 J Clin Aesthet Dermatol. 2020;13(9):21–23
J Clin Aesthet Dermatol. 2020;13(9):21–23
by Ahmed A. Saleh, MD; Aiham M. Al-Obaidi, MSc; Eman G. Behiry, MD; and Ahmed Mohammed Hamed, MD
Mr. Al-Obaidi, Dr. Saleh, and Dr. Hamed are with the Department of Dermatology and Andrology of the Faculty of Medicine at Benha University in Benha, Egypt. Dr. Behiry is with the Department of Clinical and Chemical Pathology of the Faculty of Medicine at Benha University in Benha, Egypt.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Despite the many theories that have been published over the years, the exact pathology of chronic urticaria (CU) is still largely unknown. Eosinophils have been implicated in many cutaneous disorders—serving as major effector cells, inducing tissue damage and dysfunction by releasing granule proteins, including eosinophil cationic protein (ECP), inflammatory lipid mediators, mitochondrial DNA, and eosinophil-derived neurotoxin (EDN), which is relatively neutral with some cytotoxic properties.
Objective: We sought to evaluate serum levels of EDN in patients with CU and to correlate their levels with the severity of their disease.
Participants: Fifty patients with CU and 30 matching healthy individuals, serving as controls, were recruited from the outpatient clinic of the Dermatology, Venereology, and Andrology Department of Benha University Hospitals.
Methods: 5ml of venous blood were drawn from all participants in a fasted state, stored in sterile tubes, and used to measure the serum level of EDN following the manufacturer’s instructions.
Results: The serum level of EDN was statistically significantly different in both groups, and the serum level of EDN significantly correlated with the severity of CU.
Conclusion: The significantly higher EDN level in patients with CU suggests its role in the pathogenesis of the disease, and its significant positive correlation with the severity of the disease suggests promising therapeutic solutions.
Keywords: Chronic urticaria, eosinophil-derived neurotoxin
Chronic urticaria (CU) is characterized by the persistence of spontaneously appearing skin lesions, known as wheals, that are not associated with angioedema, persist for six weeks or more, and negatively affect a patient’s quality of life. Since the pathogenesis of CU is still unclear, its treatment is empirical, with frustrating results.1
Immunoglobulin E (IgE) has been previously identified as the main mediator of the pathogenesis of CU, atopic dermatitis, asthma, and allergic rhinitis. Additionally, mast cells and basophils have been recognized as main effector cells in CU. The current focus has been directed to investigate the role of eosinophils in CU.2
Eosinophils have been implicated in many cutaneous disorders, as it is one of the major perivascular inflammatory infiltrate and extracellular granule proteins deposits (e.g., major basic protein, eosinophil cationic protein [ECP], eosinophil peroxidase, and eosinophil-derived neurotoxin [EDN]). The exact role of eosinophils is still unclear, though some studies have suggested that it contributes to pathogen defense, inflammatory response regulation, and fibrosis induction.3,4
The EDN, which is relatively neutral with cytotoxic properties, is one of the major secretory proteins present in the specific granules of human eosinophils. Its release is triggered by cytokines and other pro-inflammatory mediators, and is able to attract other immune cells.5
The aim of this study was to evaluate serum levels of EDN in patients with chronic urticaria and to correlate their levels with the severity of their disease.
Methods
This case-control study included 50 patients with CU recruited from the outpatient clinic of the Dermatology, Venereology, and Andrology Department of Benha University Hospitals between October 2018 and January 2019. Each participant signed a written informed consent before joining the study. Patients were excluded from the study if they had used topical treatments within two weeks prior or systemic treatments within one month prior to the beginning of the study. Patients with a history of any systemic disease or autoimmune disorders (e.g. Lupus erythematous, thyroid disorders, Helicobacter pylori, intestinal infections, atopy) were also excluded from this study.
A detailed history was taken from each patient to identify the cause of CU. All patients were asked about disease duration, lesion distribution, frequency of attacks, history of food additives, occupation, and previous therapeutic measures. Complete dermatological examination was performed to identify the morphology of the wheals, bruising, and signs of any systemic disease.
Following the recommendations of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum (EAACI/ GA2LEN/ EDF) activity score, the Urticaria Activity Score (UAS) was used for four consecutive days to determine disease severity for each patient by calculating the sum of the wheals score (0=absent lesions, 1=1–20 lesions, 2=20–50 lesions, and 3=more than 50 lesions in 24 hours) and the itching score (0=no itching, 1=mild itching, 2=moderate itching, and 3=severe itching). The score ranged from 0 to 6, where 0=no disease, 1–2=mild disease, 3–4=moderate disease, and 5–6=severe disease.7
After determining disease severity for each patient, 5ml of venous blood were drawn from all participants, stored in sterile tubes and left to clot; serum was then separated and stored at -20°C until it was used to measure the serum level of EDN following the manufacturer’s instructions. Serum EDN was measured using Human (EDN) ELISA Kit (Sun Red Biotechnology; Shanghai, China), with Cat. No.: 201-12-5363.
Statistical analysis. The 15th version of SPSS software (Spss Inc; Chicago, Illinois) was used for tabulation and organization of the gathered data. Chi-square test (X2) or Fisher’s exact test (FET) were used to analyze categorical data, presented as numbers and percentages. Continuous data were presented as mean±standard deviation, median, and range. Variables with normal distribution among two independent groups were evaluated using the Student t test. Associations among nonparametric variables were detected by Spearman’s correlation coefficient (rho). The cutoff value with the optimum sensitivity and specificity was detected using the ROC curve. Predictors were detected via performing a regression analysis. A P-value of less than 0.05 was considered significant.
Results
The present study included 50 patients (26 male, 24 female) with CU with a duration of disease ranging from 0.17 to 10 years (Mean±SD=2.28±2.27 years). Thirty age- and sex-matched healthy subjects (16 male and 14 female) served as a control group. Patients were between 20 and 58 years of age (Mean±SD=34.14±7.37 years), while controls were between 18 and 47 years of age (Mean±SD=31.14±8.31 years). Eleven patients (22%) were smokers, while the control group included six smokers (20%) with statistically insignificant different demographic data among both groups.
The most common precipitating factor of CU, reported by 24 patients (48%), was food additives, and the most common treatment used by patients with CU was antihistamine and corticosteroid, reported by 36 patients (72%). Most patients (n=24) experienced mild disease severity, indicated by weekly UAS evaluation, while 16 patients experienced moderate disease and 10 patients experienced severe disease.
The mean EDN serum levels were significantly higher in patients with CU than the control group (Mean±SD: 6.92±8.01ng/ml; 3.11±1.29ng/mL respectively, P= 0.012). There was non-significant relation between serum EDN levels and sex of patients, special habits, precipitating factors, and previous treatment (P= 0.535, P= 0.918, P= 0.510, and P=0.133, respectively).
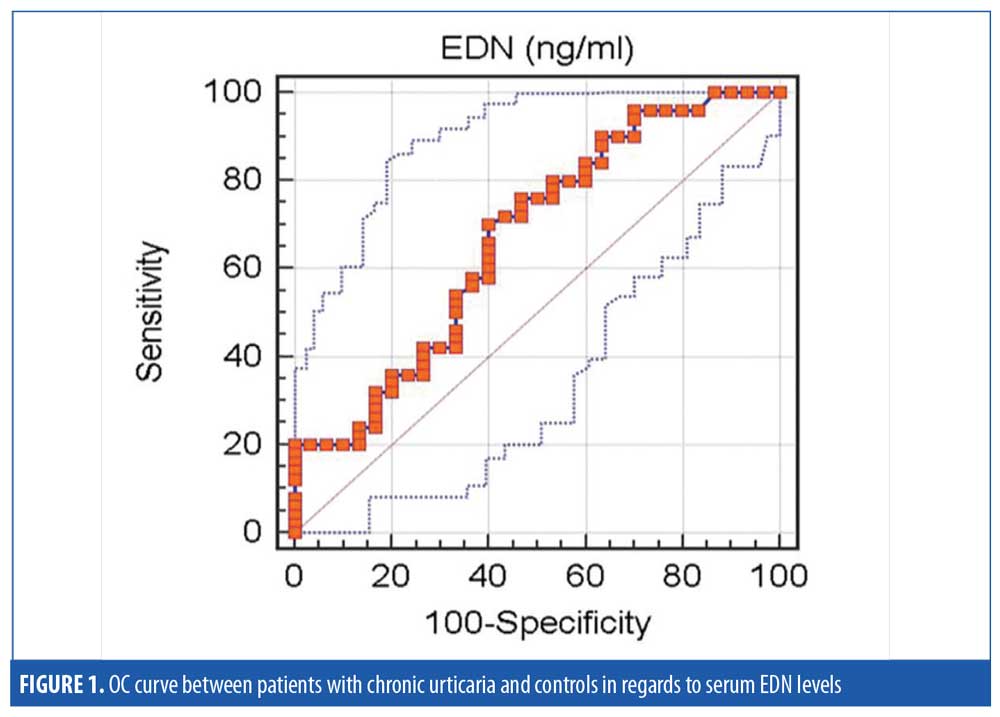
At a cut-off value of ?2.8ng/ml, serum EDN had 70-percent sensitivity and 60-percent specificity with the accuracy of 66.9 percent in the diagnosis of CU (Figure 1).
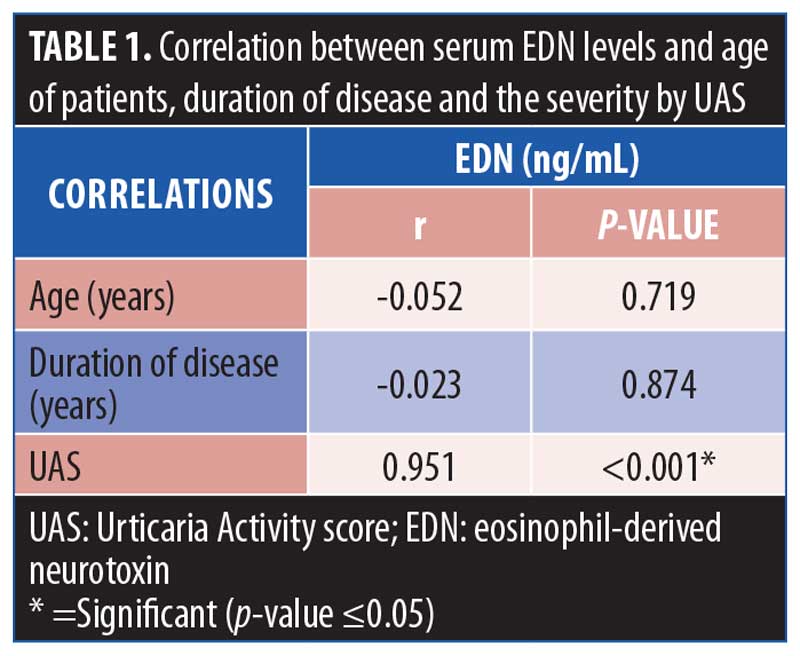
Serum EDN levels were positively correlated with the severity of the disease (r=0.951, P<0.001), while there were non-significant correlations with age and duration of disease (P>0.05) (Table 1).
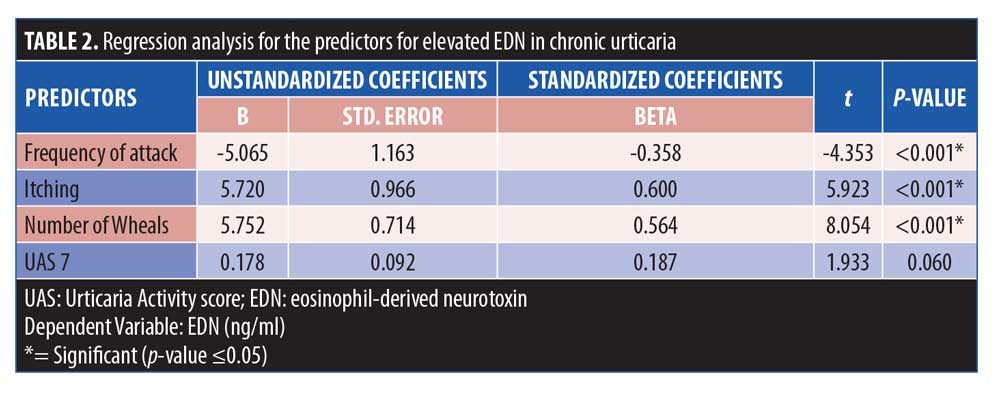
Applying regression analysis, we found that the frequency of attacks, itching, and number of wheals were significant predictors of elevated EDN in patients with CU (Table 2).
Discussion
CU is defined as a persistent or recurrent disease of the skin in which an identified trigger leads to the degranulation of mast cells. In addition to IgE and antigen complexes, a variety of IgE-independent stimuli, including neuropeptides, complements, products of infectious agents, and oxidative stress, can activate mast cell degranulation and cytokine production.8 Eosinophils have been implicated in many cutaneous disorders, being one of the major perivascular inflammatory infiltrate and extracellular granule proteins deposits (e.g., major basic protein, ECP, eosinophil peroxidase, and EDN).4
In the current study, patients with CU demonstrated a significantly higher mean serum EDN level than healthy controls. To our knowledge, the serum level of EDN in patients with CU has not been previously studied.
Detecting urticarial lesions in patients suffering from eosinophilic skin disorders, such as hypereosinophilic syndrome or Wells syndrome, as well as the elevation in the number of eosinophils during the attacks of angioedema suggest its involvement in the pathogenesis of urticaria. Eosinophils express several membrane receptors, making them sensitive to a variety of molecules and also lead to the release of various pro-inflammatory mediators (e.g., cationic proteins and leukotrienes) and also of immunoregulatory mediators, confirming their roles in immunity and inflammation.9,10 Therefore, similar to mast cells and basophils, eosinophils might be activated by allergens through crosslinking of cell-surface-bound IgE when an allergic cause of chronic urticaria can be identified.9
The key role of eosinophils in sustaining the inflammatory response is supported by the presence of a perivascular infiltration, predominantly of eosinophils, in the dermis during the late-phase reaction observed in particular forms of urticaria, such as CU. This eosinophilic infiltration is associated with infiltration of the cutaneous lesion by cationic proteins (MBP and EDN) and high levels of leukotrienes C4 and PAF-acether.11
Our results were in accordance with the study by Lorenzo et al,12 who found that the mean level of serum ECP (encoded by RNASE3 gene) closely related to the EDN (encoded by the RNASE2 gene) was significantly higher in patients with CU compared to healthy subjects.
Many studies have investigated the role of eosinophils in the pathogenesis of other allergic diseases that might be related to chronic urticaria, such as atopic dermatitis (AD) and asthma. In a study by Kim et al,13 it was reported that patients with severe AD had significantly higher mean serum EDN levels compared to patients with mild to moderate AD and healthy controls. Moreover, Gon et al,14 also reported that patients with asthma demonstrated significantly higher mean serum EDN levels than controls. These findings support the role of eosinophils and their protein EDN in the pathology of CU.
The current study showed a significant positive correlation between serum EDN levels and the disease severity of patients with chronic urticaria. These results were consistent with a previous study by Toyoda et al,15 in which the high serum ECP and the presence of necrotic eosinophils in the dermis of patients with severe CU suggest abnormal activity of eosinophils, which is responsible for a massive and rapid degranulation. However, these results were best described by Lamkhioued et al,10 who showed that the activation of eosinophils leads to degranulation, consisting of the extracellular release of the cationic proteins contained in granules. The type of cationic proteins released by eosinophils is selectively dependent on the activated receptor, a mechanism of piecemeal degranulation. Another study by Lorenzo et al12 showed that the serum ECP levels and eosinophilic numbers in the blood of patients with CU were significantly and positively correlated with disease activity and severity, suggesting the involvement of eosinophils in the pathology of CU.
Conclusion
The significantly higher serum eosinophil-derived neurotoxin level in patients with CU suggests its role in the pathogenesis of the disease, and the significant positive correlation of the serum EDN level with the severity of CU suggests promising future in its therapy.
As the results of the present study revealed a positive correlation between serum EDN levels and disease severity, and also showed that the frequency of attacks, itching, and number of wheals were significant predictors of elevated EDN in patients with CU, it’s possible that EDN could be used not only for diagnosing CU in the future, but also as a prognostic factor.
References
- Grattan C. Chronic urticarial: General Principles and Management. In: Greaves MW, Kaplan AP (eds.) Urticaria and Angioedema. New York: Marcel Dekker;2004:343–368.
- Cugno M, Marzano AV, Tedeschi A, et al. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol. 2009;148(2):170–174.
- Simon D, Simon HU, Yousefi S. Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy. 2013;68(4):409–416.
- Soragni A, Yousefi S, Stoeckle C, et al. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell. 2015.19;57(6):1011–1021.
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22.
- Mynek A, Zalewska-Janowska A, Martus P, et al. How to assess disease activity in patients with chronic urticaria?. Allergy. 2008;63(6):777–780.
- Sussman G, Hébert J, Gulliver W, et al. Insights and advances in chronic urticaria: a Canadian perspective. Allergy Asthma Clin Immunol. 2015. 11;11(1):7.
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast cell activation. Nat Rev Immunol. 2006;6(3):218–230.
- Staumont-Sallé D, Dombrowicz D, Capron M, Delaporte E. Eosinophils and Urticaria. Clin Rev Allergy Immunol. 2006;30(1):13–18.
- Lamkhioued B, Aldebert D, Gounni AS, et al. Synthesis of cytokines by eosinophils and their regulation. Int Arch Allergy Immunol. 1995;107(1–3):122–123.
- Kambe N, Kitao A, Nishigori C, Miyachi Y. Late-phase urticaria Update. Curr Allergy Asthma Rep. 2002;2(4): 288–291.
- Lorenzo GD, Mansueto M, Melluso G, et al. Blood eosinophils and serum eosinophil cationic protein in patients with acute and chronic urticaria. Mediators Inflamm. 1996;5(2):113–115.
- Kim HS, Kim JH, Seo YM, et al. Eosinophil-derived neurotoxin as a biomarker for disease severity and relapse in recalcitrant atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(5):441–445.
- Gon Y, Ito R, Hattori T, et al. Serum eosinophil-derived neurotoxin: correlation with persistent airflow limitation in adults with house-dust mite allergic asthma. Allergy Asthma Proc. 2015;36(6):e113–e120.
- Toyoda M, Maruyama T, Morohashi M, Bhawan J. Free eosinophil granules in urticaria: a correlation with the duration of wheals. Am J Dermatopathol. 1996;18(1): 49–57.

