 by Nevena Skroza, md; Ersilia Tolino, MD; Alessandra Mambrin, MD; Sara Zuber, MD; Veronica Balduzzi, MD; Anna Marchesiello, mD; Nicoletta Bernardini, MD; Ilaria Proietti, MD; and Concetta Potenza, md
by Nevena Skroza, md; Ersilia Tolino, MD; Alessandra Mambrin, MD; Sara Zuber, MD; Veronica Balduzzi, MD; Anna Marchesiello, mD; Nicoletta Bernardini, MD; Ilaria Proietti, MD; and Concetta Potenza, md
Drs. Tolino, Mambrin, Zuber, Balduzzi, Marchesiello, Bernardini, and Proietti are with the Sapienza University of Rome, Polo Pontino in Terracina, Italy. Drs. Skroza and Potenza are associate professors at Sapienza University of Rome, Polo Pontino.
Funding: No funding was provided for this study.
Disclosures: The authors have no relevant conflicts of interest relevant to the content of this article.
Abstract: Background. Acne is predominantly known as a skin disorder of the adolescent population. However, current research indicates that the prevalence of adult patients with acne, especially among women, is increasing.
Objective. The objective of this study was to evaluate differences between adults and teenagers with regard to acne prevalence, patient sex, acne severity, and quality of life. In adult patients, we considered differences in family history of acne, onset, and smoking habits.
Design. We performed a retrospective study of a total of 1,167 patients with acne who attended our outpatient clinic from January 2008 to March 2015.
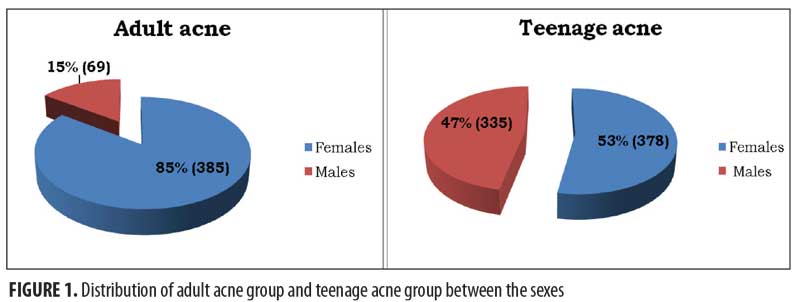
Participants. The study population was divided into two groups: adolescent acne and adult acne. Among the adult subjects, 385 were female and 69 were male; among the adolescent subjects, 378 were female and 335 were male.
Measurements. The severity of acne was recorded using the Global Acne Grading System. The impact of acne on quality of life was investigated using the Assessment of Quality of Life questionnaire.
Results. Study results show that acne in female patients was more prevalent than in male patients. The evaluation of acne severity showed that “mild acne” is the most frequent form. With regard to smoking habits, time of onset, and family history of acne, we did not find any statistically significant differences between the sexes.
Conclusion. In both sexes, there are some differences in adult acne versus the adolescent form. Treating adult acne demands a different approach to diagnosis and a tailored management plan that considers all of the variables involved.
Keywords: Adult acne, persistent acne, late onset acne, risk factor, sex differences
J Clin Aesthet Dermatol. 2018;11(1):21–25
Introduction
Acne is widely considered a chronic skin disease that primarily affects individuals going through puberty, with a prevalence among this population of almost 95 percent.1 However, although acne is principally a disorder of adolescence, current research indicates that the prevalence of adult patients with acne is increasing.2 Three different groups of acne patients can be considered: preadolescent, adolescent, and post-adolescent patients.3–5 An extensive survey carried out on a total of 1,013 men and women showed that the prevalence of acne was 50.9 percent, 35.2 percent, 26.3 percent, and 15.3 percent among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.6 According to the time of onset, two subtypes of adult acne are recognized: persistent and late-onset. Persistent acne is a continuation or relapse of the disease from adolescence into adulthood and middle age,7,8 while the late onset type involves patients aged 25 years and older who have not previously been affected by acne vulgaris. Both subtypes more frequently affect women and are often associated with inflammation, changes in pigmentation, and scarring. Late-onset acne is thought to be less common than persistent acne; in a cross-sectional study involving adult female patients diagnosed with acne at a general dermatology clinic, Schmitt et al9,10 reported that 80 percent of women showed persistent acne.This latter type of acne has been reported to be generally mild to moderate in severity and to present with more inflammatory lesions and fewer comedones compared to adolescent acne.7,11 It generally affects the face, particularly the mandibular region, the zone below the jawline, the cervical region, and sometimes the chest.12
Adult acne pathogenesis involves several endogenous and exogenous factors. Examples of the former include endocrine disorders, the chronic stimulation of innate immunity,14 and genetic predispositions,12–14 those of the latter include cosmetics,7–15 stress,14 and tobacco.7,8
Adult acne is a chronic condition that appears to impact the quality of life in adult patients more than in their younger counterparts,13–16 with considerable psychological, social, and emotional impacts6,17,18 and up to a 40-percent prevalence of psychiatric comorbidity.19
Currently, adult acne treatment should be tailored to address the specific characteristics of this adult population, including other factors beyond acne severity.
Methods
Study design. We performed a retrospective study that included 1,167 acne patients from our database who attended our outpatient clinic from January 2008 to March 2015.Patients affected by “mild,” “intermediate,” and “severe” acne were included in this study. The study population was divided into two groups: adolescent acne (aged 12–25 years) and adult acne (aged > 25 years). In the adult population, there were 385 women and 69 men, while in the adolescent population, there were 378 girls and 335 boys. A detailed history and examination was carried out for each patient, including the collection of data on sex, age, acne severity, and smoking habits; family history of acne and age of onset were selectively investigated in the adult population.
Severity of acne was recorded using the Global Acne Grading System (GAGS). This system divides the face, chest and back into six areas (forehead, left cheek, right cheek, nose, chin and chest and back) and assigns a factor to each area on the basis of severity.
The impact of acne on quality of life was investigated by administering the Assessment of Quality of Life questionnaire (AQoL) to patients. The questionnaire scale includes 19 items that measure factors regarding acne severity and psychological morbidity related to the disease. Each question in the survey is scored using a six-point scale, with 0=remarkable deterioration of quality of life and 6=little deterioration of quality of life. The total score of the questionnaire is 114 points. Measuring the impact of acne on quality of life allows us to understand the disease perception from the patient’s point of view.
Statistical analysis. Quantitative data were expressed as mean and ± standard deviation. Chi-square (x2) and Student’s t-tests were used for categorical and quantitative variables, respectively. Statistical significance was set at p>0.05. Statistical analyses were performed using the Statistical Package for Social Sciences version 21.0 software package (IBM Corp., Armonk, New York).
Results
We retrospectively analyzed data from our acne outpatient database. Out of the 1,167 patients suffering from acne, 454 (41.3%) were adults and 713 were adolescents (58.7%).Among adults, female sex was more prevalent, with 385 (85%) female patients versus 69 (15%) male patients. In the adolescents, female patients numbered 378 (53%) versus 335 male patients (47%) (Figure 1).
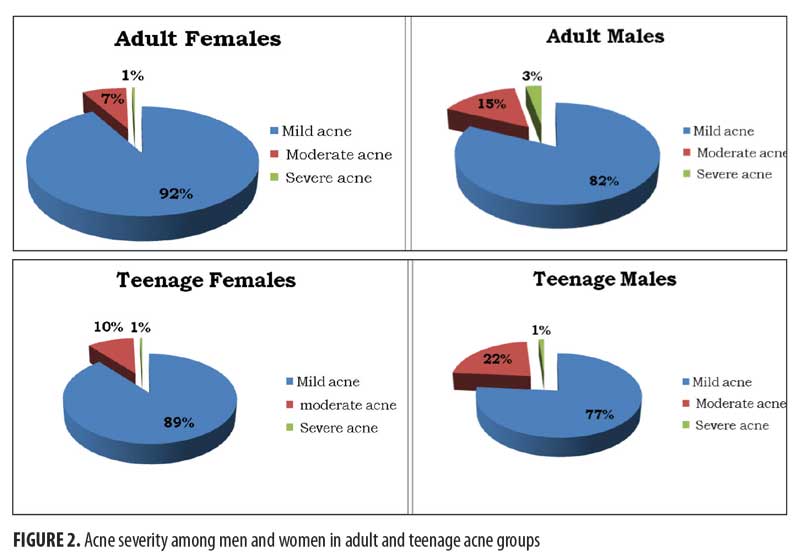
The evaluation of acne severity showed that “mild acne” was the most frequent form in all subgroups (92% in adult females, 82% in adult males, 89% in teenage females, and 77% in teenage males, respectively). Additionally, the least frequent form was “severe acne” (1% in adult females, 3% in adult males, 1% in teenage females, and 1% in teenage males). Finally, “moderate acne” was observed homogeneously in all subgroups, though both male groups showed a little higher prevalence of such in comparison with the female groups (15% in adult males and 22% in teenage males versus 7% in adult females and 10% in teenage females) (Figure 2).
Furthermore, we considered family history and onset of acne and in male and female adults (Table 1). Smoking habits were investigated in both the adolescent and adult groups. In the total of 454 patients examined, 322 (70.9%) patients (50 male [72.5%] and 272 female [70.6%]) declared a family history of acne (p=0.872). Regarding the time of onset, 321 (70.7%) out of 454 patients (43 male [62.3%] and 278 female [72.2%]) were classified as having “persistent” adult acne, while 133 (29.3%) out of 454 (26 male [37.7 %] and 107 female [27.8%]) presented with the “late-onset “ type of adult acne (p=0.129). Moreover, smoking habits were identified in 74 patients (16.3%) (17.4% male and 16.1% female) (p=0.068). Based on these data, we did not find any statistically significant differences in the considered items among the sexes.
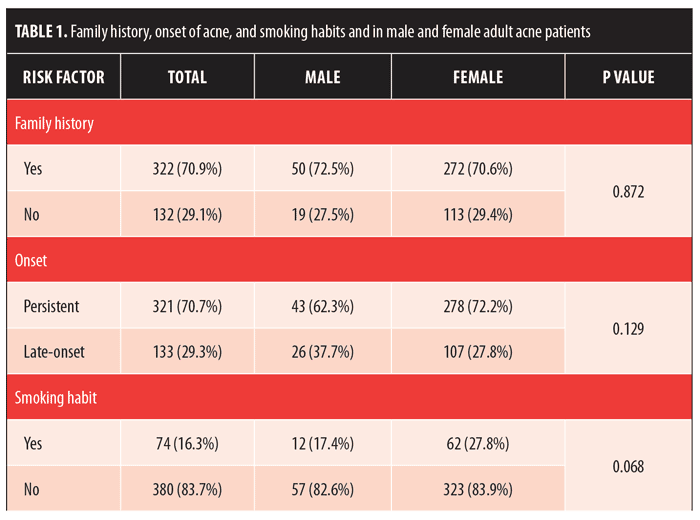
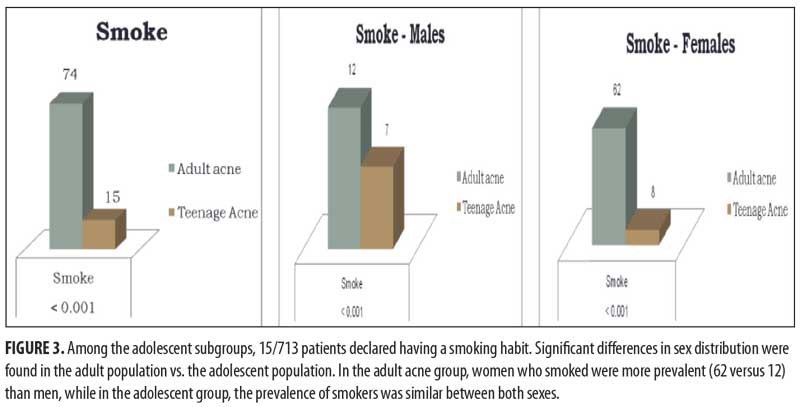
Among the adolescent subgroups, 15 of the 713 patients declared having a smoking habit. Significant differences in sex distribution were found between the adult population versus the adolescent population in terms of smoking. In the adult acne group, women who smoked were more prevalent (62 women vs. 12 men), while, in the teenager group, the prevalence of those who smoked was similar among both sexes (Figure 3). Data for family history, onset of acne, and smoking habits are summarized in Table 1.
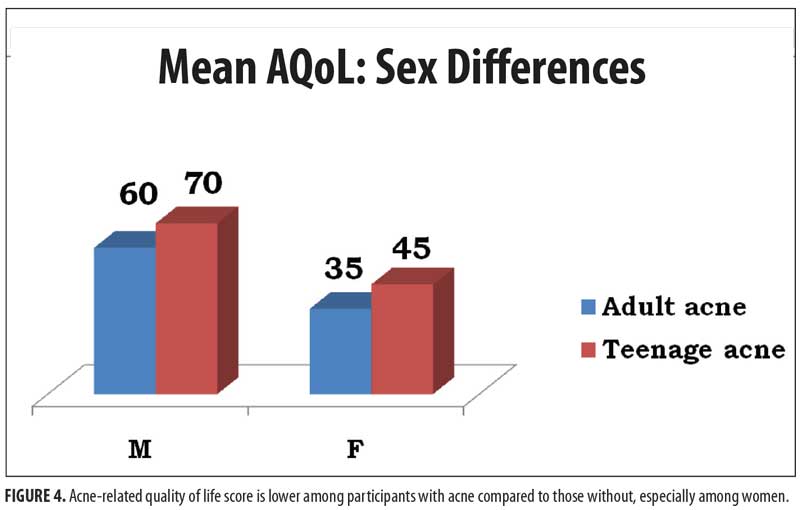
Based on the AQoL score, acne has a significantly negative impact on quality of life (Figure 4), especially in the female population (female adolescents: 45/114 points and female adults: 35/114 points; male adolescents: 60/14 points and male adults: 70 /114 points).
Discussion
Acne is a chronic inflammatory skin disease that primarily affects the face, chest, and back, with a prevalence of almost 95 percent in adolescents. Several large studies have reported a prevalence of adolescent acne ranging from 81 to 95 percent in young men and 79 to 82 percent in young women.20,21 Its prevalence is increasing in the adult population too, particularly in women, even if literature lacks exact data on the subject. The first study on adult acne prevalence by Cunliffe et al22 demonstrated that acne incidence was higher among adolescent men than adolescent women. However, Cunliffe and colleagues found that, in adults 18 years of age and older, this prevalence decreases for both sexes, but becomes more prevalent in adult women than adult men. In 1999, Goulden et al23 confirmed that clinical facial acne affects adult women more frequently than it does men. In this study, the prevalence of post-adolescent acne was three percent in men and 12 percent in women in a population of randomly selected individuals with similar demographic characteristics. The authors further noted that the mean age of patients with acne increased from 20.5 years to 26.5 years from 1989 to 1999. Moreover, 82 percent of affected people had persistent acne subtypes. In our study, which included 454 adult patients, women were predominantly affected (85%) compared to men (15%), while in younger individuals (n=713), the two sexes were similarly affected. In opposition to the study of Cunliffe et al,22 the prevalence of acne in our study population did not decline between the ages of 24 to 44 years, but did decrease after the age of 45 years.23 The prevalence of acne in adult women was also analyzed in the study by Collier et al.6 The result of this research showed that 50 percent of women were affected in the third decade of life and that acne was more common in women than in men at all ages after age 20 years (p<0.05). Variations in the prevalence of the disease among different ethnic groups have also been reported. A study on 2,895 women of different ethnicities showed that acne was more prevalent in women with darker skin types (African American: 37%; Hispanic: 32%) versus in those with lighter skin (Asians 30%; Caucasians 24%; and Indians 23%).24 Moreover, in Asians, inflammatory acne forms are more frequent than non-inflammatory forms (20% vs. 10%), whereas, in Caucasians, non-inflammatory acne is more prevalent (14% vs. 10%).
Based on the time of onset, two subtypes of adult acne can be described: persistent acne and late-onset acne. In our study, 321 (70.7%) out of 454 patients showed persistent forms of acne, while 133 (29/3%) out of 454 presented a “late onset “ type of adult acne (p=0.129).
Inflammation, change in pigmentation, and scarring characterized both subtypes, albeit there were some differences in term of lesions localization and therapy.
The clinical lesions of adult acne are typically considered to be different than those of adolescent acne.25 Furthermore, young men are more affected than young women and generally show the most severe forms of the disease, while adult acne mainly affects women presenting with seborrhea, comedones, and inflammatory lesions. The evaluation of acne severity in our study showed that “mild acne” is the most frequent form in male and female patients of both adolescent and adult ages; also, in all groups, the least frequent form was “severe acne.”
Morphologically, the classical presentation of adult acne consists of inflammatory papulo-pustular lesions in the lower half of the face. The acne usually presents gradually and is mild-to-moderate in severity, in contrast with adolescent forms. In the literature, two clinical profiles of adult acne have been described.13 One shows hyperseborrhea and non-inflammatory lesions diffuse in all areas of the face with abundant open or closed small comedones, while the other consists of predominant inflammatory lesions, long-lasting nodules, and cysts on the lower one-third of the face, neck, and jawline. This latter type of presentation has been described as “chin acne.” Capitanio et al,26 in 2010, described a new clinical presentation of adult acne called “comedonal postadolescent acne,” which is characterized by inflammatory lesions and large, prominent cyst-like comedones homogeneously distributed on the whole face with a relative absence of lesions on the lower half of the face and jawline. Khunger et al,27 in a study involving 280 Indian patients, revealed that adult acne usually presents as inflammatory papules and pustules (in 83% of patients) that are mild to moderate in severity. Moreover, in the Indian population, comedonal acne is rare in comparison with adolescent acne; however, cystic acne is not uncommon and was present in 12 percent of the patients studied. This traditional description of adult acne was recently revised by Dreno et al in 20149 with an observational, prospective international study on 374 adult female patients with acne. The results illustrate that post-adolescent acne is very similar in severity to adolescent acne in up to 90 percent of women, involving multiple areas of the face (e.g., mandibles, temples, cheeks). In 6.4 percent of cases, the women presented only inflammatory lesions and 17.1 percent had only comedonal acne. In addition, only 12 percent of women presented with lesions localized on the mandibular area.16 Truncal involvement was reported in 48.4 percent of patients, and, notably, women with truncal acne also had multiple other body areas affected. Other important additional clinical features have been reported, such as post-inflammatory hyperpigmentation (commonly present in the cheek26 [38.2%] and mandibular9 [26.1%] areas), scars, which are frequently atrophic and present in 58.8 percent of patients studied, particularly in women with mandibular presentation;9 hyperseborrhoea; and erythema.
The pathogenesis of adult female acne is very complex and remains incompletely elucidated. Similar to adolescent acne, the pathogenesis of adult female acne involves an interplay of excess sebum production, abnormal keratinization within the follicle, and bacterial colonization of the pilosebaceous duct by Proponibacterium acnes.28 Furthermore, hormones, the use of cosmetics and/or drugs, and chronic stress have been put forward as possible etiological factors. Genetics also play a strong role; Goulden et al2 showed that the majority of patients (67%) have a first-degree family history of post-adolescent acne. In our study, with respect to the total of 454 adult patients examined, 322 (70.9%) patients (50 males [72.5%] and 272 females [70.6%]) declared a family history of acne (p=0.872). In another study,9 job stress was associated with more severe forms of acne in women; women with localized acne were also more likely to report higher stress levels (6.0–6.4 vs. 5.1) and a psychologically stressful job (68.6–73.6% vs. 58.8%). In this study, it was also reported that 24.8 percent of patients were smokers; notably, there was a significantly higher proportion of subjects with severe acne among smokers in comparison with among nonsmokers (17.4%). Smoking has been related with abnormal follicular keratinization and inflammation through lipide peroxidation of sebum in comedones by inflicting oxidative stress. According to the data in our study, adult acne patients smoke more than their younger counterparts, with statistically significant results in both sexes (p<0.001).
Adult acne is mainly mild-to-moderate in severity, but treatment failure and a chronic, relapsing course may increase the self perception of symptom severity with a resulting negative impact on quality of life.
In several papers, acne severity was similar between the two sexes but quality of life was more impaired in women than in men.18–29 Acne duration seems to be an important factor responsible for the worse perception of the disease by women. Adult acne in women has been associated with depression, anxiety, psychological stress, and suicidal ideation.3 Moreover, Tan et al18 observed that other factors such as increased age and longer acne duration (> five years) are related with a greater impairment in quality of life in females.
Only a few studies investigating sex differences in adult acne have been reported in the literature; our study evaluates differences between men and women regarding acne severity, smoking habits, family history of acne, and time of onset. Significant differences in gender distribution have been found between the adult and the adolescent populations. As for prevalence, adult acne is more prevalent in women than in men, while in adolescents, the prevalence is quite similar in both sexes. Moreover, data about acne severity show that “severe acne” and “mild acne” forms are observed homogeneously in all groups; only “moderate acne” shows a slightly higher prevalence in male than in female patients.
As for smoking habits, time of onset, and family history of acne, we did not find statistically significant differences between the sexes.
Limitations. Our study presents some limitations, in that it is retrospective and monocentric in nature. Moreover, our clinical evaluation was based on the GAGS without considering the lesions and distribution patterns of acne that can differ between sexes, according to literature data.25
Conclusion
Acne is one of the most common skin disorders worldwide and occurs primarily at puberty with a prevalence of almost 95 percent. Although it is principally a disorder of adolescence, the prevalence of adult patients with acne, particularly adult women with acne, is increasing. The higher prevalence of adult acne in the female population prompted us to investigate whether other variables could present significant differences between sexes. These differences have recently gained attention in many branches of medicine, including dermatology, so we selectively addressed our interest to the different forms of acne, family history of the disease, time of onset, smoking habits, and quality of life in both sexes. This restriction could represent a limit of the present study but it also represents a starting point for further research on factors influencing adult acne. As this study reports only the results from our outpatient clinic, it would be auspicable to include experiences from other centers in order to obtain the most statistically significant data.
Our results agree with the literature data on adult acne: women are affected more by acne (85%) than men (15%); family history represents one of the main risk factors (70%); the persistent form of acne is more prevalent (70.7%) than the late-onset form; mild acne is the predominant form (87%); and there is a large number of smokers among adults with acne than among younger individuals with acne. Finally, there is evidence of a negative impact of acne on quality of life in both adult and adolescent female patients. Some differences of adult acne from adolescent acne in both sexes were noted in our study; as such, each demands a different approach to diagnosis and treatment, and we recommend a tailored management plan that considers all of the variables involved.
References
- Burton JL, Cunliffe WJ, Stafford I, Shuster S. The prevalence of acne vulgaris in adolescence. Br J Dermatol. 1971;85(2):119–126.
- Goulden V, Clark S, Cunliffe W. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136(1):66–70.
- Del Rosso JQ, Harper JC, Graber EM, et al. Status report from the American Acne & Rosacea Society on medical management of acne in adult women, part 1: overview, clinical characteristics, and laboratory evaluation. 2015;96(6):376–382.
- Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7(2):22–30.
- Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21(2):223–230.
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59.
- Rivera R, Guerra A. [Management of acne in women over 25 years of age]. Actas Dermosifiliogr. 2009;100(1):33–37.
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7(5):281–290.
- Dréno B, Thiboutot D, Layton AM, et al. Global Alliance to Improve Outcomes in Acne Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106.
- Schmitt JV, Masuda PY, Miot HA. [Acne in women: clinical patterns in different age-groups]. An Bras Dermatol. 2009;84(4):349–354.
- Addor FA, Schalka S. Acne in adult women: epidemiological, diagnostic and therapeutic aspects. An Bras Dermatol. 2010;85(6):789–795.
- Knaggs HE, Wood EJ, Rizer RL, Mills OH. Post-adolescent acne. Int J Cosmet Sci. 2004;26(3):129–138.
- Preneau S, Dreno B. Female acne—a different subtype of teenager acne?. J Eur Acad Dermatol Venereol. 2012;26(3):277–282.
- Dumont-Wallon G, Dreno B. [Specificity of acne in women older than 25 years]. Presse Med. 2008;37(4 Pt 1):585–591.
- Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatog Venereol. 2001;15(6):541–545.
- Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5(6):459–462.
- Plunkett A, Merlin K, Gill D, et al. The frequency of common nonmalignant skin conditions in adults in central Victoria, Australia. Int J Dermatol. 1999; 38(12):901–908.
- Tan JK, Li Y, Fung K, et al. Divergence of demographic factors associated with clinical severity compared with quality of life impact in acne. J Cutan Med Surg. 2008;12(5):235–242.
- Henkel V, Moehrenschlager M, Hegerl U, et al. Screening for depression in adult acne vulgaris patients: tools for the dermatologist. J Cosmet Dermatol. 2002;1(4):202–207.
- Lello J, Pearl A, Arroll B, et al. Prevalence of acne vulgaris in Auckland senior high school students. N Z Med J. 1995;108(1004):287–289.
- Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in early adolescent boys: correlations with pubertal maturation and age. Arch Dermato. 1991;127(2):210–216.
- Cunliffe W J, Gould DJ. Prevalence of facial acne vulgaris in late adolescence and in adults. Br J Med. 1979;1(6171):1109–1110.
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41(4):577–580.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25(9):1054–1060.
- Dreno B. Treatment of adolescent acne ; a new challenge. J Eur Acad Dermatol Venereol. 2015;29 Suppl 5:14–19.
- Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63(5):782–788.
- Khunger N, Kumar C. A clinic epidemiological study of adult acne: is it different from adolescent acne?. Indian J Dermatol Venereol Leprol. 2012;78(3):335–341.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–S50.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27 Suppl 1:3–8.

