 by Rebecca F. Wang, BA; Benjamin H. Kaffenberger, MD; and Jessica A. Kaffenberger, MD
by Rebecca F. Wang, BA; Benjamin H. Kaffenberger, MD; and Jessica A. Kaffenberger, MD
Ms. Wang and Drs. B. Kaffenberger and J. Kaffenberger are with the Department of Internal Medicine, Division of Dermatology, at The Ohio State University Wexner Medical Center in Columbus, Ohio.
Funding: Ms. Wang received funding from The Ohio State University College of Medicine Roessler Research Scholarship.
Disclosures: The authors have no relevant conflicts of interest to disclose.
Abstract: Background. New-onset dermatitis in the elderly can be attributed to a variety of disease processes. We defined new-onset dermatitis in which the etiology is attributed solely to age-related processes as “dermatitis of immune senescence”—a diagnosis of exclusion based on clinical presentation and further diagnostic testing.
Objective. Retrospective cohort of elderly patients with new-onset dermatitis to examine the differences in demographics, work-up, and treatments between patients with dermatitis of immune senescence and those patients ultimately given more specific diagnoses.
Methods. Four hundred and thirty-three patients aged 60 years and older with new-onset dermatitis from 2011 to 2016 at Ohio State University were identified by chart review and categorized as “dermatitis of immune senescence” or “alternate diagnosis” based on patch testing, biopsy, and physician documentation.
Results. In this subset of patients, 10.2 percent (44/433) underwent patch testing and 16.2 percent (70/433) underwent biopsy. Furthermore, 86.4 percent of patients who underwent patch testing (38/44) and 57.1 percent who underwent biopsy (40/70) were given a more specific diagnosis following their test. Use of intramuscular steroids (p<.001), oral steroids (p=.004), and antihistamines (p=.002) were significantly higher in the alternate diagnosis group.
Conclusion. The low rate of patch testing and biopsy and the high rate of diagnosis change post-procedure demonstrate an underutilization of diagnostic testing in this population.
Keywords: Dermatitis, immune senescence, elderly, aging, patch test, biopsy
J Clin Aesthet Dermatol. 2018;11(1):19–20
Introduction
New-onset dermatitis in the elderly can be attributed to a variety of disease processes, including atopic dermatitis, contact dermatitis, and stasis dermatitis.1,2 Additionally, age-related declines in normal immune tolerance and epidermal barrier repair can contribute to new-onset dermatitis.3 We defined new-onset dermatitis in which the etiology is attributed solely to these age-related processes as “dermatitis of immune senescence”—a diagnosis of exclusion based on clinical presentation and further diagnostic testing. In this review, we retrospectively analyze a cohort of elderly patients with new-onset dermatitis (ICD9 codes 691.8, 692.0-5, 692.81, 692.83, 692.84, 692.89, 692.9, 693.0-1, 693.8, and 693.9) to examine the differences in demographics, workup, and treatments between patients with dermatitis of immune senescence and those patients ultimately given more specific diagnoses.
Methods
Four hundred and thirty-three patients aged 60 years and older with new-onset dermatitis from 2011 to 2016 at Ohio State University were identified by chart review. Patients were excluded if they were diagnosed with dermatitis prior to the age of 60 years or if they received fewer than three diagnoses. Patients were categorized as “dermatitis of immune senescence” or “alternate diagnosis” based on patch testing, biopsy, and physician documentation. Differences between the groups were evaluated using Pearson’s chi-squared test and two-tailed t-tests. A p value less than 0.05 was considered statistically significant.
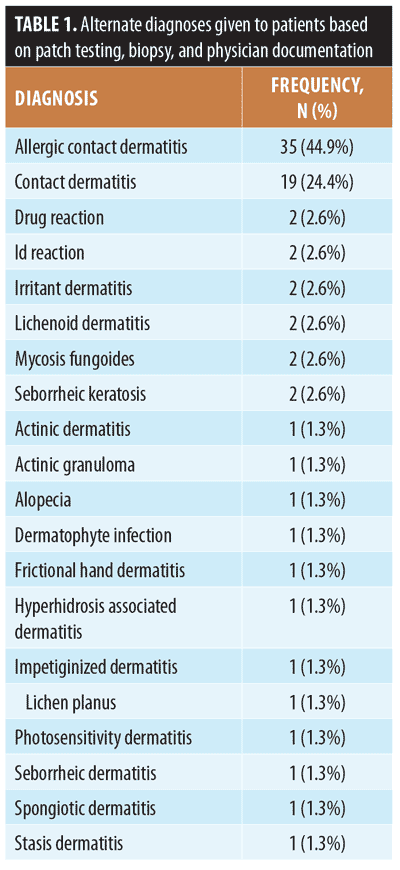
Of the patients included in this analysis, 10.2 percent (44/433) underwent patch testing and 16.2 percent (70/433) underwent biopsy. Furthermore, 86.4 percent of patients who underwent patch testing (38/44) and 57.1 percent who underwent biopsy (40/70) were given a more specific diagnosis following their test (Table 1). Demographics, prior medications, affected body surface area, predicted one-year mortality, and treatments prescribed for both groups are shown in Table 2. Interestingly, our analysis did not find marked differences between demographics, affected body surface area, laboratory workup, or mortality between patients with dermatitis of immune senescence and those patients ultimately given more specific diagnoses.
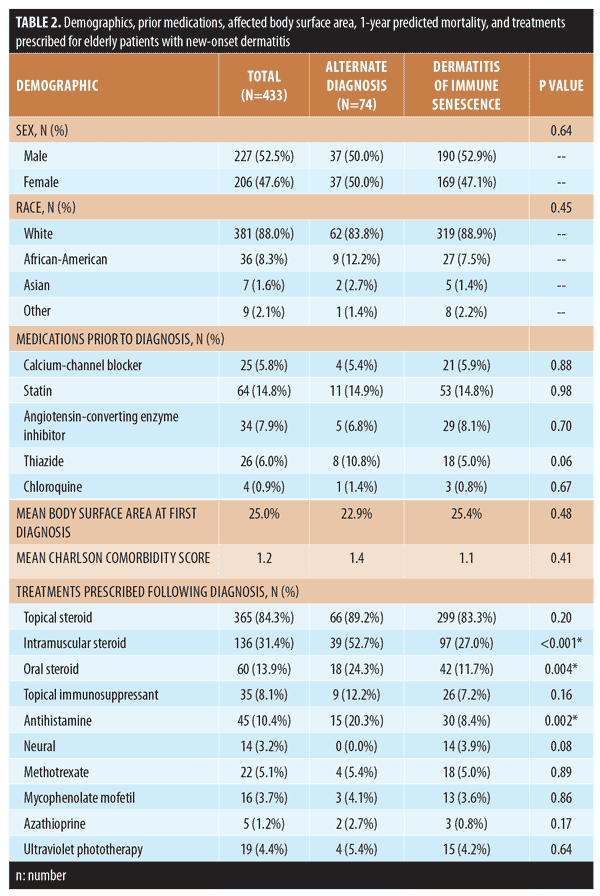
With regard to disease management, treatments were similar between the two groups with the exception of intramuscular steroid use (p<0.001), oral steroid use (p=0.004), and antihistamine use (p=0.002), which were significantly higher in the alternate diagnosis group (Table 2). Increased use of these relatively shorter-term treatments suggests that the alternative diagnosis groups might have presented a higher acuity of their dermatitis, a potential confounder. Follow-up time was also significantly different between groups. Dermatitis of immune senescence patients had a follow-up average of 659 days (standard deviation [SD]: 410), while alternate diagnosis patients had a follow-up average of 483 days (SD: 359) (p<0.001). The shorter follow-up time in patients with alternate diagnoses might indicate successful avoidance strategies or remission in the case of contact dermatitis.
Limitations of our study included the underutilization of diagnostic testing, as it is possible that with a higher patch test or biopsy rate more patients could be given a more specific diagnosis. In addition, as this was solely a characterization analysis on new-onset dermatitis, further research is needed regarding the immunopathogenesis of dermatitis of immune senescence. Finally, our study was limited by variations in coding and documentation among physicians that might have affected the interpretation of diagnoses. However, with a retrospective design, our analysis reflects real-world clinical practice and diagnostic evaluation. Given the low rate of patch testing and biopsy and the high rate of diagnosis change post-procedure, our results demonstrate an underutilization of diagnostic testing and suggest that there might be value in performing more patch tests and biopsies in this subset of patients.
References
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73–86.
- Reich A, Ständer S, Szepietowski JC. Pruritus in the elderly. Clin Dermatol. 29(1):15–23.
- Berger TG, Steinhoff M. Pruritus in elderly patients—eruptions of senescence. Semin Cutan Med Surg. 2011;30(2):113–117.

