 J Clin Aesthet Dermatol. 2021;14(12):64–65.
J Clin Aesthet Dermatol. 2021;14(12):64–65.
by Shikha Walia, BS; Mirjana G. Ivanic BA; Zainab A. Jafri, DO; and Jashin J. Wu, MD
Dr. Walia is with Lake Erie College of Osteopathic Medicine in Bradenton, Florida. Dr. Ivanic is with Meharry Medical College in Nashville, Tennessee. Dr. Jafri is with Arizona College of Osteopathic Medicin in Glendale, Arizona. Dr. Wu is with the Dermatology Research and Education Foundation in Irvine, California.
ABSTRACT: Amid the current COVID-19 pandemic, there is concern for increased risk of infection while on immunomodulatory therapy. Omalizumab, a monoclonal antibody, is an add-on therapy for the treatment of chronic idiopathic urticaria (CIU) when first line therapy alone fails to achieve appropriate response. Current understanding of the response to COVID-19 infection is largely varied and actively under investigation. In the context of a pandemic, it is important to consider the safety profile of omalizumab as it modulates the immune system. We reviewed data from pivotal Phase III clinical trials investigating omalizumab use in CIU patients with a focus on reported respiratory-related adverse events (AEs) to assess these risks. Results from three phase III trials show that omalizumab adjunct therapy does not significantly increase infection risk and severity.
Keywords: Chronic idiopathic urticaria, COVID-19, immunomodulator, omalizumab, urticaria
Chronic idiopathic urticaria (CIU), an immunologic-mediated condition resulting in hives (with/without angioedema) lasting six weeks or longer with no identifiable trigger, is difficult to treat with H1-antihistamines alone. Omalizumab has demonstrated efficacy as an add-on therapy in CIU patients with persistent symptoms and was approved by the United States Food and Drug Administration (FDA) in 2014 for the treatment of CIU in patients ages 12 years and older. Omalizumab is a monoclonal antibody that targets circulating immunoglobin E (IgE), dampening the release of cytokines from mast cells and basophils. The current understanding of the response to COVID-19 infection is largely varied and actively under investigation.1 During a viral pandemic, it is therefore important to consider the drug’s safety profile as an immunomodulator.
Potential risks presented to CIU patients on omalizumab include increased susceptibility to COVID-19 infection secondary to immunomodulatory effects and exposure during drug administration. Risk of loss of response following omalizumab discontinuation is also relevant.2 Data from pivotal phase III clinical trials investigating omalizumab use in CIU patients was used to evaluate these risks.
Methods
Two similarly designed randomized, double-blind, placebo-controlled phase III trials (ASTERIA I and ASTERIA II) investigated the efficacy and safety of omalizumab (75mg, 150mg, and 300mg doses) in CIU patients ages 12 to 75 years remaining symptomatic despite approved doses of H1-antihistamines. The main difference in design was duration of the treatment period (ASTERIA I: 24 weeks; ASTERIA II:12 weeks). A third randomized, double-blind, placebo-controlled phase III trial (GLACIAL) investigated the safety and tolerability of 300mg omalizumab in CIU patients with persistent disease despite high-dose H1-antihistamines plus H2-antihistamines, leukotriene receptor antagonists, or both (Table 1).
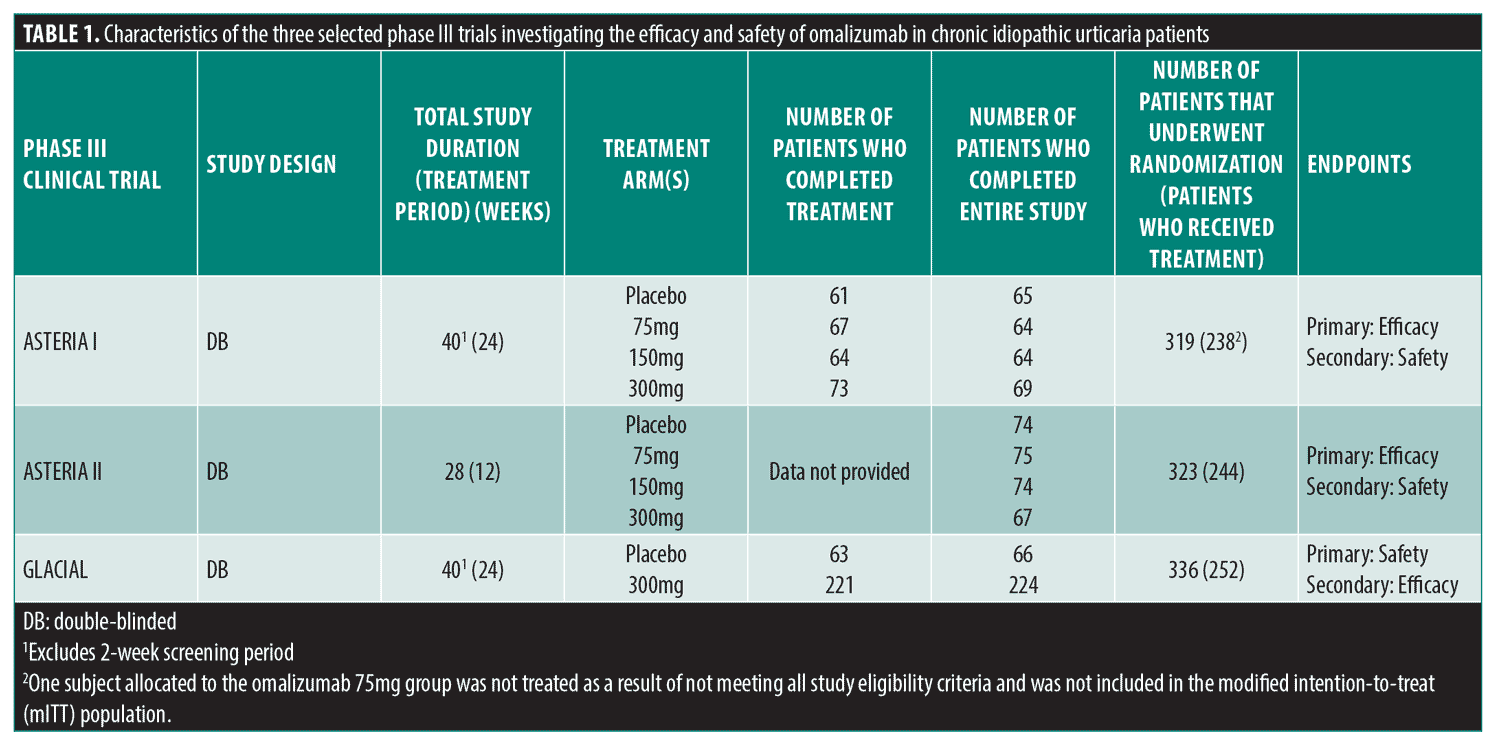
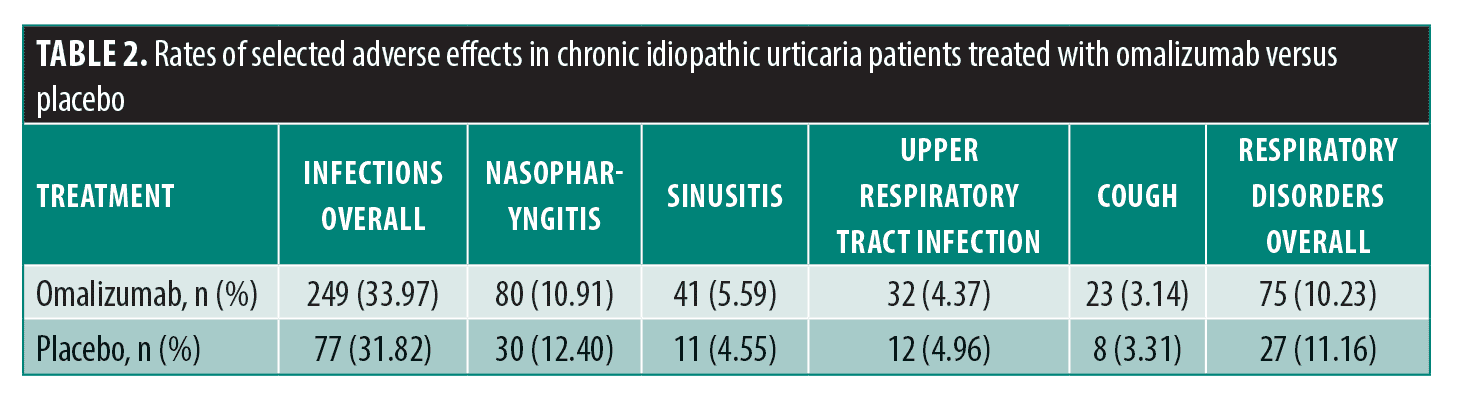
To appropriately assess risk as it relates to COVID-19, focus was placed on the following six adverse events (AEs) in each study: overall infections and infestations; nasopharyngitis; sinusitis; upper respiratory tract infection (URTI); overall respiratory, thoracic, mediastinal disorders; and cough. For both ASTERIA I and II, the dosed treatment groups were combined into one treatment group. Rates of these AEs in treatment versus placebo groups were then compared for all three trials.
Results
In ASTERIA I treatment versus placebo, there were slightly increased rates of overall infections or infestations (28.5% vs. 27.5%) and sinusitis (5.04% vs. 5.00%); the remaining AE rates were lower in the treatment group.3 An increased rate of sinusitis was observed in the treatment group compared to the placebo group (4.12% vs. 2.50%) in ASTERIA II; the remaining AE rates were lower in the treatment group.4 In GLACIAL, the rates of all six AEs were higher in the treatment group compared to the placebo group.5 Kaplan et al2 reported that while there was an increased frequency of URTI in the treatment group (7.14%) compared to placebo (2.41%), the incidence and severity of AEs were similar between the two cohorts (Table 2).
Limitations. Our analysis is limited by reported AE data from clinical trials, and by the selected AEs, as the manifestations of COVID-19 extend beyond the respiratory system.
Conclusion
Collectively, these data do not suggest increased susceptibility to respiratory infection in CIU patients receiving omalizumab. Priority should be placed on individual patient risk of COVID-19 infection balanced with omalizumab’s benefits in CIU, particularly in disease burden and quality of life.
References
- Catanzaro M, Fagiani F, Racchi M, et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Sig Transduct Target Ther. 2020;5(1):84.
- Kaplan A, Ferrer M, Bernstein JA, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol. 2016;137(2):474–481.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study [published correction appears in J Invest Dermatol. 2015 Mar;135(3):925]. J Invest Dermatol. 2015;135(1):67–75.
- Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–935.
- Maurer M, Rosén K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria [published correction appears in N Engl J Med. 2013;368(24):2340–2341]. N Engl J Med. 2013;368(10):924–935.

