 by Deganit Barak-Shinar, PhD, and Lawrence J. Green, MD
by Deganit Barak-Shinar, PhD, and Lawrence J. Green, MD
Dr. Barak-Shinar is Vice President of Clinical, Regulation and Quality Assurance at Kamedis Ltd. in Tel-Aviv, Israel. Dr. Green is Associate Clinical Professor of Dermatology at The George Washington University School of Medicine in Washington, DC.
Funding: This study was wholly funded by Kamedis Ltd.
Disclosures: Dr. Barak-Shinar is an employee of Kamedis Ltd. Dr. Green serves on the medical advisory board for Kamedis Ltd.
Abstract: Objective. The aim of this study was to evaluate the safety and efficacy of an herbal and zinc pyrithione shampoo and a scalp lotion (Kamedis Derma-Scalp Dandruff Therapy, Kamedis Ltd., Tel Aviv, Israel) for the treatment of scalp seborrheic dermatitis and dandruff.
Design. This was an interventional, open-label, safety and efficacy study.
Setting. This open-label study was conducted at Consumer Product Testing Company Inc. in Fairfield, New Jersey. At the baseline visit (Day 0), an examination of the scalp was conducted by a board-certified dermatologist. The entire scalp was evaluated for evidence of seborrheic dermatitis using the Adherent Scalp Flaking Score with a 10-point scale. Only subjects with evidence of moderate-to-greater seborrheic dermatitis or moderate-to-greater dandruff were deemed qualified for inclusion in the study.
Participants. Fifty subjects were recruited and included in the study.
Measurements. Study subjects were evaluated by the same dermatologist for erythema and flaking at Days 0, 14, 28, and 42 using a five-point scale for each parameter. At each time point, a total severity score was calculated based on the findings of the evaluations. Following the scalp evaluation, each subject had a standardized digital photograph taken of his or her scalp. Each subject was also asked to answer a satisfaction questionnaire regarding the product treatment enhancement and characteristics.
Results. A reduction in both parameters evaluated was seen at all time points. Statistical significance was achieved at each time point when compared with the baseline visit. In addition, the subjects expressed a high degree of satisfaction with the treatment. No adverse events were reported during this study. Conclusion: The study showed that the herbal zinc pyrithione shampoo and scalp lotion provided improvement in the main symptoms of seborrheic dermatitis.
Keywords: Seborrheic dermatitis, dandruff, scalp, shampoo, lotion, Kamedis, erythema, loose flaking, total severity score
J Clin Aesthet Dermatol. 2018;11(1):26–31
Introduction
Moderate-to-severe seborrheic dermatitis (SD) and dandruff is a common skin condition that can affect the scalp and is characterized by erythema and scaling that often itches. Milder SD can appear as itchy, flaking skin without visible inflammation.
It is estimated that the prevalence range of SD can occur in 50 to 70 percent of the population.1–3 Even though the prevalence is high, the exact cause of the skin condition is still not fully understood.1–3 Available therapies temporarily control the symptoms but do not cure the condition. The pathogenesis of SD is considered to be caused by a combination of three main factors: sebaceous gland function, presence and proliferation of Malassezia furfur yeast (Pityrosporum ovale) on the skin, and the individual immunological response to this yeast. Reducing Malassezia counts with antifungal agents is effective in providing relief for SD and dandruff.4–5
Available treatments for the control of scalp SD are divided into three main classes according to their mechanism of action: antifungal agents, flake-removing agents, and anti-inflammatory agents. The majority of the commercially available treatments contain antifungal agents, which are active compounds that can be used against the Malassezia furfur yeasts. Zinc pyrithione and selenium sulfide are over the counter products that reduce Malassezia furfur proliferation. Salicylic acid can cause keratolysis and help improve the flaking seen in SD.6–13 Over the counter and prescription topical steroids and immunomodulators are anti-inflammatory and have also been shown to improve SD.14–16
Very few clincal studies have considered herbal-based shampoos for the treatment of dandruff. However, herbal-based shampoos that contain anti-fungal agents have been found to be effective and tolerable for dandruff treatment .17
In this study, we present a new herbal-based SD treatment kit that includes antifungal agents for the treatment of SD. Kamedis Derma-Scalp Dandruff Therapy shampoo and scalp lotion (Kamedis Ltd., Tel-Aviv, Israel) includes a therapeutic shampoo and a pre-treatment scalp lotion. The products are based on herbal botanical ingredients18–27 that have antifungal, antibacterial, and anti-inflammatory effects. Zinc pyrithione is also used in the shampoo to augment the antifungal activity of the herbal ingredients.
The aim of this open-label study was to evaluate the safety and efficacy of the new herbal-based shampoo and scalp lotion (Kamedis Ltd., Tel-Aviv, Israel) in subjects with moderate-to-severe scalp SD or moderate-to-severe dandruff during and following a treatment period of six weeks.
Methods
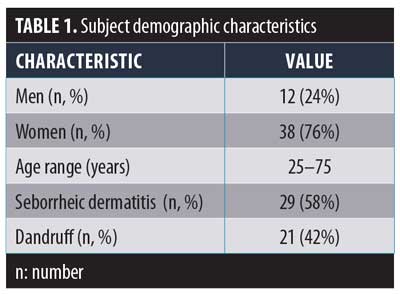
Fifty subjects (38 women and 12 men) aged 20 to 75 years were recruited for the study. Forty-nine subjects completed the single-center, open-label study. Table 1 outlines the demographic characteristics of the participants. One subject did not complete the study due to personal reasons unrelated to the study product. The clinical study was conducted at Consumer Product Testing Company (CPTC) Inc. in Fairfield, New Jersey.
All subjects signed an informed consent form and a photographic release form. Institutional review board approval was obtained for this study. Qualified subjects were classified as having moderate-to-severe SD based on a board-certified dermatologist’s evaluation using the Adherent Scalp Flaking Score (ASFS).28 Using ASFS, the dermatologist examined the entire scalp for evidence of SD using the following scoring system: 0 = no scaling, 2 = slight scaling, 4 = some scaling, 6 = moderate scaling, 8 = heavy scaling, and 10 = very heavy scaling. Only subjects with evidence of moderate or greater SD (score ? 6) or moderate to greater dandruff (score ? 6) were qualified for the study. For 14 days prior to the initiation of the study, subjects were instructed to wash their hair with Johnson’s Baby Shampoo (Johnson & Johnson, New Brunswick, New Jersey) provided by the facility and to use only non-medicated conditioner. Subjects were instructed to avoid using any other shampoo or any styling aids, such as hair sprays, mousses, or gels.
At the baseline visit (Day 0), the 50 subjects who were previously identified as having moderate-to-severe SD were evaluated for scalp erythema and scaling.
The erythema evaluation used the following scale: 0=none, 1=mild, 2=moderate, 3=marked, and 4=severe. The loose flaking evaluation used the following scale: 0=none or barely perceptible, 1=mild, 2=moderate, 3=severe, and 4=very severe.
Following the scalp evaluation, each subject had a standardized digital photograph taken (Nikon D90 camera, Nikon Inc., Tokyo, Japan) with fixed local lens on a stereotactic head device (Canfield Scientific Inc., Parsippany, New Jersey).
Each qualified subject was supplied with the aforementioned baby shampoo as well as the study shampoo and scalp lotion kit. Each subject was instructed to use the dandruff therapy shampoo at least twice per week and to use the scalp lotion daily before bedtime, leaving it on overnight prior to shampooing the following morning.Subjects were instructed to use this system for a consecutive period of 42 days. The subjects were instructed to use the study shampoo a minimum of twice per week up to daily use. Baby shampoo could be used on days that the study shampoo was not used, in cases of scalp dryness. In addition, each subject was instructed to record the application time of the treatment products in a daily diary.
After the baseline visit (Day 0), subjects visited the clinic for two follow-up visits at Day 14 and Day 28 and for a final visit at Day 42. A flexibility of two days was allowed. In the case of an adverse event or any reaction problems, the subjects were asked to immediately contact the testing facility and to come in for an unscheduled visit.
At each of the two follow-up visits and at the final visit, the board-certified dermatologist evaluated the scalp irritation and loose flaking. A digital photograph of the subject scalp was captured.
A total severity score was calculated for each subject per each visit—specifically for Day 0, Day 14, Day 28, and Day 42—by summing the scores for erythema and loose flaking.
At the final visit, the subject completed a product-assessment questionnaire. The questionnaire included several questions on satisfaction with the product. The questionnaire used the following five-point scale: 5=liked it extremely well; 4=liked it really well; 3=somewhat liked it; 2=neither liked nor disliked it; or 1=did not like it. The attributes included overall satisfaction, speed of results, ease of use, texture, color, and odor.
Results
Forty-nine subjects completed the study. One dropped out due to personal reasons unrelated to the study.
Physician assessments from each subject visit were presented with respect to the erythema and loose flaking indices. Improvement percentages were compared with those from baseline using the nonparametric Dunn Test. Analysis showed statistically significant reduction between all tested pairs when compared with baseline (according to parameters and visits when considering an alpha level of 5 percent in the probability distribution plot). This reduction supports the observation that symptoms improve with each visit and especially from baseline.
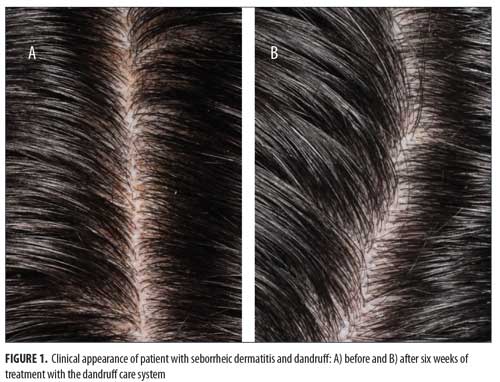
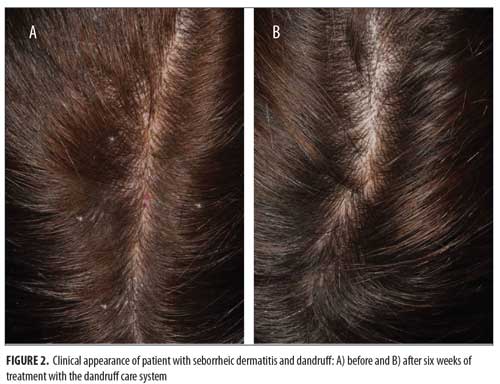
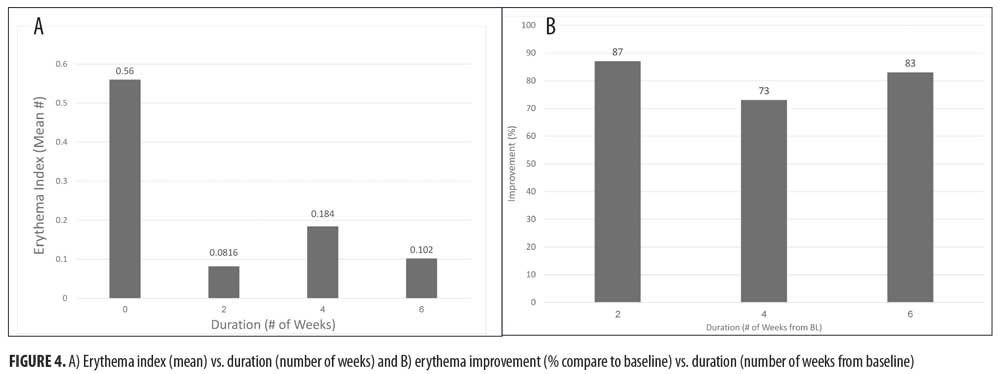
At all time points, erythema and loose flaking showed significant clinical improvement in all participants compared with baseline. In addition, clear improvement was visually evident in all participants (Figures 1–3).Specifically, the erythema mean index presented (Figure 4A) was reduced from 0.56 at baseline (Week 0) to 0.0816 at the next visit (Week 2). The index showed a minor increase at the following two visits with values of 0.184 and 0.102 at Weeks 4 and 6, respectively. The erythema parameter improved by 87 percent after two weeks and remained at an 83-percent improvment after six weeks of treatment (p<0.05 as compared with baseline). The largest percent improvement in all parameters occurred between baseline and Week 2, suggesting a fast onset of action (Figure 4B).
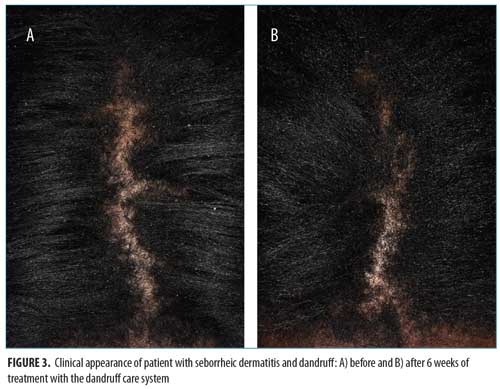
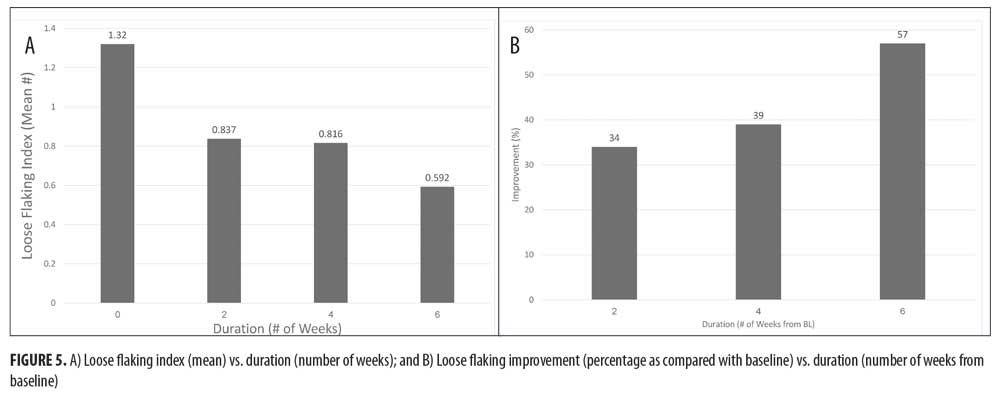
Loose flaking mean index (Figure 5A) reduced from 1.32 at baseline to 0.837 at Week 2 and 0.592 at Week 6, showing an improvement of 34 percent after two weeks, 39 percent after four weeks, and 57 percent after six weeks of treatment (Figure 5B) (p<0.05 as compared with baseline).
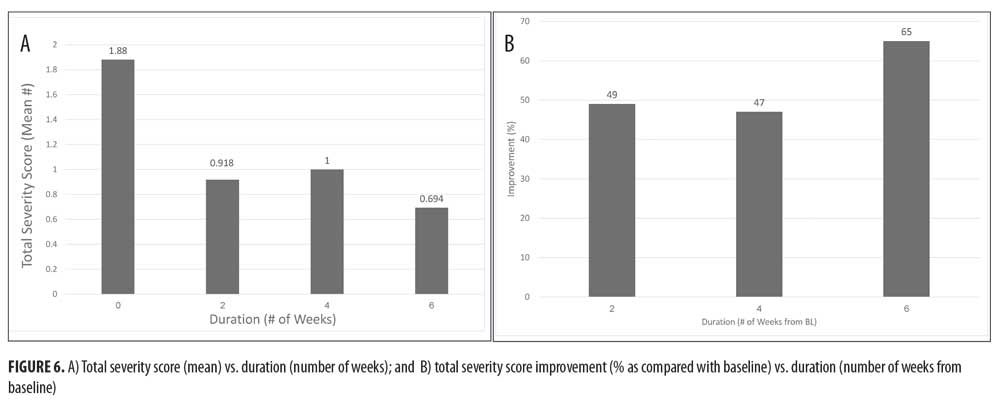
The average total severity score was improved by 49 percent following two weeks of treatment and by 65 percent after six weeks of treatment (p<0.05 as compared with baseline), while the mean score changed from 1.88 at baseline to 0.694 after six weeks (Figures 6A and 6B). The evaluated parameters maintained their improvement until the end of the six-week study.
The statistical analysis of the improvement values clarifies the significant decrease of the evaluated parameters in comparison with the baseline pre-application scores. All symptoms showed significant improvement after two weeks of treatment, and then showed maintenance or further improvement until the end of the study period. The method performances were chosen and validated, since sphericity test showed that the repeated measures effect might be negligible over time. Therefore, the data could be compared using the cumulative mean to the baseline.
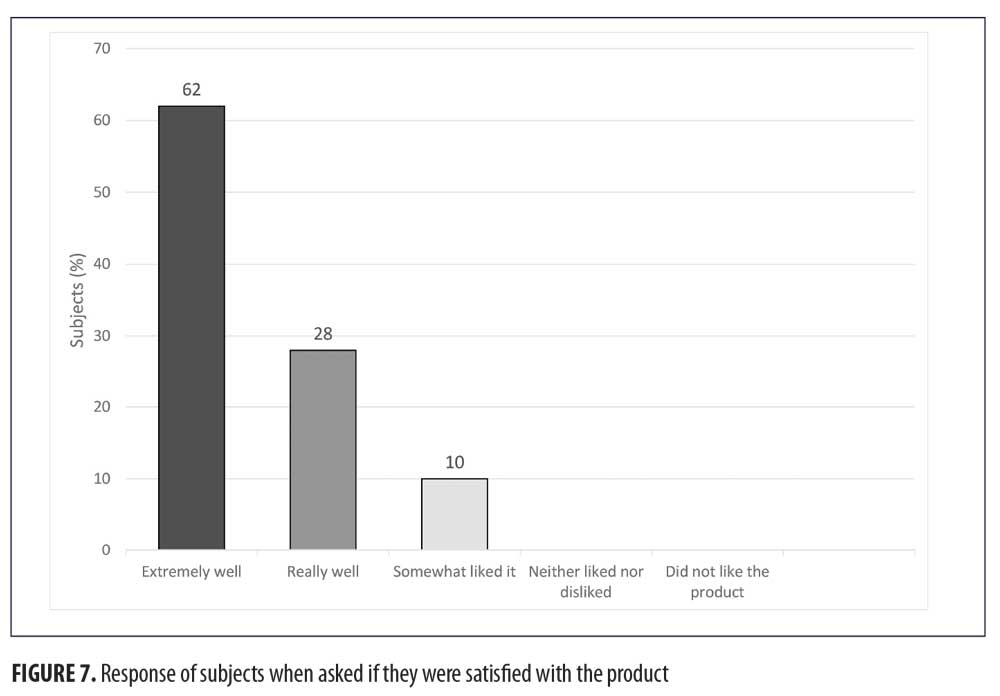
When the subjects were asked how they liked the product, 62 percent (n=30) of the subjects liked the product extremely well, 28 percent (n=14) liked it really well, and 10 percent (n=5) somewhat liked it. Zero participants responded with “neither liked nor disliked the product” or “did not like the product.”(Figure 7)
Results regarding the characteristics of the product, including cosmetics attributes (fragrance, easy to foam, easy to rinse), feel on the skin, texture, color, and odor yielded a very good satisfaction rate (67% [n=33] of the subjects listed it extremely well and really well).
No adverse experiences or events occurred during the course of the trial.
Discussion
In this study, treatment with the study shampoo and scalp lotion significantly improved all evaluated parameters of scalp SD and dandruff when compared with baseline while presenting no safety concerns.
As an antifungal agent, numerous studies have shown that zinc pyrithione, ketoconazole,6 and selenium sulfide7 improve SD. Herbal-botanical compounds can expand the variety of treatment options available to treat SD.12,17 One example of this approach is a shampoo treatment based on an extract of Rosa centifolia petals and epigallocatechin gallate (EGCG) incorporated with climbazole and zinc pyrithione. According to the publication authors, these ingredients inhibit sebum secretion and have anti-inflammatory and antioxidant effects.12 In addition, tea tree oil shampoo has been shown to have antifungal effects and has shown to be clinically effective in treating dandruff caused by the yeast Pityrosporum ovale.17
The study treatment products, composed of shampoo and scalp lotion, are a combination of commonly used active ingredients and unique herbal botanical ingredients. Zinc pyrithione is a commonly used antifungal agent in the treatment of dandruff and SD. The herbal botanical ingredients in both products, especially in the scalp lotion, offer potential anti-inflammatory, antimicrobial, and antifungal ingredients, such as Phellodendron amurense bark extract, Portulaca oleracea extract, Sapindus mukorossi fruit extract, and Indigofera tinctoria extract.18–26 In addition, the treatment products contain ingredients for sebum secretion reduction (Rheum palmatum root extract27), which might assist in improving and relieving the severity of SD.
Conclusion
This six-week, open-label safety and efficacy study demonstrated that using an herbal and zinc pyrithione-based shampoo and scalp lotion improved the symptoms and severity of scalp SD and dandruff symptoms. All factors demonstrated clinically meaningful reductions during the study, especially from baseline to the first visit at Week 2. A major improvement from baseline was demonstrated at each parameter in every symptom measured. Loose flaking showed additional improvement toward the end of the study, which contributed to the additional improvement demonstrated in the total severity score. This improvement after longer period duration suggests a low adaptation rate of the subjects to the ingredients, which suggests the product will remain effective for a longer treatment time. A vehicle-controlled study would be the next step in better demonstrating the efficacy of the study therapy. This design will be added to future investigations.
Subjects were extremely satisfied with the speed of improvement, the ease of use of the products, and the cosmetic characteristics of the shampoo and lotion. No safety issues were encountered.
References
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2):1–22.
- Grimalt R. A practical guide to scalp disorders. J Investig Dermatol Symp Proc. 2007;12(2):10–14.
- Schwartz JR, Messenger AG, Tosti A, et al. A comprehensive pathophysiology of dandruff and seborrheic dermatitis – towards a more precise definition of scalp health. Acta Derm Venereol. 2013;93(2):131–137.
- Bergbrant IM. Seborrhoeic dermatitis and Pityrosporum yeasts. Curr Top Med Mycol. 1995;6:95–112.
- Dawson TL Jr. Malassezia globosa and restricta: breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J Investig Dermatol Symp Proc. 2007;12(2):15–19.
- Piérard-Franchimont C, Goffin V, Decroix J, Piérard GE. A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrheic dermatotis. Skin Pharmacol Appl Skin Physiol. 2002;15(6):434–441.
- Rapaport M. A randomized, controlled clinical trial of four anti-dandruff shampoos. J Int Med Res. 1981;9(2):152–156.
- Bulmer AC, Bulmer GS. The antigugal action of dandruff shampoos. 1999;147(2):63–65.
- Turlier V, Viode C, Durbise E, et al. Clinical and biochemical assessment of maintenance treatment in chronic recurrent seborrheic dermatitis: randomized controlled study. Dermatol Ther (Heidelb). 2014;4(1):43–59.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6(2):44–49.
- Dall’Oglio F, Lacarrubba F, Verzì AE, Micali G. Noncorticosteroid combination shampoo versus 1% ketoconazole shampoo for the management of mild-to-moderate seborrheic dermatitis of the scalp: results from a randomized, investigator-single-blind trial using clinical and trichoscopic evaluation. Skin Appendage Disord. 2016;1(3):126–130.
- Kim YR, Kim JH, Shin HJ, et al. Clinical evaluation of a new-formula shampoo for scalp seborrheic dermatitis containing extract of Rosa centifolia petals and epigallocatechin gallate: a randomized, double-blind, controlled study. Ann Dermatol. 2014;26(6):733–738.
- Emtestam L, Svensson Å, Rensfeldt K. Treatment of seborrhoeic dermatitis of the scalp with a topical solution of urea, lactic acid, and propylene glycol (K301): results of two double-blind, randomised, placebo-controlled studies. 2012;55(5):393–403.
- Kircik LH. Treatment of scalp and facial seborrheic dermatitis with desonide hydrogel 0.05%. J Clin Aesthet Dermatol. 2009;2:32–36.
- Katsambas A, Antoniou C, Frangouli E, et al. A double blind trial of treatment of seborrhoeic dermatitis with 2% ketoconazole cream compared with 1% hydrocortisonecream. Br J Dermatol. 1989;121(3):353–357.
- Stratigos JD, Antoniou C, Katsambas A, et al. Ketoconazole 2% cream versus hydrocortisone 1% cream in the treatment of seborrheic dermatitis. A double-blind comparative study. J Am Acad Dermatol. 1988;19(5 Pt 1):850–853.
- Satchell AC, Saurajen A, Bell C, Barnetson RS. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47(6):852–855.
- Cuéllar MJ, Giner RM, Recio MC, et al. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. 2001;72(3):221–229.
- Yu HH, Kim KJ, Cha JD, et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8(4):454–461.
- Quan H, Cao YY, Xu Z, et al. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob Agents Chemother. 2006;50(3):1096–1099.
- Chan K, Islam MW, Kamil M, et al. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. Sativa (Haw.) Celak. J Ethnopharmacol. 2000;73(3):445–451.
- Lim YH, Kim IH, Seo JJ. In vitro activity of kaempferol isolated from the Impatiens balsamina alone and in combination with erythromycin or clindamycin against Propionibacterium acnes. J Microbiol. 2007;45(5):473–477.
- Ibrahim M, Khan AA, Tiwari SK, et al. Antimicrobial activity of Sapindus mukorossi and Rheum emodi extracts against H pylori: In vitro and in vivo studies. World J Gastroenterol. 2006;12(44): 7136–7142.
- Anisimov MM, Shcheglov VV, Strigina LI, et al. Chemical structure and antifungal activity of a number of triterpenoids. Biol Bull Acad Sci USSR. 1979;6(4):464–468.
- Choi J, Jung HJ, Lee KT, Park HJ. Antinociceptive and anti-inflammatory effects of the saponin and sapogenins obtained from the stem of Akebia quinata. J Med Food. 2005;8(1):78–85.
- Kunikata T, Tatefuji T, Aga H, et al. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur J Pharmacol. 2000;410(1): 93–100.
- Cha TL, Qiu L, Chen CT, et al. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65(6): 2287–2295.
- Bacon RA, Mizoguchi H, Schwartz JR. Assessing therapeutic effectiveness of scalp treatments for dandruff and seborrheic dermatitis, part 1: a reliable and relevant method based on the adherent scalp flaking score (ASFS). J Dermatolog Treat. 2014;25(3):232–236.

