 J Clin Aesthet Dermatol. 2022;15(4):13–19.
J Clin Aesthet Dermatol. 2022;15(4):13–19.
by Robyn Siperstein, MD; Jose Raul Montes, MD; and AnnMari Speranza, MS
Dr. Siperstein is with the University of Miami in Miami, Florida. Dr. Montes is with the Ophthalmology Department at the University of Puerto Rico in San Juan, Puerto Rico. Dr. Speranza is with the University of Tampa in Tampa, Florida.
FUNDING: Funding for editorial assistance was provided by X-Medica, LLC.
DISCLOSURES: Dr. Siperstein is a paid consultant, trainer, and recipient of research grants from Allergan & Galderma. Dr. Montes is a speaker and trainer for Allergan/Abbvie, Galderma and MERZ. Ms. Speranza does not have any conflicts to disclose.
ABSTRACT: Objective. This retrospective review assesses the efficacy and safety of low-dose triamcinolone (1mg/cc) added to hyaluronic acid fillers to decrease swelling after infraorbital injection.
Methods. This retrospective analysis includes 447 patients who underwent 706 infraorbital hyaluronic acid filler treatments from April 2013 to March 2020 by a single injector. Short-term post-procedural swelling (less than or greater to 2 weeks) was assessed through follow-up phone calls, which were documented in patient charts. The effect of triamcinolone, filler type, volume, and patient characteristics on the rate of post-procedure swelling were analyzed.
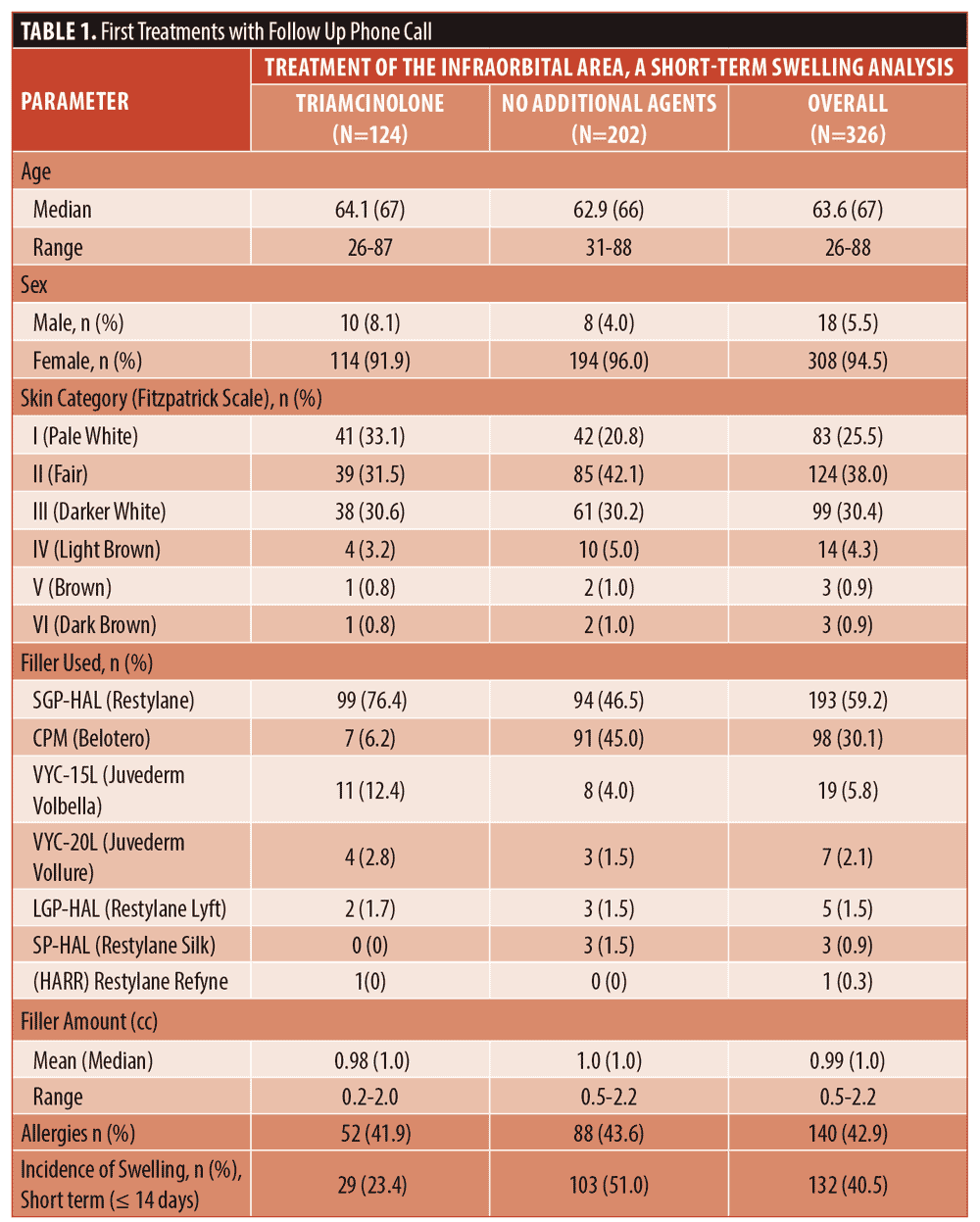
Results. Swelling after infraorbital hyaluronic acid filler occurred in over half of the patients (51%, 103/202), but significantly decreased (23%, 29/124) when 1mg/cc of triamcinolone was mixed with the filler (x2[1, N=326]=24.296, p<0.00001). The incidence of swelling was directly correlated with the amount of hyaluronic acid filler injected (37% less than or greater to 0.55cc, 51% 0.56–1cc, and 60% >1cc) [x2[1, N=95]=3.9231, p=.048]. There was no significant difference in patient age, sex, Fitzpatrick skin type, or history of allergies on incidence of post-procedure swelling. Adverse events were limited to expected injection-site reactions, and there were no reports of hypopigmentation or atrophy from the addition of triamcinolone.
Limitations. This is a retrospective study that used patient reporting for short-term post procedure swelling.
Conclusion. This is the first retrospective study showing the safety and efficacy of a novel technique adding low-dose triamcinolone (1mg/cc) to hyaluronic acid filler to reduce post-procedure swelling within the first few weeks following infraorbital injection. Additionally, using lower volumes is also effective at reducing post-procedure swelling. Larger, randomized, controlled trials are needed to support our findings.
Keywords: Infraorbital filler, tear trough filler, hyaluronic acid filler, triamcinolone, post-procedure swelling, filler complications, swelling prevention, edema, swelling.
The periorbital area is one of the most requested areas for aesthetic facial improvement since it is often the focus of our attention when judging age and disposition of an individual. The periorbital area is one of the first areas to show signs of aging. In general, a youthful attractive face has smooth contours from the eyes to the cheeks. With age, the eyes and cheeks start to compartmentalize through positional changes in both bone and soft tissue, and with involutional changes retaining ligaments become more evident.1 For nonsurgical correction of volume loss, hyaluronic acid (HA) fillers are a valuable tool; however the United States (US) Food and Drug Administration (FDA) approval for fillers is largely restricted, and only recently has one hyaluronic acid filler (VYC-15L) received FDA approval for use in the infraorbital area. Thus, study data on the infraorbital area is both limited and needed.
The tear trough is recognized as the most challenging area to treat with hyaluronic acid filler.2, 3 One of the reasons treatments of the infraorbital area pose a challenge is the high risk of swelling. In many studies, the most common complication of infraorbital hollow treatment is localized swelling.4 In a review article by Hirmand,5 the author evaluated 100 cases in which variable but subtle edema was among the most common complication following hyaluronic acid filler with resolution taking anywhere from 2 to 3 weeks to resolve.5 In a study evaluating supraperiosteal placement of (Belotero Balance [Merz Pharma Group]) in the infraorbital area with small depots, all patients experienced some swelling and bruising, which resolved within 2 to 8 days.6 Patients with swelling usually do not show signs of inflammation, such as erythema, pain, or heat.7
Clinically, it is not always possible to predict whether a patient is prone to swelling following infraorbital injection. Indeed, in one retrospective review, only a small minority of patients who experienced swelling had a pre-procedural history of fluid retention, seasonal allergies, previous eyelid or midface surgery, or festoons on exam.7 Thus, it is critical that the high incidence of both short-term and long-term swelling be discussed with all patients.7 For patients, swelling is problematic due to discomfort and the need for extended social downtime. In clinical practice, patients often cite the need to attend work, school, or social events as a reason why they cannot have procedures or need to postpone them, and options to prevent this downtime is a very common request.
In dermatology, triamcinolone is widely used intralesionally on all areas of the body for a variety of diagnoses, including the periocular area for conditions such as infantile hemangiomas.8 In ophthalmology, local treatment with low-dose topical or injectable steroids is common and is considered safe and effective not only around the eye, but also inside the eye. In certain cases, corticosteroids are used topically an adjuvent treatment for corneal ulcers9, as well as injected for chalazions10, hemangiomas8, macular edema11, cataract surgery12, and vitrectomy.
Based on the history of the safe use of low-dose, short-term triamcinolone in various fields over the last 60 years, and the need in some patients for prevention of swelling, patients in the author’s practice were offered low-dose triamcinolone as an off-label additive to their HA filler for infraorbital injections beginning in 2013. While anecdotal experience has been positive, the objective of this retrospective analysis was to better characterize the effect of triamcinolone on post-procedure swelling, and to better understand other variables that may affect patient propensity to experience swelling in this area.
Methods
There were 447 patients which underwent a total of 706 infaorbital hyaluronic acid treatments by the author (252 with triamcinolone and 454 without) over a seven year period from April 2013 to March 2020. The majority of patients (62%) had one treatment, while one quarter (25%) of the patients had two treatements and the remaining13% had three or more treatments.
The same injection technique was used in all procedures, with a 25-gauge needle to create 1 to 3 pilot holes and a 27-gauge cannula to deliver the product in a retrograde manner. For patients with multiple treatments over the seven year period, the first analysis included only the 1st treatment to reduce confounding variables. Patients were included in the analysis regardless of the type of filler used.
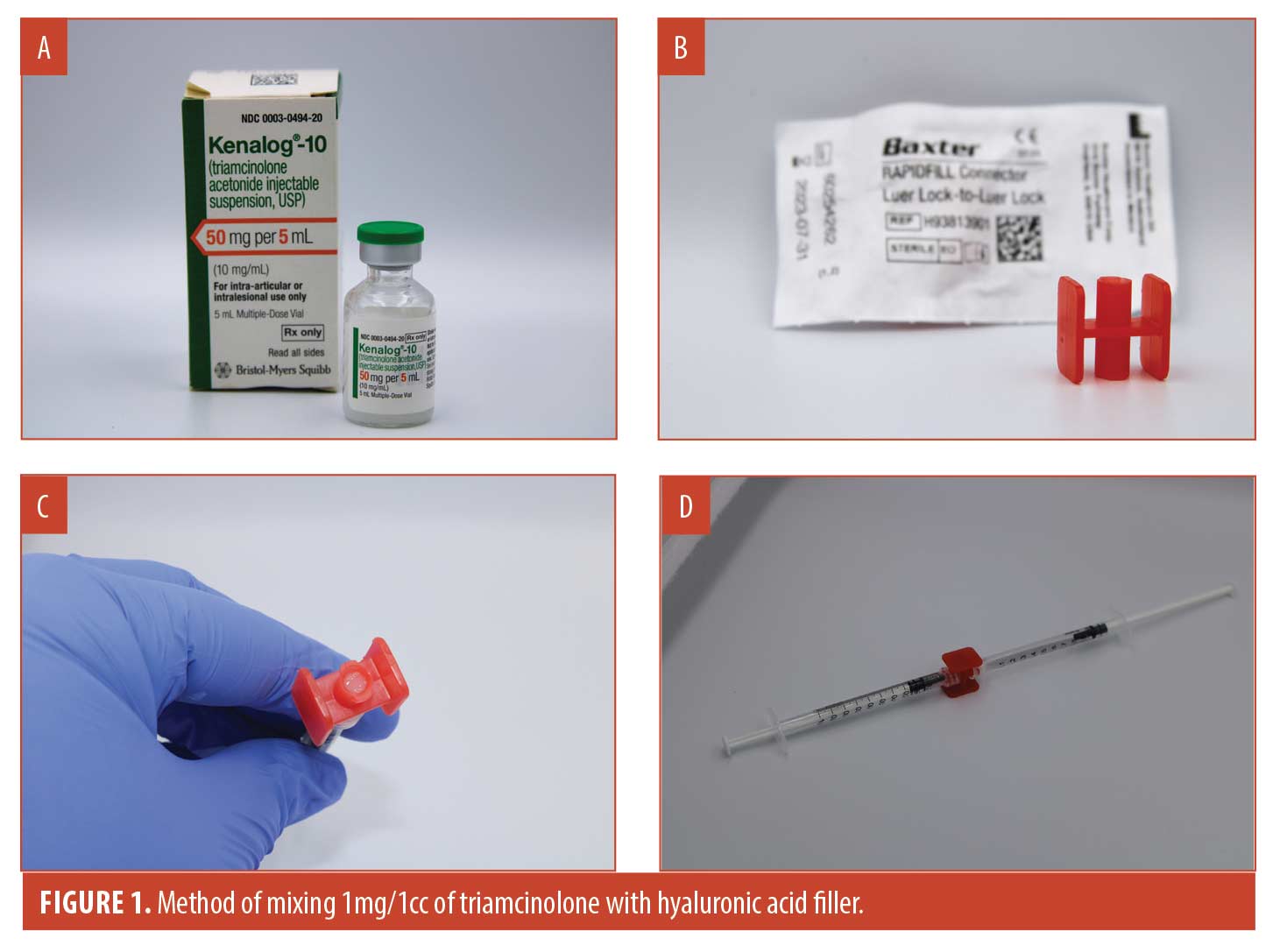
For patients who received triamcinolone mixed with a 1cc syringe of hyaluronic acid filler (final concentration 1mg/1cc), 0.1cc of triamcinolone acetonide (Figure 1a) Kenolog-10 (Bristoll-Meyers Squibb) was pulled into a 1.0cc syringe and then pushed into a Baxter Rapidfill Connector (Figure 1b), until the center of the connector was filled entirely (measures approximately 0.1cc) to ensure no air was remaining (Figure 1c). The hyaluronic filler syringe was then attached to the other end of the connector and the filler was mixed back and forth approximately ten times until there was a uniform consistency of color (Figure 1d). The filler was entirely drawn back into the original hyaluronic acid syringe and a 27-gauge cannula was attatched for injection (video online demonstrating the technique of mixing triamcinolone into hyaluronic acid filler). Due to the propensity of oral glucocorticoids to alter blood glucose levels, in an abundance of caution, patients with diabetes were instructed to closely monitor their blood glucose levels following treatment and to report to the office if there was any change in their usual levels.
Incidence of short-term post-procedural swelling (less than or equal to 14 days after the procedure) was determined by blind evaluators who reviewed documentation of follow-up phone calls in the chart (n=495) which were initiated within 1-2 days following the procedure. Patients were asked, “Are you having any side effects after your treatment?”. Any contact made with the patient greater than two weeks following the procedure was not included.
Since the use of triamcinolone significantly reduced short-term post-procedural swelling, only first treatments without triamcinolone with documented follow-up phone calls were analyzed for secondary variables (n=202). If there was a trend toward significance with secondary variables without the power to reach significance, then the calculations were run a second time using all treatments without triamcinolone if there was no swelling before retreatment. The only exception was review of filler types, since first and subsequent treatments were often done with different filler types which would confound the results if all treatments were analyzed.
Since HA fillers are commercially available in 0.5cc, 0.55cc, and 1cc syringes, the analysis of the effect of volume on the incidence of swelling was carried out by grouping patients injected with 0.55cc or less, 0.56-1cc, and more than 1cc. For those patients treated both with and without triamcinolone at least four months apart, blinded evaluators recorded patient preference if it was documented in the chart.
The following statistical methods were done. Descriptive summaries of categorical outcomes included the number and percent of treatments. Descriptive summaries of continuous measures included the number of patients (n), mean, median, minimum, and maximum. When analyzing short-term swelling among different types of fillers, fillers with at least five first treatments with documented follow-up phone calls within 14 days were included in the chi square test for significance. Chart review was done in accordance with the declaration of Helsinki. Patient consents including photo consents were documented in the chart and provided.
Results
Demographics. Short-term follow-up information on swelling was available for 326 of the 447 first treatments. For these 326 patients, the demographics, product information, history of allergies and swelling are in Table 1.
Short-term swelling after first treatment. A total of 51 percent (103/202) of patients after treatment with infraorbital hyaluronic acid filler alone reported post-procedure swelling within the first 14 days, compared to 23 percent (29/124) in those with triamcinolone mixed with their filler (x2[2, n=326]=24.296, p<.00001). The amount of filler injected in each treatment group was similar, with an average of .997cc (median 1cc) in the group without triamcinolone and an average of .976cc (median 1cc) in the group with triamcinolone.
Secondary variables. The analysis of secondary variables using 1st treatments without triamcinolone (n=202) showed a trend with more swelling after larger amounts of infraorbital HA filler, however it was not significant (x2 [1, n=202]=2.448, p=0.294).
The numbers of patients in the smallest (less than or equal to 0.55cc) and largest (>1cc) volume groups was small with the majority of treatments (78%, 157/202) belonging to the 0.56cc to 1cc group. Since there was a trend with 1st treatments, all treatments without triamcinolone were then analyzed and a significant difference was detected as shown in Figure 2 (x2 [1, n=316] =6.360, p=0.042).
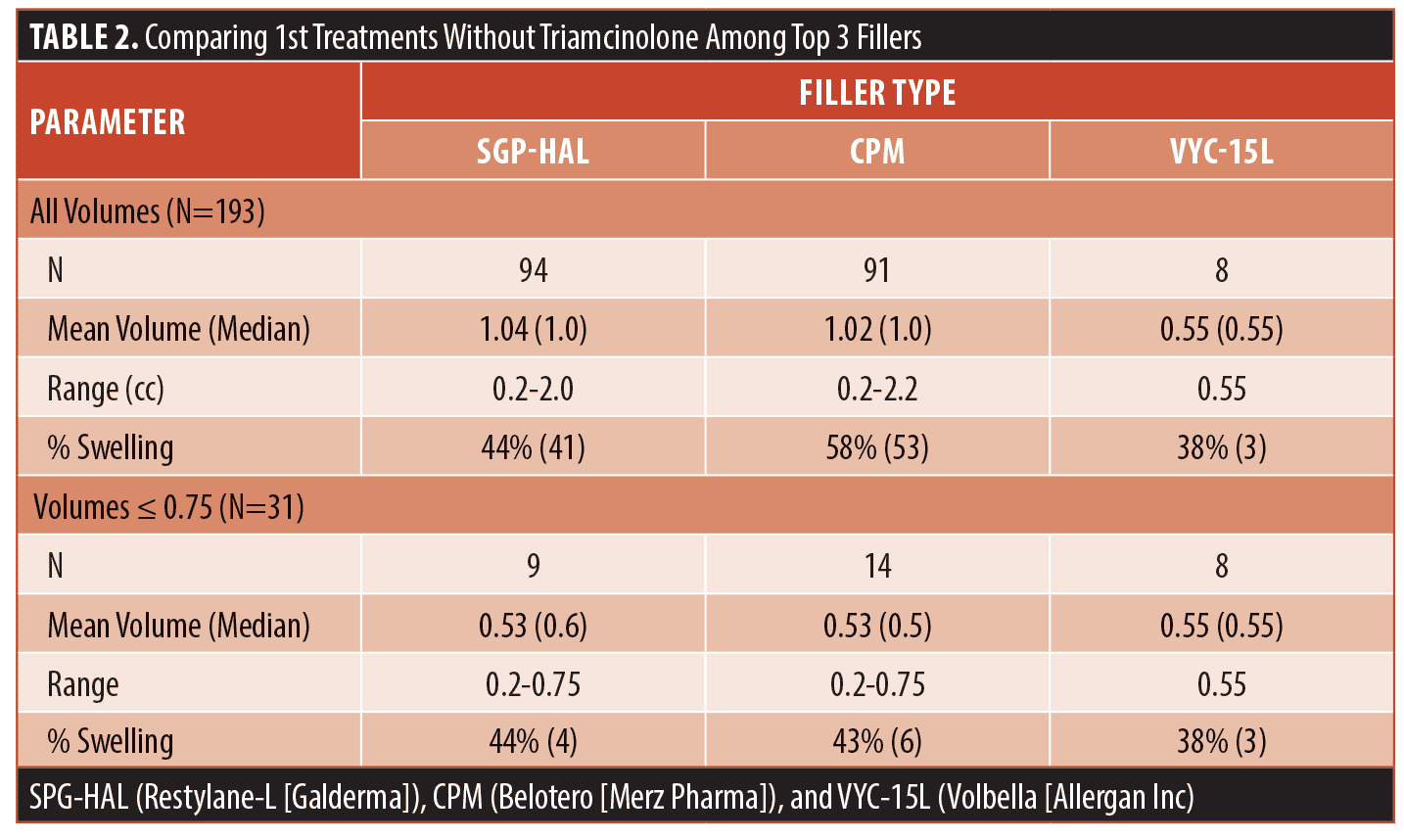
Filler type was analyzed by examining the rate of swelling for the three fillers, VYC-15L (Volbella [Allergan Inc]), SGP-HAL (Restylane-L [Galderma]) and CPM (Belotero [Merz Pharma]), which had data on more than five patients without triamcinolone. There was less post-procedural swelling for VYC-15L (38%), then SGP-HAL (44%) and Belotero (59%); however, it was not significant [X2(2,n=193)=5.1624, p=0.076] and it is very important to note that all first treatments with VYC-15L were with 0.55cc, while the most frequently injected amount for other brands was 1cc. Since volume significantly affected rates of swelling, to adjust for volume injected, all those with first treatments using 0.75cc or less were analyzed so that the three main filler types had similar average injection amounts, as seen in Table 2. The incidence of swelling for HA brands were less variable when comparing similar volumes (38% for VYC-15L, 43% for SGP-HAL, and 44% for CPM) and was not significant (X2, [2, N=31]=0.093, p=0.955).
The rate of short-term swelling in first time treatments was unaffected by the patient’s gender, with 50 percent [4/8] of men and 51 percent [99/194] of women experiencing short-term swelling (X2, [1, N=202]=0.51, p=0.822). Similarly, the rate of swelling was also unaffected by the history of allergies with 52 percent (46/88) of those with allergies and 51 percent (58/111) of those without allergies reporting short-term post-procedure swelling (X2, [1, N=202]=0.012, p=0.911). Reported allergies included iodine, several antibiotics, anesthetics, adhesive, several types of opioids, valium, epinephrine, diphenhydramine, ibuprofen, ACE inhibitor, and lorazepam. Analysis of each individual allergy did not reveal any significant trends. Similarly, Fitzpatrick skin type (FST) did not appear to affect the incidence of short-term swelling, with 51.1 percent (96/188) of first treatments without triamcinolone with FST I-III patients resulting in swelling, and 50 percent (4/8) of treatments with FST IV-VI patients resulting in swelling (X2, [1, N=202]=0.004, p=0.947).
Of the 202 first treatments in the infraorbital area without triamcinolone, 85 of those included treatment with neuromodulators in the periorbital area on the same day as the filler injection. Of these treatments, 51.2 percent (44/85) of those who had neuromodulators experienced post-procedure swelling compared to 50.4 percent (59/117) without, a difference that was not statistically significant (X2, [1, N=202] =0.011, p=0.915). Finally, the mean age for patients who had short-term swelling was 63.1 years versus 65.2 years for those without swelling.
Adverse events. There were no cases of skin atrophy, problems with vision, skin ulceration, or hypopigmentation reported by patients on follow-up phone call or by the blinded evaluator upon review of patient photos in their charts. Except for patient intolerance for prolonged swelling (n=7) and granuloma formation (n=2), there were no other adverse events that resulted in patients having their filler removed or any other intervention. The two granulomas recorded, one with VYC-15L and one with SGP-HAL, resolved completely without further adverse events once they were injected with hyaluronidase. One patient with severe type 1 diabetes and two patients with type 2 diabetes had notes in their charts that their glucose levels following treatment did not change. The patient with type 1 diabetes reported she checked her glucose levels every two hours after the procedure for the first six hours and then every 3 to 4 hours for the next two days with no changes from her values the three days prior.
Patient preference. There were 52 patients who had both treatments with and without triamcinolone spaced at least four months apart. Of these 52 patients, 33 had notes in their chart regarding which treatment they preferred. The majority, 63.6 percent (21/33), preferred the technique with triamcinolone due to the decreased swelling, while 12 (36%) did not notice a difference between the two techniques, and none (0%) preferred the technique without triamcinolone.
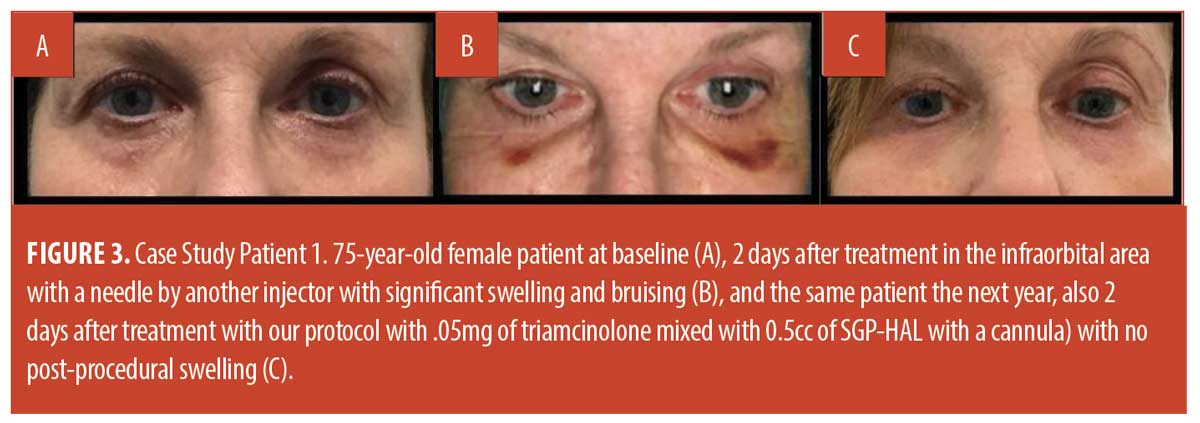
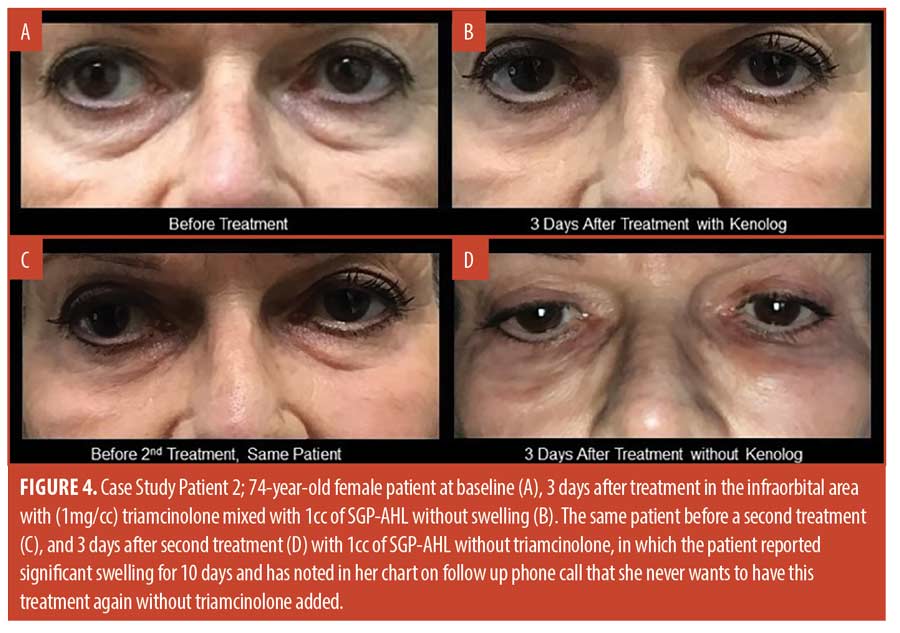
Discussion
Immediate post-procedure swelling with infraorbital hyaluronic acid filler is a common side effect, occurring in over 50 percent of patients in this review. Social downtime from swelling not only affects the patient’s ability to undergo a procedure, but also their satisfaction with the procedure. In general, swelling most often dissipates without intervention within 3 to 10 days; however, for many patients, this amount of social downtime is prohibitive. Most patients request to be given medications or procedures that will limit their downtime.
Interventions commonly offered to patients to prevent and treat post-procedural swelling, include bromelain, arnica, cold compression, antihistamines, diuretics, decreasing salt intake, sleeping upright, ibuprofen, and topical or oral corticosteroids. However, most of these recommendations lack definitive supporting literature. A literature review published in 2019 of 11 clinical trials with oral arnica, two clinical trials with topical arnica, and seven clinical trials with oral bromelain revealed that results are inconsistent (9 of 20 studies were positive), and there remains insufficient data to support the use of arnica or bromelain. Furthermore, while ice is widely used and was recommended by an expert consensus in 2018,13 ice or cool packs have not been shown to effectively reduce swelling14 on their own, but were found to be helpful when used along with compression for bruising.15
Oral corticosteroids are sometimes used, primarily in cases of severe post-procedural swelling, usually after larger, more invasive treatments. One paper reviewed the use of single IV bolus of 1g of methylprednisolone given intraoperatively during facial surgery, which resulted in less edema, a shorter duration of swelling, and less requirements for analgesia without any adverse events.16 Griepentrog7 has reported that the addition of oral prednisone (30mg) before infraorbital hyaluronic acid filler eliminated post-procedural swelling in those previously known to swell with the same technique.
The mechanism of action of steroids is multifactorial. Corticosteroids can decrease the size and permeability of vessels resulting in a decrease of fluid from the capillaries. They also reduce the inflammatory reaction by downregulating cytokine pathways, modulating leukocytes and macrophages, stabilizing mast cell granules, and inhibiting the release of arachidonic acid reducing prostaglandins.17 Although oral steroids are a highly effective and an inexpensive option for managing inflammation, side effects for high-dose long-term treatments can be considerable. With high doses and long-term use, often only needed for chronic debilitating conditions, oral corticosteroids are associated with development of diabetes, osteoporosis, hypertension, gastritis, depression, insomnia, weight gain, skin thinning, and fluid retention.18 For these reasons, oral steroids are not recommended routinely before minimally invasive procedures such as fillers for prevention of swelling.
In this review, the off-label addition of low-dose triamcinolone to hyaluronic acid fillers (1mg/cc) placed with a cannula in the infraorbital area, in over 252 treatments, appears to be an effective and safe practice for reducing short-term swelling by more than half, with a significant reduction from 51 percent to 23 percent (p<0.00001) without any additional reported side effects in those with multiple medical conditions, skin types (I–VI), and ages (26–88 years). Given the high incidence of swelling with infraorbital treatments and the inability to predict who will have swelling, the use of this low-dose and low-risk protocol as a preventative option beforehand was an ideal solution in a clinical practice setting. By decreasing patient side effects, their willingness to undergo treatments increased, as well as overall patient satisfaction and retention.
This review not only analyzed if low-dose triamcinolone was safe and effective, but also reviewed other factors that could affect the rate of swelling. Selecting the correct filler to use in this area is the first step and likely one of the most important factors, as the hydrophilicity of some fillers can lead to significant edema. In this review, the top three most commonly used types of hyaluronic fillers for the infraorbital area were analyzed and included SGP-HAL (Restylane-L [Galderma, Fort Worth, Texas]), CMP (Belotero [Merz Pharma, Frankfurt, Germany]), and VYC-15L (Volbella [Allergan, Dublin, Ireland]). These fillers along with a newer filler to market, RH2 (Teoxane, Geneva, Switzerland]), are known to be the least hydrophilic. Furthermore, the use of a cannula to reduce the amount of trauma, and limiting superficial placement of the filler, are both additional factors that play a role in the reduction of post-procedural swelling.
Since triamcinolone significantly decreases post-procedure swelling, secondary variables were analyzed only in patients not treated with triamcinolone with documented follow-up phone calls (n=202). Similar to another retrospective review,7 our analysis did not identify any patient characteristics that may be used clinically to predict swelling or inform swelling risk for individual patients. For example, age, sex, history of allergies, and Fitzpatrick skin type did not affect patients post-procedure risk of swelling according to our review; however, most patients were elderly (mean age of 62.4 years) and the majority were female with Fitzpatrick Skin Types I–III, which may limit the applicability of these findings to some degree.
As hypothesized in previous publications13,19, when comparing three groups (less than or equal to 0.55cc, 0.56–1cc, and >1cc), the volume significantly affected short-term post-procedure swelling with more swelling occurring with larger volumes. This emphasizes the importance of using the least amount possible, and if larger amounts are needed, spacing out treatments with less at each visit.
The analysis of brands of HA filler in our review was complicated by the fact that VYC-15L was launched in a 0.55cc syringe, and therefore nearly all the patients treated with this HA type were those who required less filler. Since we were able to show the volume of filler significantly decreases swelling and the average amounts of filler injected with VYC-15L was much lower (mean of 1.04 for SGP-HAL and 1.02 for CPM, compared to 0.55 for VYC-15L), we included a final comparison of filler brands with only treatments less than 0.75cc. This cutoff amount was chosen as it created three groups with similar mean volumes; however, that significantly decreased the power. Once that change was made, the differences in the rate of swelling became smaller and were not significant, showing that the volume injected is a more important factor affecting swelling compared to the brand of filler. A larger data set was not available due to many individual patients being treated with multiple HA types over several treatments, forcing the analysis of only first treatments. A larger prospective study is warranted utilizing 1cc of each of the commonly used filler types since that is the volume used most often in clinical practice.
Because neuromodulators have a delayed onset of action, it follows that the impact of concomitant neuromodulators was not significant. However, future reviews assessing swelling from two weeks to three months post-procedure may inform whether neuromodulators have an impact on prolonged or delayed onset swelling. Similarly, neuromodulators administered two weeks before infraorbital filler may also have an effect, perhaps due to the diminished muscle mobility in the infraorbital area slowing dissipation of swelling but was beyond the scope of this review.
Regarding safety, most dermatologists use injectable triamcinolone daily to decrease inflammatory conditions in doses of 2.5 to 40mg/cc locally without concerns for long-term side effects. Acute side effects in the skin or subcutaneous tissues are caused by injecting too large a dose (>20mg per session)20, but are generally transient, and resolve spontaneously within 6 to 12 months or within several weeks to months with the use of saline.21,22 While eyelid fat atrophy and iris and eyelid depigmentation have occurred on the eyelid from direct cortisone injections,23, 24 the intralesional doses used in those case reports were high (less than or equal to 40mg of triamcinolone), which is more than 40 times the dose injected in one area while this protocol uses a total of 1mg spread out bilaterally under both infraorbital areas. Steroid induced atrophy can be avoided using low concentrations and volumes, injection beneath the dermis, and limiting re-injection to greater than 6 weeks22; all criteria that are met in the clinical protocol described here.
Caution is needed when mixing triamcinolone as there are many higher concentrations commercially available that should not be used. Finally, there is a case report of an injection with 0.5cc of methylprednisolone 80mg/cc (40mg) injected with a needle into a chalazion on the eyelid immediately after surgery causing retinal occlusion25, however, the same safety concerns exist with hyaluronic acid filler alone. Since the average particle size of triamcinolone (<50 microns)26 is less than the particle size of HA fillers (250-1000 microns), the addition of triamcinolone to HA fillers is not likely to add any potential risk already present with HA filler injections. Injecting small aliquots slowly with a cannula, in a retrograde manner with low force, while constantly moving should always be considered to decrease the chance of vascular occlusion.
Taken together, the findings of this review support the use of low-dose triamcinolone with fillers in the infraorbital area to reduce swelling and highlight the importance of lower injection volumes in preventing swelling. Future prospective studies are an important need in this area of aesthetics. Additionally, since the lip is a small enclosed space with a propensity to swell similar to the undereye, another study examining the utility of this protocol to prevent lip swelling after hyaluronic acid filler is currently underway.
Many of the limitations of this review have been discussed above and include that this is a retrospective review that relies on patient reporting, rather than assessment by a clinician. However, it is the patient’s perception of swelling that leads to social downtime, and is equally, if not more important to measure.
Conclusion
Post-procedure swelling following treatment of the infraorbital area with HA filler is a common side effect without predictable patient characteristics. Swelling can cause social downtime, which is an important factor patient satisfaction and retention. This is the first retrospective study showing the safety and efficacy of a novel technique adding low-dose triamcinolone (1mg/cc) to hyaluronic acid filler to reduce post-procedure swelling within the first few weeks following infraorbital injections with no reported additional side effects. Furthermore, using lower volumes also was effective in reducing swelling.
Acknowledgments
Editorial assistance was provided by Ginny Vachon, PhD of Principal Medvantage, LLC in Atlanta, Georgia. This editorial assistance support was provided by X-medica, LLC. No outside funding or support beyond this was provided for the review.
References
- Ko, A.C., B.S. Korn, and D.O. Kikkawa, The aging face. Surv Ophthalmol, 2017. 62(2): p. 190-202.
- Matarasso, S.L., et al., Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg, 2006. 117(3 Suppl): p. 3S-34S.
- Sharad, J., Dermal Fillers for the Treatment of Tear Trough Deformity: A Review of Anatomy, Treatment Techniques, and their Outcomes. J Cutan Aesthet Surg, 2012. 5(4): p. 229-38.
- Steinsapir, K.D. and S.M. Steinsapir, Deep-fill hyaluronic acid for the temporary treatment of the naso-jugal groove: a report of 303 consecutive treatments. Ophthalmic Plast Reconstr Surg, 2006. 22(5): p. 344-8.
- Hirmand, H., Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg, 2010. 125(2): p. 699-708.
- Huber-Vorlander, J. and M. Kurten, Correction of Tear Trough Deformity With a Cohesive Polydensified Matrix Hyaluronic Acid: A Case Series. Plast Surg Nurs, 2015. 35(4): p. 171-6.
- G Griepentrog, M.L., C Burkat, B Lemke, J Rose, Periorbital Edema Following Hyaluronic Acid Gel Injection: A Rectrospective Review. The American Journal of Cosmetic Surgery, 2011. 28(4).
- Couto, J.A. and A.K. Greene, Management of problematic infantile hemangioma using intralesional triamcinolone: efficacy and safety in 100 infants. J Plast Reconstr Aesthet Surg, 2014. 67(11): p. 1469-74.
- Palioura, S., et al., Role of steroids in the treatment of bacterial keratitis. Clin Ophthalmol, 2016. 10: p. 179-86.
- CM Jermak, J.D., J Heffez, and GA Peyman, Triamcinolone Acetonide in Ocular Therapeutics. Survey of Ophthalmology, 2007. 52(5): p. 501-521.
- Chang, A.A., et al., Safety and Efficacy of Intravitreal Preservative-Free Triamcinolone Acetonide (Triesence) for Macular Edema. J Ocul Pharmacol Ther, 2015. 31(9): p. 563-9.
- Reddy, J.K., et al., Safety & efficacy of single subconjunctival triamcinolone 5 mg depot vs topical loteprednol post cataract surgery: less drop cataract surgery. Int J Ophthalmol, 2019. 12(5): p. 774-778.
- Urdiales-Galvez, F., et al., Treatment of Soft Tissue Filler Complications: Expert Consensus Recommendations. Aesthetic Plast Surg, 2018. 42(2): p. 498-510.
- King, M., Management of Edema. J Clin Aesthet Dermatol, 2017. 10(1): p. E1-E4.
- Nestor, M.S., G.R. Ablon, and M.A. Stillman, The use of a contact cooling device to reduce pain and ecchymosis associated with dermal filler injections. J Clin Aesthet Dermatol, 2010. 3(3): p. 29-34.
- Habal, M.B., Prevention of postoperative facial edema with steroids after facial surgery. Aesthetic Plast Surg, 1985. 9(2): p. 69-71.
- Weiner, G., Savvy steroid use, in EyeNet Magazine.
- Jakobiec, A., Principles and Practice of Ophthalmology. Corticosteroids in ophthalmic practice, ed. B.S. Abelson MB. 2008: Saunders Elsevier.
- Funt, D.K., Avoiding malar edema during midface/cheek augmentation with dermal fillers. J Clin Aesthet Dermatol, 2011. 4(12): p. 32-6.
- Shapiro, J., Current treatment of alopecia areata. J Investig Dermatol Symp Proc, 2013. 16(1): p. S42-4.
- Shiffman, M.A., Letter: Treatment of local, persistent cutaneous atrophy after corticosteroid injection with normal saline infiltration. Dermatol Surg, 2010. 36(3): p. 436.
- El-Amawy, H.S. and S.M. Sarsik, Saline in Dermatology: A literature review. J Cosmet Dermatol, 2020.
- Park, J. and M. Chang, Eyelid Fat Atrophy and Depigmentation After an Intralesional Injection of Triamcinolone Acetonide to Treat Chalazion. J Craniofac Surg, 2017. 28(3): p. e198-e199.
- Al-Mahdi, H., Iris depigmentation: an unusual complication of intralesional corticosteroid injection for capillary hemangioma. Middle East Afr J Ophthalmol, 2010. 17(1): p. 100-2
- Hosal, B.M. and G. Zilelioglu, Ocular complication of intralesional corticosteroid injection of a chalazion. Eur J Ophthalmol, 2003. 13(9-10): p. 798-9
- Benzon HT, C.T., McCarthy RJ, Benzon HA, Walega DR, Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology, 2007. 106(2): p. 331-8.

