 J Clin Aesthet Dermatol. 2020;13(9):26–32
J Clin Aesthet Dermatol. 2020;13(9):26–32
by Hilary Baldwin, MD
Dr. Baldwin is the Medical Director of the Acne Treatment and Research Center in Brooklyn, New York and is with Rutgers Robert Wood Johnson Medical Center in New Brunswick, New Jersey.
ABSTRACT: Acne vulgaris is the most common dermatological disease in the United States, affecting up to 85 percent of teenagers. While the American Academy of Dermatology has established guidelines regarding acne treatment in general, the variability among acne treatments, even within a given class, prevents establishment of a straightforward regimen. For example, moderate to severe acne is generally treated with an oral antibiotic, although several options are available—both across and within antibiotic classes. The aim of this review is to report the efficacy and safety data available for commonly prescribed oral antibiotics. While there are currently no data to support superiority of one drug over another, there are substantial differences in safety profiles and brand-specific features that may make one antibiotic preferable over another.
Keywords: doxycycline, minocycline, sarecycline, azithromycin, antibiotic resistance
Acne vulgaris is the most common skin disorder encountered in dermatology practice in the United States, affecting approximately 85 percent of teenagers and sometimes persisting into and throughout adulthood.1–3 Although not life-threatening, acne can have a significant adverse psychological and physical impact.4 Many patients with acne report feelings of depression, anxiety, emotional stress, or poor self-image, and severe acne can lead to permanent scarring in up to 20 percent of cases.4,5
Acne is a disease of the pilosebaceous unit with a complex pathology. Currently, there are thought to be at least four synergistic, biological mechanisms that contribute to acne pathogenesis, which is primarily inflammatory in nature.6,7 These include increased sebum production, follicular hyperkeratinization, local inflammatory cascades, and microbial proliferation of Cutibacterium acnes (or C. acnes, formerly Propionibacterium acnes).8,9 The complex interplay of these biological pathways makes effective treatment of acne difficult. Nonetheless, there are a variety of therapeutics available, each targeting one or more of these pathogenic processes.2–4
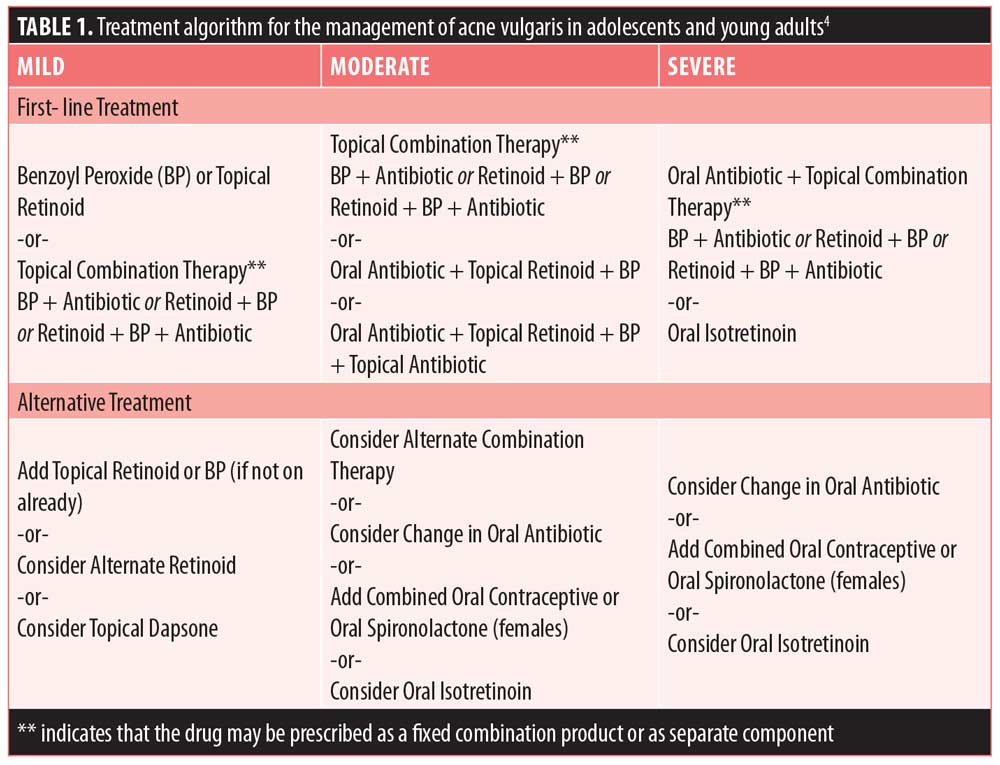
Pharmacological treatments for acne include a variety of topical and systemic agents. Topical treatment (e.g., benzoyl peroxide, antibiotics, and retinoids) is generally used as first-line treatment in cases of mild-to-moderate acne with comedonal lesions and inflammatory lesions.4 Systemic treatment (e.g., oral antibiotics and hormonal therapy) can be used as first-line treatment in cases of moderate-to-severe acne, in combination with a topical agent.2,4 While it is outside the scope of this review to discuss each treatment type in detail, Table 1 provides a summary of recommended treatment options. This review will focus on the efficacy and safety of oral antibiotics that are commonly used or available to treat acne.
Oral Antibiotics
The American Academy of Dermatology supports the use of oral antibiotics for treating moderate and severe acne, and oral antibiotics have been a mainstay of acne treatment for over 50 years.4 It is well-accepted that antibiotics are efficacious in reducing acne severity and have an overall acceptable safety profile.
In recent years/decades, however, there has been growing concern regarding the development of antibiotic resistance.10 As such, some previously employed antibiotics (e.g., erythromycin and clindamycin) are no longer used clinically because of their high rates of resistance.11 The concern is serious enough that the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) now actively promote campaigns aimed to confront antibiotic resistance.10,12 The CDC encourages antibiotic stewardship, which asserts that physicians must act responsibly when prescribing antibiotics, with the hope that this will limit the development and/or expansion of antibiotic resistance.10 The WHO’s “global action plan” has five strategic objectives, aimed both at the community (e.g., improving awareness and understanding) and providers (e.g., optimizing use).12
Apart from the tetracycline class of antibiotics (doxycycline, minocycline and sarecycline), the potential benefit of oral antibiotics often outweighs the potential risks, and they remain a mainstay of moderate-to-severe acne treatment. With currently available clinical studies and data, there is a consensus that no one antibiotic is superior to another regarding efficacy.13,14 The safety profiles, however, differ considerably.15,16 In this article, we will review previous research for several antibiotics currently used, including doxycycline, minocycline, azithromycin, and trimethoprim-sulfamethoxazole, and one recently introduced to the market, sarecycline. Studies included were those that evaluated treatment response in patients with acne.
Among acne studies, there are several metrics of efficacy that are commonly reported.17 Perhaps most frequently used is the change in inflammatory lesions from baseline to endpoint, expressed as either absolute change or percent change. Similarly, the absolute or percent change in non-inflammatory lesions (i.e., open or closed comedones) might be recorded, although this is less common in studies of oral antibiotics, as they are generally more effective for inflammatory lesions. A common subjective measure is the investigator’s global assessment (IGA), which considers the quality and quantity of acne lesions. While the IGA is a five-point scale based on acne severity (ranging from 0=clear to 4=severe), results are generally dichotomized in trials such that a participant has IGA “success” (score of 0 or 1; clear or almost clear, respectively) or IGA “failure” (score >1). Change in inflammatory lesions will be the emphasis of efficacy results for this review.
This review will begin by summarizing studies of tetracycline-class antibiotics, followed by azithromycin and trimethoprim-sulfamethoxazole.
The tetracyclines. Among oral antibiotics used to treat acne, tetracycline has the longest history, having been discovered in the 1940s and FDA-approved in 1953. While effective, its side effect profile, need for frequent dosing, and susceptibility to antibiotic resistance have made it unpopular, and it is no longer considered a standard treatment regimen.14 There are, however, several tetracycline derivatives that were chemically adapted to provide additional benefits.11 Thus, this review will focus not on tetracycline as a drug, but rather on the individual drugs that fall within the tetracycline class (i.e., doxycycline, minocycline, and sarecycline).
Each of these “second-generation” tetracyclines share the same mechanisms of action (MOA); the chemical is transported into bacterial cells, binds the 30S unit of the ribosome, and inhibits protein synthesis. In addition to their antibacterial action, the tetracyclines demonstrate multiple anti-inflammatory properties.18 The therapeutic effect of tetracyclines in acne might be due, at least in part, to reduction in neutrophil chemotaxis and inhibition of proinflammatory cytokines and matrix metalloproteinases.19 The anti-inflammatory actions may be particularly important considering research by Jeremy et al20 that showed inflammation is present far in advance of clinically recognizable lesions—known as the prelesional folliculocentric inflammatory phase. While all tetracyclines share these anti-microbial and anti-inflammatory MOAs, there is significant variability regarding side effect profiles, discussed below, that account for the preferential prescription of one over the other.16,18,21
Doxycycline. The first tetracycline derivative to come to market was doxycycline, approved by the FDA in 1967 and still one of the most commonly used antibiotics in clinical practice.14,22 Relative to its parent tetracycline, doxycycline is more lipophilic, making it optimal for penetrating and accumulating in the sebaceous gland—where C. acnes resides and proliferates.11 Two formulations of doxycycline are available—doxycycline hyclate and doxycycline monohydrate, which are different salt forms of the same active drug. Doxycycline hyclate is more water-soluble than doxycycline monohydrate and might be more ulcerogenic and susceptible to causing gastrointestinal (GI)-associated side effects.23,24 Their differences are more relevant for manufacturing purposes than for assessing efficacy, however.25
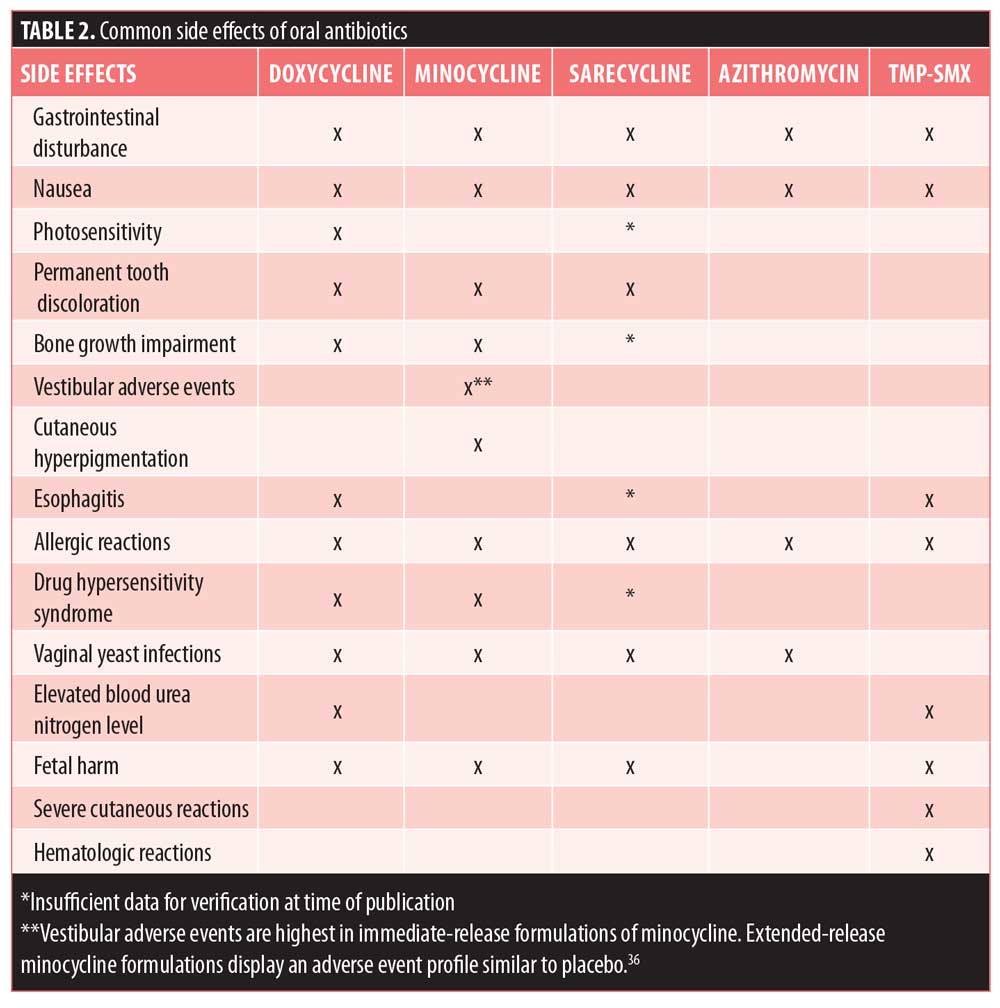
The side effect profile of doxycycline is superior to that of tetracycline, although there are potential adverse events that should be taken into consideration (Table 2). Most of doxycycline’s side effects are mild and/or can be prevented with proper measures.14 Doxycycline is frequently associated with phototoxicity; it interacts with ultraviolet (UV) rays, making an individual more susceptible to severe sunburn. Gastrointestinal disturbance is a common side effect of doxycycline treatment and might present as nausea, vomiting, and/or diarrhea. Doxycycline is amenable to being taken with food—which might lessen GI discomfort—although its absorption is reduced by approximately 20 percent with food.11 When it is taken to treat acne, however, this reduction in absorption is believed to have minimal clinical relevance. Pill esophagitis—inflammation or esophageal ulcers—can occur when taking doxycycline. Like other side effects, however, it is largely avoidable if patients take medication with a large glass of water and do not lie down shortly after ingesting medication.14 Lastly, there is the potential for permanent tooth discoloration in individuals with developing teeth. This is a side effect shared by all tetracycline derivatives which are not recommended for children younger than eight years of age and women during pregnancy.26 Similarly, all tetracyclines have been shown to impair bone growth by forming a stable calcium complex and thus should be avoided in children and during pregnancy.27
Doxycycline has a long history of safe and effective treatment in acne patients. An early study by Plewig et al28 (1970) evaluated the efficacy of doxycycline in 62 patients in a double-blind crossover study. A “good” or “excellent” response in the reduction of inflammatory lesions was reported in 33 percent of patients receiving doxycycline. Statistically, improvement from baseline was significant with doxycycline treatment, but not placebo. In a clinical study by Moore et al,29 662 patients were randomized to one of three treatments—a 40mg dose of modified-release doxycycline, a 100mg dose of doxycycline, or placebo. After 16 weeks of treatment, reduction in lesion count and success rate were evaluated. For both outcome measures, doxycycline treatment was superior to placebo. Interestingly, the lower dose of modified-release doxycycline was more effective in reducing the number of overall lesions, suggesting that a large part of the efficacy of doxycycline stems from its role as an anti-inflammatory agent.29
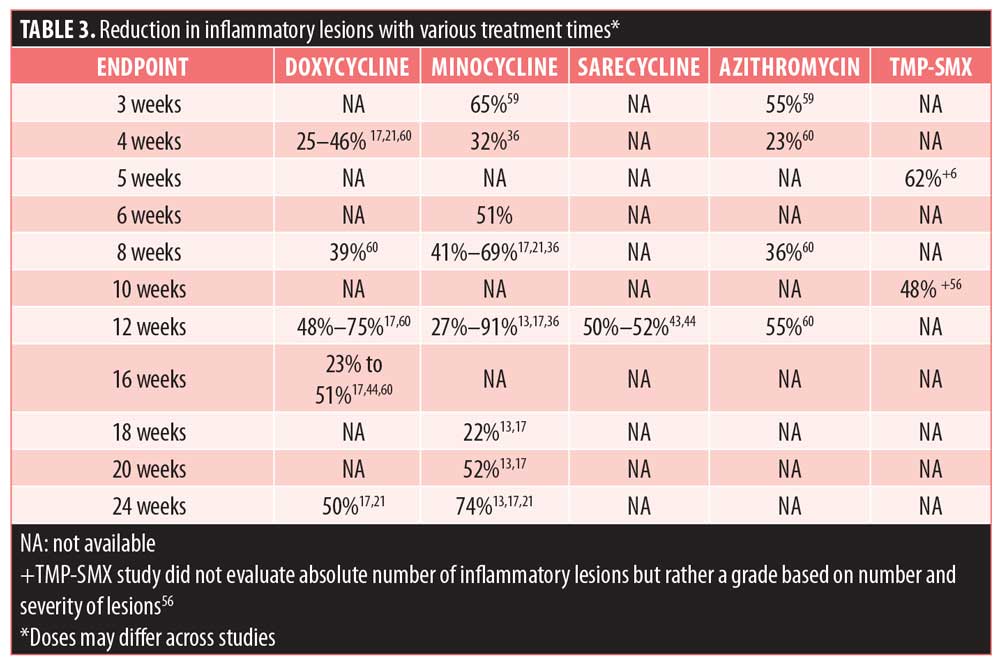
A review article that gathered results from tetracycline treatment studies for acne between 1962 and 2006 showed that doxycycline was consistently effective, although results were variable between studies.17 The largest reduction in inflammatory lesions was 75 percent, and the lowest reduction in inflammatory lesions was 23 percent.17 Efficacy results, reported as reduction in inflammatory lesions, are summarized in Table 3.
Due to the popularity of doxycycline, several brands are available, each boasting additional benefits aside from their efficacy in treating acne. Acticlate is available as a functionally scored tablet, and dosage can be easily modified without requiring a new prescription.30 Doryx is enteric-coated, which makes it more resistant to breakdown by stomach acids.31 There is also Doryx MPC (modified polymer coating), extends absorption even longer, potentially reducing GI-related side effects.32 Targadox is the smallest branded doxycycline and does not require pill splitting.33 Its excipients are gluten-free, lactose-free, vegan, and non-GMO, making it amenable to most patient dietary restrictions/preferences.33 All brands may be taken once daily with or without food.
Minocycline. Another tetracycline derivative, minocycline, was FDA-approved after doxycycline, in 1971, and continues to be a popular treatment option for moderate to severe acne.14,22 Of the tetracyclines, minocycline is the most lipophilic, making it easily accessible for penetrating and accumulating in the sebaceous gland, where C. acnes colonizes.11 The high absorption rate of minocycline is beneficial in multiple facets—it may be taken with food, which may increase patient adherence; lower doses are required; and less active drug remains in the GI tract, which might limit the disturbances commonly seen with doxycycline. Consumption with dairy products does not decrease the bioavailability of minocycline. Photosensitivity is rare with minocycline. Because the permeability of minocycline is so high, however, it is more susceptible to crossing the blood-brain barrier, which can promote acute vestibular adverse events, such as dizziness and vertigo.11,14 In addition, although rare, there is the potential for severe adverse effects, like hyperpigmentation (sometimes irreversible), drug hypersensitivity, Stevens-Johnson syndrome, and autoimmune, lupus-like reactions (Table 2).26
In the last couple of decades, extended-release (ER) minocycline formulations have come to the market. Rather than the rapid absorption and high peak concentration seen with immediate-release (IR) minocycline, ER formulations regulate the rate at which minocycline is released and systemically available.34,35 Although the efficacy for ER and IR minocycline is similar, reported severe adverse events are reduced with the ER formulation.14,35
Unlike doxycycline and immediate-release minocycline, extended-release minocycline underwent traditional Phase III studies and has been evaluated in two randomized, double-blind, placebo-controlled trials.36 Combined, 924 subjects received weight-based minocycline or placebo for 12 weeks, at which time two primary endpoints were assessed: mean percent change in inflammatory lesions and percentage of subjects with an Evaluator’s Global Severity Assessment (EGSA) of clear or almost clear. Across the two studies, mean percent improvement in inflammatory lesions ranged from 43.1 to 45.8 percent for minocycline, compared to 30.8 to 31.7 percent for placebo.36 In patients treated with minocycline, 15.9 to 17.3 percent achieved success, compared to only 7.9 to 9.5 percent treated with placebo.36 No effect was seen on non-inflammatory lesions. A review article that compiled results from minocycline (both immediate-release and extended-release) treatment studies for acne between 1962 to 2006 showed that, similar to doxycycline, the efficacy was quite variable between studies.13 The most significant reduction in inflammatory lesions was 91 percent and the lowest reported reduction in inflammatory lesions was 22 percent.
The adverse events associated with immediate-release minocycline (primarily vestibular) have caused a surge in marketed extended-release minocyclines that are dosed based on patient weight.35 Solodyn and Ximino are commonly prescribed, and MinoLira was most recently launched to the market. Solodyn was first to market with an extended-release formulation and was FDA-approved in 2006.37 Ximino, approved in 2012, is the first and only extended-release minocycline available in a capsule form, which some people prefer to tablets.38 MinoLira is the first dual-release minocycline tablet used for treating acne and was FDA-approved in 2017.39 Each of the three brands promotes once-daily, weight-based dosing and can be taken with or without food.
Sarecycline. Sarecycline is the newest tetracycline derivative introduced into the market and was FDA-approved in 2018. As a tetracycline derivative, it shares the same protein synthesis inhibitory MOA as those previously described. However, sarecycline claims to be a narrow-spectrum antibiotic, which might lower the chance of developing antibiotic resistance.3,40 Recently, it was shown that C. acnes strains displayed a low propensity for the development of resistance to sarecycline, with a spontaneous mutation frequency being 10-10 at 4-8 X MIC. Clinical relevance has yet to be explored.40 Sarecycline is also indicated to treat acne in patients as young as nine years of age, whereas other tetracyclines lack this indication.41 Thus, sarecycline treatment might be preferred in young children, although it is important to note that children aged 9 to 12 comprised only one percent of the clinical study population, and additional research is warranted.42 Sarecycline comes with the same warnings of tooth discoloration and impaired bone growth as other tetracycline derivatives. Like minocycline, its dosing is weight-based, once-daily, and can be taken with or without food.41
In its Phase II clinical trial evaluating 285 patients, after 12 weeks of 1.5mg/kg treatment, inflammatory lesions were reduced by 52.7 percent, compared to 38.3 percent in placebo-treated patients.43 Two larger, identically designed Phase III clinical trials of 1,702 completed patients also showed an approximate 50-percent reduction in inflammatory lesions, compared to approximate 35-percent in placebo-treated patients.44 Additionally, 22 percent of participants achieved IGA success at 12 weeks.44 The drug was generally well-tolerated with minimal side effects in comparison to placebo.
Seysara is the first and only sarecycline available.
Azithromycin. Unlike any previously discussed antibiotics, azithromycin is a macrolide antibiotic. Macrolides also inhibit protein synthesis, although in a different way from tetracyclines—by binding the 50S subunit of the ribosome.45 Azithromycin is a derivative of erythromycin. As mentioned previously, erythromycin was once used as an effective acne therapy, but widespread antibiotic resistance has rendered it less useful.3,11 Azithromycin is used to treat serious systemic infections, and its use for acne is generally reserved for select cases, such as in patients for whom tetracyclines are contraindicated.46
An important differentiator of azithromycin from other antibiotics is its safety profile during pregnancy and lactation (Table 2). Animal studies show that the drug crosses the placenta but is not associated with fetal harm.47 Thus, this may be the preferred treatment if pregnancy must be taken into consideration.48 Additionally, azithromycin has rapid uptake from circulation, followed by a slow release.49 The extended half-life is conducive to less frequent dosing, which might improve adherence.50 The bioavailability of azithromycin is decreased when taken with food.
Papers evaluating the efficacy of azithromycin date back to 1997. The case series included only three patients, and in each patient, azithromycin reduced total number of inflammatory lesions.51 A larger trial (N=64) examining different azithromycin doses supported the early findings. The Global Acne Grading System was the outcome measure, and for all doses, mean acne score significantly decreased at the end of the first (30%–33%), second (46%–50%), and third month of treatment (70%–76%) when compared to baseline.52 Numerous dosing regimens have been recommended since then, some of which call for infrequent dosing, which might be more convenient for patients.3
There are no specific brands marketed for the treatment of acne.
Trimethoprim/sulfamethoxazole (TMP-SMX). TMP-SMX was approved by the FDA in 1973. The two antibiotics work synergistically to inhibit folate synthesis in bacteria.53 While not indicated for acne, it is frequently used off-label.54 Because it is the agent most commonly used to treat adults with community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infections, it should be used sparingly due to the concern of antibiotic resistance.
TMP-SMX is also reserved for third-line therapy due to the possibility of rare, though severe and potentially life-threatening, adverse events, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).53 TMP-SMX is one of the few medications considered “high” risk for inducing SJS or TEN, and risk is highest when the drug has been recently initiated and in patients infected with HIV.3,55 In both diseases, severe epidermal necrosis causes extensive skin detachment and is life-threatening.55
Data regarding the use of TMP-SMX in treating acne are limited. However, a double-blind study of 43 patients by Hersle 56 (1972) showed that the acne score (a calculation by the authors based on lesion number and severity) decreased by 62 percent after just five weeks of treatment with TMP-SMX, compared to 9 percent in placebo-treated patients. TMP-SMX might be particularly effective in treating in patients with acne who were refractory to tetracycline treatment.53,57
While TMP-SMX treatment of acne is off-label, two brands are commonly used: Bactrim and Septra. Both are also available as a double-strength formulation. There are no features that would suggest superiority of one brand over the other.
Head-to-head Trials
A systematic review by Simonart17 evaluated randomized trials and was designed to specifically compare the efficacy of different tetracyclines. The review, however, reported no significant difference in number of either inflammatory (n=32 trials) or non-inflammatory (n=23 trials) lesions. A randomized, double-blind study by Olafsson58 compared doxycycline and minocycline treatment effect in 64 patients with acne over 12 weeks. A significant reduction in all types of acne lesions (pustules, papules, open comedones, and closed comedones) was observed at 12 weeks, and doxycycline and minocycline were found to be equally effective. Similarly, a meta-analysis by Kim48 evaluated results from six trials that compared oral azithromycin pulse therapy and doxycycline daily therapy. Four outcome measures were compared at 12 weeks: remaining inflammatory lesions, remaining non-inflammatory lesions, patients’ self-assessment, and investigators’ assessment. In each case, there was no significant difference between groups. Even after a sensitivity analysis was conducted to account for potential bias, neither outcome measure—inflammatory lesions nor investigators’ assessment—significantly differed between groups.48
An extensive minocycline-focused Cochrane review published in 2012 concluded that minocycline is an effective treatment for moderate to moderately severe acne, but there was no evidence that it is better than other commonly used acne treatments, including oral doxycycline or macrolide antibiotics.13 Reviews by Del Rosso14 and Kircik21 similarly concluded that doxycycline and minocycline display a similar efficacy to one another. Studies have compared azithromycin to either doxycycline or minocycline.14,21,59 In each case, both drugs were deemed effective and there were no significant group differences.45,60
Comparative trials with TMP-SMX are limited because it is used off-label and as a third-line agent. Sarecycline has not yet been studied as a direct comparator to any other oral antibiotic.
Discussion
At present, doxycycline, minocycline, and sarecycline are are the most commonly prescribed antibiotics for acne treatment, and both have a long history of efficacy and safety.22 While the identification of “the best” oral antibiotic for acne would certainly simplify a treatment regimen, data do not exist that permit such a conclusion to be drawn.
Inconsistencies across trials make it impossible to justly compare products.3 Methodological variation is commonly seen in randomization, blinding, number of participants, study duration, dosing, and primary/secondary outcome measures. Additionally, it is important to keep in mind that most studies included in this review, by necessity, assessed treatment outcome following monotherapy. However, in clinical practice, oral antibiotics are rarely prescribed as monotherapy. The multifactorial pathophysiology of acne calls for the use of several agents to attack the disease from numerous directions. The combination of oral antibiotics with benzoyl peroxide, combination antibiotics, and topical retinoids is standard of care.3 Additionally, oral antibiotic monotherapy is discouraged due to the potential development of antibiotic resistance.2,4
When choosing an oral antibiotic for treating acne, there are no data identifying a clearly superior product. Rather, prescriber choice might come down to personal or patient preference, such as delivery vehicle (e.g., capsule vs. tablet), dietary restrictions, gastrointestinal sensitivity, sun exposure, and past experiences with antibiotics. The tetracycline class is probably superior to other antibiotics due to its dual action of antibacterial and anti-inflammatory activities. Additionally, tetracyclines are generally inexpensive and well-tolerated. Between doxycycline, minocycline, and sarecycline, side effect profile might be a determining factor.61 Doxycycline is generally associated with more upfront, bothersome side effects, like GI upset and photosensitivity.27 Immediate-release minocycline is associated with a high incidence of vestibular adverse events, but the extended-release formulations and sarecycline have reduced these dramatically and instead show a side effect profile similar to placebo.35,36 The risk of longer-term, more serious side effects (hyperpigmentation, lupus-like drug eruption, and hypersensitivity syndromes), although rare, are more common with minocycline.26 Azithromycin might be a preferred treatment in patients who are pregnant, although in practice it is rarely utilized. TMP-SMX is an effective treatment for non-responders or in individuals who cannot tolerate tetracycline-class drugs.54
Regardless of treatment choice, it is critical to keep in mind the CDC’s initiative toward antibiotic stewardship. Antibiotics should always be prescribed responsibly and only when they are necessary to treat disease.10 Antibiotic monotherapy is discouraged, and antibiotic therapy for long-term management is not recommended for most patients.2,4 Rather, the addition of a topical agent to systemic antibiotic treatment might show a favorable efficacy and safety profile.62,63 Once systemic antibiotic treatment is discontinued, the continued use of topical retinoids or benzoyl peroxide and topical retinoid might be helpful for long-term maintenance therapy.64,65
Conclusion
While azithromycin and TMP-SMX are efficacious, tetracyclines (notably doxycycline, minocycline, and now sarecycline) remain the preferred first-line treatment option for acne due to their established safety and efficacy. No one antibiotic has been shown to be superior to another. Rather, each is associated with different side effects, and treatment choice is based on provider and patient preferences.
Acknowledgements
7West Communications provided editorial assistance for the preparation of this manuscript.
References
- Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131:S163–S186.
- Thiboutot DM, Dreno B, Abanmi A, et al. Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78: S1–S23.
- Marson JW, Baldwin HE. An overview of acne therapy, Part 1: Topical therapy, oral antibiotics, laser and light therapy, and dietary interventions. Dermatol Clin. 2019;37(2):183–193.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016:74:945–73.
- Halvsorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131(2):363–370.
- Fox L, Csongradi C, Aucamp M, et al. Treatment modalities for acne. Molecules. 2016;21(8):E1063.
- Lavers I. Diagnosis and managements of acne vulgaris. Nurse Prescribing. 2014;12(7):330–336.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2012;168:474–485.
- Dreno B, Pecastaings S, Corvec S, et al. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32(Suppl 2):5–14.
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. https://www.cdc.gov/drugresistance/Threat-Report-2013/pdf/ar-Threats-2013-508.pdf.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: Pharmacokinetic and pharmacodynamic perspectives. J Clin Aesthet Dermatol. 2011;4(2):40–47.
- World Health Organization site. Antibiotic resistance fact sheet. 5 Feb 2018. Available from: https://www.who.int/en/news-room/fact-sheets/detail/antibiotic-resistance.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database of Syst Rev. 2012;8:CD002086.
- Del Rosso JQ. Oral Doxycycline in the management of acne vulgaris: Current perspectives on clinical use and Rerent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8(5):19–26.
- Bienenfeld A, Nagler AR, Orlow SJ. Oral antibacterial therapy for acne vulgaris: An evidence-based review. Am J Clin Dermatol. 2017;18:469–490.
- Park H, Skopit S. Safety Considerations and monitoring in patients treated with systemic medications for acne. Derm Clin. 2016;34:185–193.
- Simonart T, Dramaix M, Maertelaer. Efficacy of tetracyclines in the treatment of acne vulgaris: a review. Br J Dermatol. 2008;158:208–216.
- Griffin MO, Fricovsky E, Ceballos G, et al. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299(3):C539–C548.
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2003;139:459–64.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–27.
- Kircik LH. Doxycycline and Minocycline for the management of acne: A review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol. 2010;9(11):1407–1411.
- Barbieri S, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155(3):290–297.
- Pages F, Boutin J, Meynard J, et al. Tolerability of Doxycycline Monohydrate vs Chloroquine-proguanil in Malaria Chemoprophylaxis. Trop Med Int Health. 2002;7(11):919–24.
- Carlborg B, Farmer JC. Esophageal corrosion tests with Doxycycline Monohydrate tablets. Curr Ther Res. 1983;34(1):110–116.
- Krout C, Lio PA. Tetracyclines: history and current formulation review from a dermatology perspective. Pract Dermatol. 2015;50–54.
- Ochsendorf F. Minocycline in acne vulgaris: benefits and risks. Am J Clin Dermatol. 2010;11(5):327–341.
- Tan H. Antibacterial therapy for acne: A guide to selection and use of systemic agents. Am J Clin Dermatol. 2003;4(5):307–314.
- Plewig G, Petrozzi JW, Berendes U. Double-blind study of Doxycycline in acne vulgaris. Arch Dermatol. 1970;101:435–8
- Moore A, Ling M, Bucko A, et al. Efficacy and safety of subantimicrobial dose, modified-release Doxycycline 40mg versus Doxycycline 100mg versus placebo for the treatment of inflammatory lesions in moderate and severe acne: A randomized, double-blinded, controlled study. J Drugs Dermatol. 2015;14(6):581–586.
- Acticlate Package Insert. West Chester, PA. Aqua Pharmaceuticals.
- DORYX® Package Insert. Greenville, NC. Mayne Pharma.
- DORYX® MPC Package Insert. Greenville, NC. Mayne Pharma.
- Targadox® Package Insert. Scottsdale, AZ. Journey Medical Corporation.
- Plott RT, Wortzman MS. Key bioavailability features of a new extended-release formulation of Minocycline Hydrochloride tablets. Cutis. 2006;78(suppl 4):6–10.
- Del Rosso JQ. Weight-based dosing and extended-release formulation of Minocycline tablets: Is there clinical significance? J Clin Aesthet Dermatol. 2009;2(1):44–47.
- Fleischer AB, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(4 Suppl):21–31.
- Solodyn® Package Insert. Scottsdale, AZ. Medicis Pharmaceutical Corp.
- XiminoTM Package Insert. Cranbury, NJ. Sun Pharmaceutical Industries.
- MINOLIRATM Package Insert. Princeton, NJ. Promius Pharma, LLC.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:e01297–18.
- SeysaraTM Package Insert. Irvine, CA. Allergan Pharmaceuticals International Limited.
- Almirall SA. Study to evaluate safety and efficacy of Sarecycline in treatment of acne. https://clinicaltrials.gov/ct2/show/results/NCT02320149. Apr 2019.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of Sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: Results of a Phase 2, dose-ranging study. J Drugs Dermatol. 2018;17(3):333–338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral Sarecycline 1.5mg/kg/day is effective for moderate to severe acne vulgaris: Results from two identically designed, Phase 3, randomized, bouble-blind clinical trials. J Drugs Dermatol. 2018;17(9):987–996.
- Riddle CC, Amin K, Schweigher ES. A review of Azithromycin for the treatment of acne vulgaris. Cosmetic Dermatol. 2007;20(5):299–302.
- Walsh TR, Efthimiou J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:e22–32.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254–262.
- Kim JE, Park AY, Lee SY, et al. Comparison of the efficacy of azithromycin versus Doxycycline in acne vulgaris: A meta-analysis of randomized controlled trials. Ann Dermatol. 2018;30(4):417–426.
- Peters DH, Friedel HA, McTavish D. Azithromycin: a review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1992;44: 750–799.
- Srivastava K, Arora A, Kataria A, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–434.
- Fernandez-Obregon AC. Azithromycin for the treatment of acne. Int J Dermatol. 1997;36(3):234–240.
- Naieni FF, Akrami H. Comparison of three different regimens or oral azithromycin in the treatment of acne vulgaris. Indian J Dermatol. 2006;51(4):255–257.
- Bhambri S, Del Rosso JQ, Desai A. Oral Trimethoprim/Sulfamethoxazole in the treatment of acne vulgaris. Cutis. 2007;79:430–434.
- Amin K, Riddle CC, Aires DJ, Schweiger ES. Common and alternate oral antibiotic therapies for acne vulgaris: a review. J Drugs Dermatol. 2007;6(9): 873–880.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
- Hersle K. Trimethoprim-Sulphamethoxazole in acne vulgaris: A double-blind study. Dermatologica. 1972;145:187–191.
- McCarty M, Del Rosso JQ. Chronic Administration of oral Trimethoprim-Sulfamethoxazole for acne vulgaris. J Clin Aesthet Dermatol. 2011;4(8):59–66.
- Olafsson JH, Gudgierson J, Eggertsdottir GE, et al. Doxycycline versus Minocycline in the treatment of acne vulgaris: a double-blind study. J Dermatol Treat. 1989;1:15–17.
- Sardesai VR, Deka YT. Comparison of efficacy of oral Azithromycin with oral Minocycline in the treatment of acne vulgaris. Clin Dermatol Rev. 2017;1:37–40.
- Kus S, Yucelten D, Aytug A. Comparison of efficacy of Azithromycin vs. Doxycycline in the treatment of acne vulgaris. Clin Exp Dermatol. 2005;30(3):215–20.
- Smith K, Leyden JJ. Safety of Doxycycline and Minocycline: A systematic review. Clin Ther. 2005;27(9):1329–42.
- Thiboutot DM, Shalita AR, Yamauchi PS, et al. Combination therapy with Adapalene gel 0.1% and Doxycycline for severe acne vulgaris: A multicenter, investigator-blind, randomized, controlled study. Skinmed. 2005;4(3):138–46.
- Tan J, Humphrey S, Vender R, et al. A treatment for severe nodular acne: a randomized investigator-blinded, controlled, noninferiority trial comparing fixed-dose Adapalene/benzoyl peroxide plus Doxycycline vs oral Isotretinoin. Br J Dermatol. 2014;171:1508–1516.
- Thiboutot DM, Shalita AR, Yamauchi PS, et al. Adapalene gel, 0.1%, as maintenance therapy for acne vulgaris. A randomized, controlled, investigator-blind follow-up of a recent combination study. Arch Dermatol. 2006;142:597–602.
- Leyden J, Thiboutot MD, Shalita AR, et al. Comparison of Tazarotene and Minocycline maintenance therapies in acne vulgaris: A multicenter, double-blind, randomized, parallel-group study. Arch Dermatol. 2006;142(5):605–612.

