/study/NCT03104374. NLM identifier: NCT03104374. Accessed December 26, 2020.
- RINVOQ™ (upadacitinib) Meets Primary and All Ranked Secondary Endpoints in Phase 3 Study in Psoriatic Arthritis [press release]. Chicago, IL: AbbVie; October 31, 2019. Available at: https://news.abbvie.com/news/press-releases/rinvoq-upadacitinib-meets-primary-and-all-ranked-secondary-endpoints-in-phase-3-study-in-psoriatic-arthritis.htm. Accessed December 31, 2020.
- Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503-2512.
- Schmieder GJ, Draelos ZD, Pariser DM, et al. Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: phase II, randomized, double-blind, placebo-controlled study. Br J Dermatol. 2018;179(1):54-62.
- Pfizer. A Phase 2, Randomized, Double-blind, Placebo-controlled Study To Evaluate Safety And Efficacy Of Pf-04965842 In Subjects With Moderate To Severe Psoriasis. Available at: https://clinicaltrials.gov/ct2/show/study/NCT02201524. NLM identifier: NCT02201524. Accessed January 30, 2020.
- David M, Gospodinov DK, Gheorghe N, et al. Treatment of Plaque-Type Psoriasis With Oral CF101: Data from a Phase II/III Multicenter, Randomized, Controlled Trial. J Drugs Dermatol JDD. 2016 01;15(8):931-938.
- David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26(3):361-367.
- Zanin-Zhorov A, Weiss JM, Trzeciak A, et al. Cutting Edge: Selective Oral ROCK2 Inhibitor Reduces Clinical Scores in Patients with Psoriasis Vulgaris and Normalizes Skin Pathology via Concurrent Regulation of IL-17 and IL-10. J Immunol. 2017;198(10):3809-3814.
- Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384(9959):2036-2045.
- D’Ambrosio D, Freedman MS, Prinz J. Ponesimod, a selective S1P1 receptor modulator: a potential treatment for multiple sclerosis and other immune-mediated diseases. Ther Adv Chronic Dis. 2016;7(1):18-33.

