 J Clin Aesthet Dermatol. 2021;14(6 Suppl 1):S11–S14
J Clin Aesthet Dermatol. 2021;14(6 Suppl 1):S11–S14
by Devan Vaghela, MBBS, MRCP; Emma Davies, RN, INP; Gillian Murray, MPharm, PG Dip, Clin Pharm, INP; Cormac Convery, MB ChB, MSc, MASLMS; and Lee Walker, BDS, MFDS, RCPSG, MJDF, RCS, ENG
Dr. Vaghela is with the Department of Microbiology at Norfolk and Norwich University Hospitals, United Kingdom, and with V&A Aesthetics in London, United Kingdom; Ms. Davies is Clinical Director of Save Face in Cardiff, United Kingdom; Dr. Murray is with Clinical Academic Kings College in London, England; Dr. Convery is with The Ever Clinic in Glasgow, Scotland; and Dr. Walker is with B City Clinic in Liverpool, England. All authors are founding board members of the Complications in Medical Aesthetics Collaborative (CMAC).
FUNDING: No funding was provided for the preparation of this article.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: The Complications in Medical Aesthetics Collaborative (CMAC) is a not-for-profit organization established to promote best patient outcomes through educating clinicians who perform nonsurgical cosmetic procedures in the prevention, diagnosis, and management of complications that can arise. The organization is a global community sharing information, learning, experience, and data to promote best practice. Herpes simplex is common in the general population. The risk of reactivation due to trauma is acknowledged in the literature. Reactivation of herpes simplex 1 following cosmetic procedures is considered rare, but there are no reliable data on the incidence. Although self-limiting, symptoms can be painful and distressing for patients who have undergone a procedure to improve their appearance. Clinicians should document the steps they’ve taken to avoid reactivation, and should be confident in their management plan and patient care if a patient suffers a reactivation. The authors have referred to the available literature and experience to provide a user-friendly guideline to reduce risk of herpes simplex 1 reactivation through appropriate patient screening, promote accurate diagnosis and an understanding of differentials, and provide management plans for prophylaxis to minimize adverse sequalae. In cases of post-procedure outbreak, the guideline explains the management options, including patient education and support when prescription medications are not indicated.
Keywords: Herpes dermal fillers, herpes simplex cosmetic treatments, herpes diagnosis and treatment. dermal fillers herpetic infections, cold sores diagnosis and treatment, aciclovir prophylaxis dermal fillers
Herpes simplex virus type 1 (HSV-1) is known to cause symptoms commonly called “cold sores.” HSV-1 is usually a benign viral infection, with many people having no symptoms at all. Infection occurs from close contact, such as kissing; in countries of high socioecominc status, 40 to 60 percent of the population have acquired the infection by the age of 70 years.1 Recurrence of cold sore symptoms typically occurs 2 to 3 times a year, with most attacks occurring within the first year of symptoms.2 According to the literature, HSV-1 reactivation following dermal filler injection is rare. A case series of 138 patients in six centers in the United States found that two patients (1.45%) developed herpes-like infections within two weeks of dermal filler treatment.3
In a 500-patient study, postoperative infection with HSV-1 was seen in 7.4 percent of patients postablative laser procedure, regardless of prior history.4 Prophylaxis is recommended with or without a history of HSV-1 for patients undergoing ablative laser resurfacing procedures.5 Further data related to cosmetic procedures and HSV-1 reactivation are needed.
This guideline focuses on the diagnosis, risk assessment, and management strategies to reduce risk of HSV-1 occurrence following cosmetic procedures and optimize outcomes.
Classification
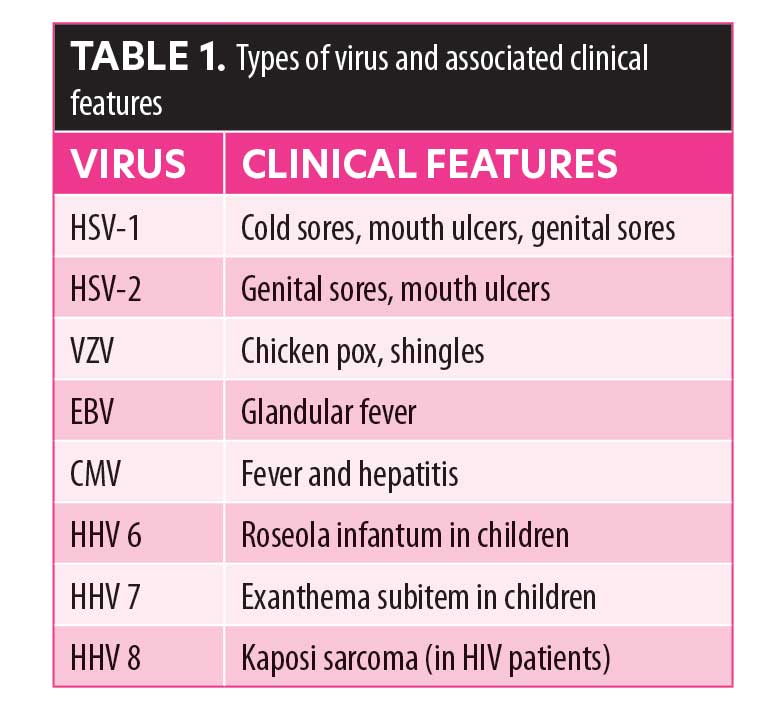
HSV-1 is a member of the Herpesviridae family (order Herpesvirales), and part of the Baltimore Class 1 group, which are characterized by an icosahedral capsid enclosed in a glycolipid envelope. Other members of this family include varicella zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human herpes virus 6, 7, and 8 (HHV 6, 7, and 8).2 The common presentations of these viruses are described in Table 1.
Pathophysiology
HSV-1 infection in healthy adults is, for the most part, benign and self-limiting, causing infection of the lips, cheeks, or nose.
The virus is transmitted via personal contact with an individual who is actively shedding the virus and a seronegative individual. The virus must come into contact with the mucosal surface or skin abrasion. Viral replication occurs locally in the mucosa of the mouth with an incubation period ranging from 2 to 12 days. The capsid undergoes retrograde travel through the nerve to the trigeminal ganglion (for oral lesions) where latency is established. Triggering factors associated with recurrence include physical trauma, ultra-violet radiation, fatigue, extremes of temperature, mouth or lip trauma, and dental or surgical procedures.6
Reactivation can be provoked by direct trauma with a needle during an aesthetic procedure, tissue manipulation, dermal injury, and/or an inflammatory reaction. Viral reactivation tends to occur in the treated area but can occur in neighboring areas; the most common sites are the perioral area and the nasolabial folds.7 The pattern of reactivation and subsequent eruption depend upon the causative virus.
Minimizing the Risks
It is important to document a detailed medical history of all patients prior to dermal filler procedures, including current medications that the patient is taking. Patients who are immunocompromised or on immunosuppressant treatment should undergo an individual risk assessment prior to having any procedure with dermal fillers. Patients should be screened for a history of previous herpetic outbreaks, including cold-sores (HSV-1).
Patients should be asked specifically if they have ever had a cold sore, and, if there is a positive history, a more detailed assessment should follow, such as 1) location of the lesions, 2) frequency and duration of symptoms, 3) known trigger factors, 4) prodromal symptoms, 5) whether they have been provoked by previous procedures, 6) whether they have used any antiviral medications and their success/lack of success, and 7) complications that may have arisen as a result (including post-herpetic neuralgia, cranial or peripheral nerve palsies, encephalitis, myelitis, visual loss)8
If the patient reports a history of herpes infections, an examination of the lips for early presentation or recently healing lesions is recommended. Treatment should be delayed until infection has run its course and the skin is fully healed. If the patient has prodromal symptoms, an active lesion, or is likely to be exposed to triggers within two weeks, aesthetic procedures should be delayed until risk factors are minimized and symptoms have completely resolved.9 Risk of post-herpetic neuralgia (pain persisting 120 days after disease onset) increases with patient age, and it has been estimated that 13 to 40 percent of patients over the age of 60 years will have post-herpetic neuralgia six months after their outbreak.8 A lower threshold for the use of prophylaxis and prompt treatment of an outbreak for these patients is recommended.
Refer to dermal filler manufacturer training guidelines for specific cautions and contraindications related to HSV-1.
Diagnosis
Cold sores. Diagnosis of cold sores is based on clinical history and presentation alone. Lesions can be preceded by prodromal symptoms, which might include fever, general malaise, sore throat, and cervical and submandibular lymphadenopathy, particularly in primary (rather than recurrent) infections.10 Symptoms signaling the onset of the lesions within 6 to 48 hours include pain, burning, tingling, itching, and altered sensation.
Herpetic lesions. Herpetic lesions appear initially as thin-walled, intra-epidermal vesicles that eventually burst, crust, and then heal. They are typically circular ulcerations covered by a yellowish film with surrounding erythema. There is often some weeping from the ulcerations. In otherwise healthy individuals, the recurrence of symptoms is usually mild and self-limiting, typically healing in 5 to 7 days without scarring.
Differential Diagnosis
The appearance of a herpetic outbreak can sometimes be confused with a bacterial infection, such as impetigo, so ensuring the correct diagnosis is essential to treat the complication effectively. When a blistering reaction occurs outside of the areas typical of herpes eruptions or in a high-risk area for vascular occlusion, vascular compromise should be excluded. The timing and history of the presentation is important; following a vascular occlusion, subsequent tissue ischemia and the progression of symptoms will include delayed capillary refill and reticulation, as distinct from the symptoms of a herpetic infection.11
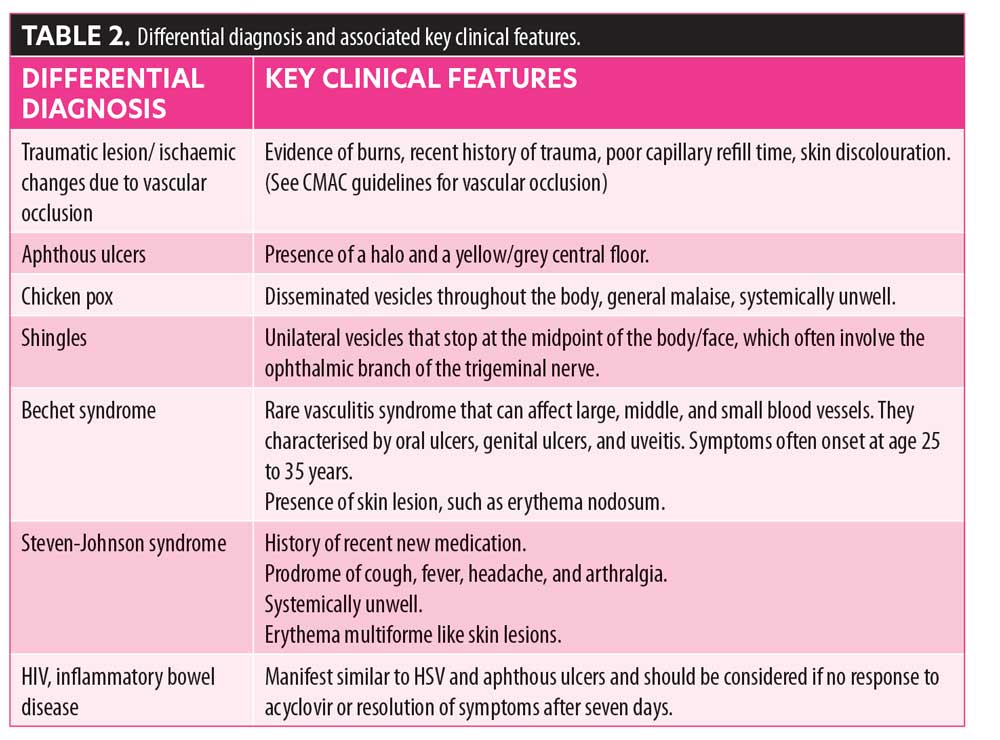
Other conditions that can present with similar symptoms include traumatic lesions (mechanical, thermal, or chemical), aphthous ulcers, chicken pox, shingles, syphilis, Bechet syndrome, Stevens-Johnson syndrome, human immunodeficiency virus (HIV), and inflammatory bowel disease (Table 2).
Treatment of Active Infection
Routine treatment for healthy individuals with mild-to-moderate recurrent episodes of HSV-1 is not recommended.Most episodes of herpes labialis are self-limiting, the evidence on the benefits of oral antivirals is limited, and oral treatment needs to be initiated at the onset of prodromal symptoms (which might be challenging to do). This is relying on patients reporting symptoms in a timely fashion, prompt access to prescribed antivirals, and patient adherence to treatment.
Antiviral drugs inhibit viral replication. These guanine nucleoside analogues are converted into their active drug component within an infected cell by the action of viral thymidine kinase. Most viral replication occurs within the first 24 hours of infection. Prompt treatment (at prodromal stage, prior to lesions erupting) is recommended to limit epithelial damage and possible secondary complications.
Oral agents available in the United Kingdom and the United States include aciclovir and valaciclovir. In the United States, valociclovir is more readily available (covered by the majority of insurance providers). Valaciclovir is a prodrug of acyclovir and has greater bioavailability and absorption, but it is more expensive. Oral acyclovir is a safe drug with a minimal side effect profile and does not require monitoring. While side effects can occur, they are rare. For further information on contraindications, cautions, drug interactions, and adverse effects, see the electronic Medicines Compendium or the British National Formulary.12,
Resistance to aciclovir is very rare in immunocompetent people. If symptoms worsen or do not improve significantly in 5 to 7 days, then an alternative diagnosis should be considered. Patients should be referred for medical review and investigation of systemic causes of ulcers, such as HIV, inflammatory bowel disease, Bechet, complex apthosis, and systemic lupus erythematosus (SLE). Oral antivirals taken early (before the lesion appears) in a recurrent episode might be more effective than a placebo at reducing symptom duration and healing time. Treatment is not a cure and will only prevent lesions if administered at the prodromal stage. Once the lesions have appeared, symptoms might be lessened in duration by one day.13
Indications for treatment with oral antiviral medicines include healthy individuals with any of the following: an episode of primary oral herpes simplex infection; recurrent herpes labialis; severe, frequent, or persistent lesions; and/or recurrent gingivostomatitis (rare), depending on clinical judgement. In immunocompromised individuals with an episode of primary or recurrent oral herpes simplex infection, use of oral antiviral medications should be based on best clinical judgement.
When an oral antiviral drug is indicated, clinicians should advise their patients to take the medication from the time of onset of prodromal symptoms, before vesicles appear, if possible, until lesions have healed, for a minimum of five days.
When a patient reports a cold sore eruption following a low-risk procedure, clinicians should educate patients on management of symptoms and prevention of spread and further reinfection and should continue to monitor and document the eruption until resolved. Patients might present with concurrent or subsequent superimposed bacterial infection with impetigo surrounding crusted lesions. Bacterial infections are often responsible for symptoms worsening rather than failure of antiviral treatment, and following clinical assessment antibiotics should be considered. (Please refer to the CMAC guidelines on Management of Acute Skin Infections.)
Treatment of HSV-1
In immunocompetent patients with moderate symptoms, such as painful swallowing, multiple lesions, and buccal mucosa/pharyngeal lesions, 200mg Aciclovir PO five times a day is recommended. In immunocompromised patients or those with poor absorption, 400mg Aciclovir PO five times a day is recommended.
Prophylaxis
Clinical decision making and risk assessment should consider the patient-specific history and assessment. While there is no definitive evidence base on the actual risk of reactivation following minimally invasive procedures or in the actual benefit of prophylactic regimes, CMAC advocates anti-HSV prophylaxis for patients who have previously had a herpetic outbreak following an aesthetic procedure,,14 when the procedure breaches the integrity of the skin, such as medical needling, chemical peels, dermabrasion and microdermabrasion, dermal filler injections to the lips or nasal labial folds, and routinely for CO2 laser resurfacing procedures. Please note that immunocompromised/immunosuppressed patients should be treated with caution.
ACICLOVIR 400MG (twice a day): To commence two days before treatment and to continue for seven days.
If previous history of failure on aciclovir prophylaxis (with good adherence) or deemed to be high risk of reactivation including immunosuppression:
VALACICLOVIR 500MG (once a day): For two days before and to continue for seven days.
There are no randomized controlled trials to indicate optimum time to commence episodic prophylaxis. Based on a review of the literature, a reasonable protocol would not be before two days prior to treatment and no later than the day of treatment and continue for five days, or until skin (post procedure) has healed.
Oral antivirals do not prevent progression of latent infection. If the patient suffers a recurrence despite prophylaxis, revert to treatment of active infection protocol. Provide self-help advice on the management of symptoms and the prevention of autoinoculation and transmission to others.
Follow-up
All patients presenting with a herpetic eruption postprocedure should be supported to manage their condition, as per patient advice sheet, and carefully monitored. Photographs should be taken to objectively assess over time. Refer to primary care for further investigation, if symptoms do not improve after seven days or if they worsen.The patient should be offered appropriate prophylactic treatment for subsequent treatments in the future.
Identifying and communicating potential risks with the patient as part of the assessment and consent process, good record keeping, and good follow-up and support all play their part in preventing a complication from becoming a complaint.
Acknowledgment
Complications in Medical Aesthetics Collaborative (CMAC) provides support and education to medical aesthetic clinicians. Members of the organization are part of a collaboration that aims to capture data to help improve patient safety. For more details, please see www.cmac.world.
References
- Chayavichitsilp P, Buckwalter JV, Krakowski AC, Friedlander SF. Herpes simplex. Pediatr Rev. 2009;30(4):119–129.
- Bentley JM, Barankin B, Guenther LC. A review of common paediatric lip lesions: herpes simplex/recurrent herpes labialis, impetigo, mucoceles, and hemangiomas. Clin Pediatr (Phila).
2003;42(6):475–482. - Food and Drug Administration. Restylane injectable gel. In: Summary of safety and effectiveness data. 2003
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. An evaluation of 500 patients. Dermatol Surg. 1998;24:315–320
- Nestor MS. Prophylaxis for and treatment of uncomplicated skin and skin structure infections in laser and cosmetic surgery. J Drugs Dermatol. 2005;4(6):s20–25.
- Cuddy SR, Schinlever AR, Dochnal S, Seegren PV, Suzich J, Kundu P et al. Neuronal hyperexcitabilityy is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1 https://elifesciences.org/articles/58037 [Last Accessed June 2021]
- 7. Gazzola R, Pasini L, Cavallini M. Herpes virus outbreaks after dermal hyaluronic acid filler injections. Aesthet Surg J. 2012;32(6):770–772.
- Gilden DH, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342(9):635–645.
- Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008;34:S92–S99.
- Cuddy SR, Schinlever AR, Dochnal S, Seegren PV, Suzich J, Kundu P et al. Neuronal hyperexcitabilityy is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1 https://elifesciences.org/articles/58037 [Last Accessed June 2021]
- Murray G, Convery C, Walker L, Davies E. Guideline for the management of hyaluronic acid filler-induced vascular occlusion. Journal of Clinical and Aesthetic Dermatology site. Jcadonline.com/online-exclusives. Accessed
- 12. Electronic Medicines Compendium site. Latest medicine updates. https://www.medicines.org.uk/emc. Accessed 18 May 2021.
- 16Opstelten W, Neven AK, Eekhof J. Treatment and prevention of herpes labialis. Can Fam Physician. December 2008;54(12):1683–1687.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316.
