 J Clin Aesthet Dermatol. 2022;15(4):20–25.
J Clin Aesthet Dermatol. 2022;15(4):20–25.
by Christopher White, DO, FAAD, and Richard Miller, DO, FAOCD
Dr. White is with Dermatology Partners in Strongsville, Ohio. Dr. Miller is with the Department of Dermatology, HCA Healthcare/USF Morsani College of Medicine and the Largo Medical Center, in Largo, Florida.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Vitiligo is a disfiguring disease that frustrates both patients and clinicians due to its difficulty to treat. Topical Janus kinase inhibitors (TJKI) may offer an alternative treatment option that are safe and well-tolerated for all anatomic sites.
Objective. The objective of this review was to evaluate the published clinical reports regarding the use of TJKI for the treatment of vitiligo and to summarize the formulations, dosing strategies, efficacy, and safety of this emerging therapeutic class.
Methods. This is a review of the National Library of Medicine (via PubMed) and Scopus through April 2021.
Results: The initial search revealed 45 potential articles; eight articles comprising 201 total patients met our inclusion criteria. Our analysis indicates that TJKI offer a viable therapeutic alternative, with similar efficacy to topical corticosteroids (TCS) and topical calcineurin inhibitors (TCI). The beneficial effects of TJKI are most pronounced on facial skin and when combined with narrow-band ultraviolet B (NB-UVB) therapy.
Limitations. Many publications reporting the use of TJKI have small sample sizes and do not use standardized scoring systems to evaluate disease extent and treatment response.
Conclusion. Vitiligo remains a difficult disease to treat, and TJKI are an appealing new treatment option. While the aggregate data on the topic remain sparse, we offer a synthesized review of the literature to date and a glimpse into the ongoing investigations regarding this emerging drug class.
Keywords: topical Janus kinase inhibitor, topical JAK inhibitor, topical tofacitinib, topical ruxolitinib, vitiligo treatment
Vitiligo, with a prevalence of 0.5 to 2.0 percent, is a common acquired depigmenting disorder.1 Well-demarcated white macules and patches develop due to melanocyte destruction. The condition is seen equally in both sexes and across races, with an average age of onset between 10 to 30 years.2 The appearance of vitiliginous lesions can be particularly devastating to patients with skin of color, where stark pigment contrasts are more readily identified, compared lighter skin phenotypes; in certain cultures, vitiligo may even be confused with infectious processes, such as leprosy, worsening the stigma of disease.2
Clinically, vitiligo is most widely classified as segmental or nonsegmental, with the more common nonsegmental variant typically broken down based on anatomic distribution.3 Nonsegmental disease is more likely to be bilateral with crossing of the midline, and displays slowly progressive, chronic depigmentation.4 Segmental disease is often unilateral, rarely crosses the midline, and undergoes rapid disease development and stabilization.4 Diagnosis is often made readily, though involved areas are best visualized with the aid of a Wood’s lamp, which accentuates amelanocytic tissue due to autofluorescence of underlying dermal collagen.5 While rarely needed, skin biopsy can be used to confirm diagnosis.
While the pathophysiology of vitiligo is multifactorial, significant clarity into etiology has been offered in recent years. Environmentally stressed melanocytes in susceptible individuals release warning signals to surrounding cells, triggering cytotoxic T-cell and interferon-gamma production, which mediate melanocyte destruction and loss of clinical pigment.6-8 Many of the activation and transcription steps required for this process are communicated via the Janus kinase/signal transducer and activator of transcription (JAK/STAT) cascade, making this an attractive target for interrupting disease and potentiating repigmentation.9 At the time this review is conducted, Janus kinase inhibitors (JAKI) carry no United States (US) Food and Drug Administration (FDA) approved indications for dermatologic disease, though they have been successfully employed off-label.10 In this review, we aim to review the applications, treatment regimens, and efficacy of TJKI for the treatment of vitiligo.
Methods
The National Library of Medicine (via PubMed) and Scopus were searched on May 2, 2021 using the following search criteria: “jak inhibitor*” or “Janus kinase inhibitor*” or “tofacitinib” or “ruxolitinib” or “upadicitinib” or “baricitinib” and “topical” and “vitiligo” with no date restrictions. Inclusion criteria included any publication reporting the use of TJKI for the treatment of vitiligo in humans; all trials, case reports, and case series were included. All languages were included. Exclusion criteria included review articles and publications focusing on nontopical (e.g. oral, microneedling) or pharmacologic applications (Figure 1).
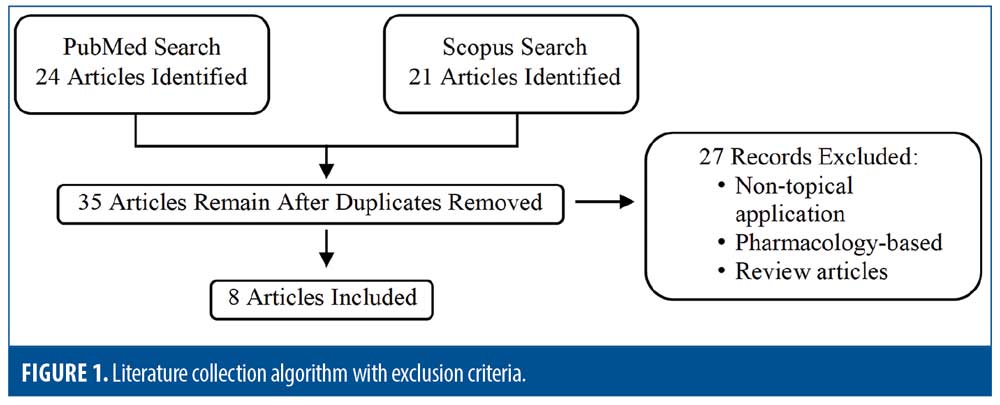
Results
The aforementioned search criteria yielded 45 publications. After removing duplicates and articles containing exclusion criteria, eight studies remained, comprising of of 201 total patients. One randomized clinical trial (RCT) was identified, along with one cohort study, two pilot studies, one case series, and three case reports.
Rothstein et al11 performed the first identified pilot study, using ruxolitinib 1.5% cream twice daily to vitiliginous lesions in 12 patients. Patients were required to wash out any previous treatments, could not use any concomitant therapies during the trial, and were relegated to treating less than 10 percent body surface area (BSA); the primary endpoint was improvement in Vitiligo Area Severity Index (VASI) scores at 20 weeks. Of the nine patients who finished the trial, 27-percent improvement was seen in mean VASI scores, with nonfacial vitiligo showing nonstatistically significant repigmentation. Significant repigmentation in facial lesions was seen, with 76-percent VASI improvement starting at a mean eight weeks. Interestingly, disease duration and BSA involvement did not appear to impact response, suggesting that even longstanding lesions may benefit from treatment.11
To determine if the results improved with time, the above study was extended to 52 weeks, with optional narrowband ultraviolet-B (NB-UVB) therapy permitted. Eight of the nine participants from the previous study enrolled in the extension, three of whom added NB-UVB to their regimen. Overall, VASI improvement for the cohort was 37.6 percent, with facial lesions showing 92-percent improvement and nonfacial, nonacral skin showing 12.6-percent improvement; it was not easily discernable which responders with facial disease received concomitant NB-UVB therapy. Notably, five participants maintained their response at the six-month mark, suggesting a previously refuted level of treatment durability with this drug class.12,13
In a case report, Joshipura et al14 reported the successful treatment of facial vitiligo with topical 1.5% ruxolitinib cream twice daily; the patient had 90-percent involvement at presentation and achieved near-complete repigmentation. The authors did note that sun-protected sites did not demonstrate repigmentation until they were exposed to environmental UV light.14
McKesey et al15 conducted a pilot study on 11 patients with facial vitiligo treated with 2% tofacitinib cream twice daily and NB-UVB therapy three times weekly for 2 to 4 months. The entire cohort had previously failed at least three months of treatment with topical corticosteroids (TC) or topical calcineurin inhibitors (TCI) with either NB-UVB or scheduled sunlight exposure. Mean VASI improvement was 70 percent, with all 11 patients showing good to excellent repigmentation.15
Mobasher et al16 evaluated 16 patients via a nonrandomized cohort study. Patients were treated with 2% tofacitinib cream twice daily; 15 patients had previously been treated with TCS, TCI, and/or phototherapy. Concurrent therapies were allowed. Thirteen patients displayed partial repigmentation, with four patients improving more than 90 percent, five improving 25 to 75 percent, and four improving 5 to 15 percent. In accordance with other reports, facial lesions responded best to treatment, while nonfacial lesions showed an average 16-percent improvement; contrastingly, no clear synergism was identified with simultaneous NB-UVB therapy, and disease duration negatively impacted treatment response. One patient with axillary lesions experienced 95-percent repigmentation, challenging the concept that concomitant phototherapy is required for treatment response. Overall, Fitzpatrick Skin Types 4 to 6 experienced greater improvement than lighter phenotypes.16
A pediatric case of facial segmental vitiligo treated with TJKI was reported by Olamiju et al.3 A four-year-old boy who had previously failed TC and was transitioned to tofacitinib 2% cream twice daily and home NB-UVB three times weekly. Freckling was identified at four weeks with complete repigmentation at six months. Following treatment discontinuation, repigmentation remained for six months before symptoms reemerged.3
In a randomized, controlled, Phase II trial, Rosmarin et al17 compared effectiveness of TJKI in 157 patients with Vitiligo. A facial VASI (F-VASI) score improvement of at least 50 percent at 24 weeks was the primary endpoint. Patients were randomized into five groups receiving either ruxolitinib 1.5% cream twice daily, ruxolitinib 1.5% cream once daily, ruxolitinib 0.5% cream once daily, ruxolitinib 0.15% cream once daily, or vehicle alone. By Week 24, the primary endpoint was reached by more patients given any dose of ruxolitinib, though the 1.5% twice daily cohort fared best with 45 percent of participants reaching the goal; this effect was improved by the 52-week point, with 52 percent of patients in the 1.5% twice daily cohort achieving at least 50-percent F-VASI improvement. Of note, 33 percent of patients receiving 1.5% twice daily ruxolitinib acheived 90-percent F-VASI improvement by Week 52.17
Yagi et al18 reported the use of twice daily topical delgocitinib in two patients with vitiligo; dosage was not reported. The first patient, with an eight-year history of neck involvement that had been recalcitrant to NB-UVB, achieved marked repigmentation at the eight-week mark. The second patient had a 30-year history of vitiligo affecting 20-percent BSA; she had no apparent repigmentation at 12 weeks.18
Recently, Ferreira et al19 reported a case of a 17-year-old patient with generalized vitiligo who failed multiple treatments over the course of 15 years. The patient was transitioned to topical tofacitinib 2% cream applied twice daily combined with NB-UVB three times weekly; significant repigmentation of facial skin was identified after nine months of treatment without any clinical or laboratory abnormalities.19
Discussion
Many theories have been proposed to explain the etiology of vitiligo. Neural dysregulation, autoimmunity, oxidative destruction of melanocytes, and diminished expression of keratinocyte-derived factors needed for melanocyte survival have all been implicated.20,21 Cytomegalovirus, human immunodeficiency virus, Epstein-Barr virus, and chronic hepatitis have been linked to development or worsening of disease.4,20
It is most probable that a combination of these mechanisms intertwines with genetic factors and environmental exposures to stress melanocytes. Stressed melanocytes warn neighboring cells via release of soluble heat shock protein 70 (HSP70), damage-associated molecular patterns (DAMPs), and other factors, stimulating a variety of cells, including keratinocytes, dendrocytes, and macrophages.7 Some of these cells directly activate other local cells to produce inflammatory chemokines, such as CXCL10, while others function as antigen-presenting cells in order to polarize naïve T-cells into Type 1 T helper cells (TH1).4,6,7 These polarized TH1 cells release the potent T-cell chemoattractant, interferon-gamma, which upregulates CXCL10 and CXCR3 production and mediates cytotoxic T lymphocyte (CTL) and natural killer (NK) cell activity.6,22,23 CXCL10 is a selective ligand for CXCR3, which is heavily expressed on TH1 cells; binding of CXLCL-10 with CXCR3 further upregulates TH1 and subsequent CTL activity, which facilitates melanocyte destruction.7,8,22
Many of the aforementioned steps, from interferon-gamma and CXCL10 release to T-cell activation as a whole, are mediated by the JAK/STAT signaling cascade, which intercedes the transcription of various genes upon extracellular ligand binding.24 Upon binding, JAK phosphorylates STAT, which then translocates to the nucleus to regulate transcription of effector molecules.24 This intimate relationship between the JAK/STAT pathway and vitiligo pathogenesis make blockade of these cascades an attractive therapeutic option. This theory has been reinforced by the identification of abundant interferon-gamma, CXCL10 chemokines, and CXCR3 receptors within lesional skin.21,25 Furthermore, transplantation of CXCR3 deficient T-cells or neutralization of the interferon-gamma or CXCL10 signaling cascades has shown disease reversal in murine models.6,8,11
Historical treatment approach. The two primary focus points in treating vitiligo are to arrest active disease and induce repigmentation.4 Initial therapy often relies on TC and TCI due to their potent anti-inflammatory properties, while cases of rapidly progressive disease may require intermittent courses of oral corticosteroids.4,17 On meta-analysis, potent TC achieved greater than 75-percent repigmentation in 40 to 56 percent of nonsegmental vitiligo.26 Analysis of TCI has been more conflicting, though a large meta-analysis reported greater than 75-percent repigmentation was seen in only 18 percent of 520 patients.27 While TC and TCI have shown promising results, each carries its own drawbacks. With high-potency TC, the strength required to achieve maximum results may cause skin atrophy and hypopigmentation; these medications are also unsafe for consistent use on intertriginous and facial skin where these adverse effects are seen more readily. Similarly, while multiple studies have reinforced the safety of TCI, the FDA required a black box warning regarding the risk for cancer, particularly lymphomas, associated with these medications may have a significant impact on patient acceptability and adherence to treatment. TCI are also associated with application site burning, sometimes hindering their use.17
Topical prostaglandin analogs have shown promising results in early studies, achieving greater than 50-percent repigmentation in 42.9 percent of cases at 24 weeks, while topical vitamin D analogs and antioxidants, such as catalase/dismutase superoxide, have shown conflicting efficacy.4,28
Phototherapy, photochemotherapy, and laser therapy may be added for their synergism with topical treatments or in cases where topical medications are unlikely to be tolerated due to greater than 10-percent BSA involvement.4 Systemic medications, such as afamelanotide, minocycline, methotrexate, azathioprine, cyclosporine, L-phenylalanine, khellin, and supplements (e.g., ginkgo biloba, zinc, and Polypodium leucotomos) have been used in resistant disease with variable success; many systemic therapies have shown effectiveness in arresting disease but not repigmentation.29 Surgical grafting and melanocyte-keratinocyte transplantation have demonstrated effectiveness for unrelenting disease, but their high cost and technical requirements restrict accessibility.1 In cases of entirely recalcitrant generalized disease, pharmaceutical depigmentation via monobenzyl ether of hydroquinone (MBEH) may be performed to camouflage affected areas by depigmenting the surrounding, uninvolved skin.4
TJKI. While no JAK inhibitors are currently approved for dermatologic indications, oral formulations have been used off-label for conditions including alopecia areata, vitiligo, psoriasis, atopic dermatitis, hidradenitis suppurativa, pyoderma gangrenosum, granuloma annulare, connective tissue diseases, sarcoidosis, cutaneous lymphoma, and graft-versus-host disease.9,30 These oral treatments have been generally successful, though side effects including serious infections, malignancy, cytopenia, elevated lipid levels, and thromboses have prompted the investigation into topical preparations for improved safety profiles.30
The growing literature reporting the use of TJKI in the treatment of vitiligo identifies a well-tolerated and effective therapeutic option that performs similarly to TC and superiorly to TCI when used as monotherapy.4,11,15,16 Interestingly, it also seems that TJKI formulations perform comparably to their oral counterparts, suggesting no loss in efficacy when transitioning from oral to topical preparations; it should be noted that this may not hold true for patients with greater than 10-percent BSA involvement.31
Like TC and TCI, TJKI seem to work synergistically with other treatments, particularly NB-UVB therapy, which supports a multimodal therapeutic approach.26,28,32 It is thought that TJKI suppress local inflammation while NB-UVB stimulates melanocyte activity.1 With their strong efficacy in facial disease, TJKI offer superior or comparable alternatives to TCI and TC, respectively, in patients unable or unwilling to tolerate these drug classes.17,28,32 McKesey15 also demonstrated that TJKI combined with NB-UVB offer treatment options to patients failing TC and TCI therapy. No other topical medication has proven repeatedly effective in this population.15
Despite the apparent benefits of TJKI, certain shortcomings must be discussed. Namely, their lack of efficacy for nonfacial disease. It is speculated that the thicker epidermis and more inconsistent environmental UV exposure of nonfacial skin may explain this effect.18,23 Base alterations, such as using a liposomal formulation, have been studied to improve drug delivery, but no consistent benefit has been reported.9,23 Additionally, though TJKI efficacy does not appear to be fully reliant on concomitant UV exposure, combination therapy may offer the greatest benefit, and additive patient instructions and cost often correlates to decreased prescribing and patient adherence.11,15,16,18
Safety and adverse events. In contrast to oral formulations, TJKI are generally well-tolerated and have an excellent safety profile. Application site erythema, transient acneiform eruptions, skin exfoliation, folliculitis, pruritus, and peripheral hyperpigmentation have been frequently reported; these effects are self-limited and, in the case of hyperpigmentation, seem to herald treatment response.10,11,15-17 Narla et al33 reported the development of myalgias in two patients treated with 1.5% ruxolitinib cream, which resolved upon drug discontinuation.33 One study identified a minimal elevation in serum cholesterol among 40 percent of patients treated with a TJKI for alopecia areata; contrastingly, Rosmarin, et al identified no clinically relevant laboratory alterations in their study.9,17,34 Overall, TJKI have not been found to be systemically absorbed, thus avoiding the more concerning adverse events that have been linked to oral variants.10
Prescribing and future outlook. The difficulty in prescribing TJKI is likely their most problematic concern. Since no topical applications have yet to receive FDA approval, providers must rely on compounding pharmacies to obtain prescriptions. These compounds are rarely covered by insurance providers and can be prohibitively expensive. Furthermore, varying strengths and formulations may lead to inconsistent treatment results. This limitation may be changing soon, as the TRuE-V1 (NCT04052425) and TrUE-V2 (NCT04057573) clinical trials studying the use of ruxolitinib 1.5% cream as a vitiligo treatment reached their primary completion dates in April 2021, with study completion dates set for October 2021; the study’s primary endpoint measured 75-percent VASI improvement of facial disease at 24 weeks, and the study sponsor recently reported that this goal was achieved.35-38
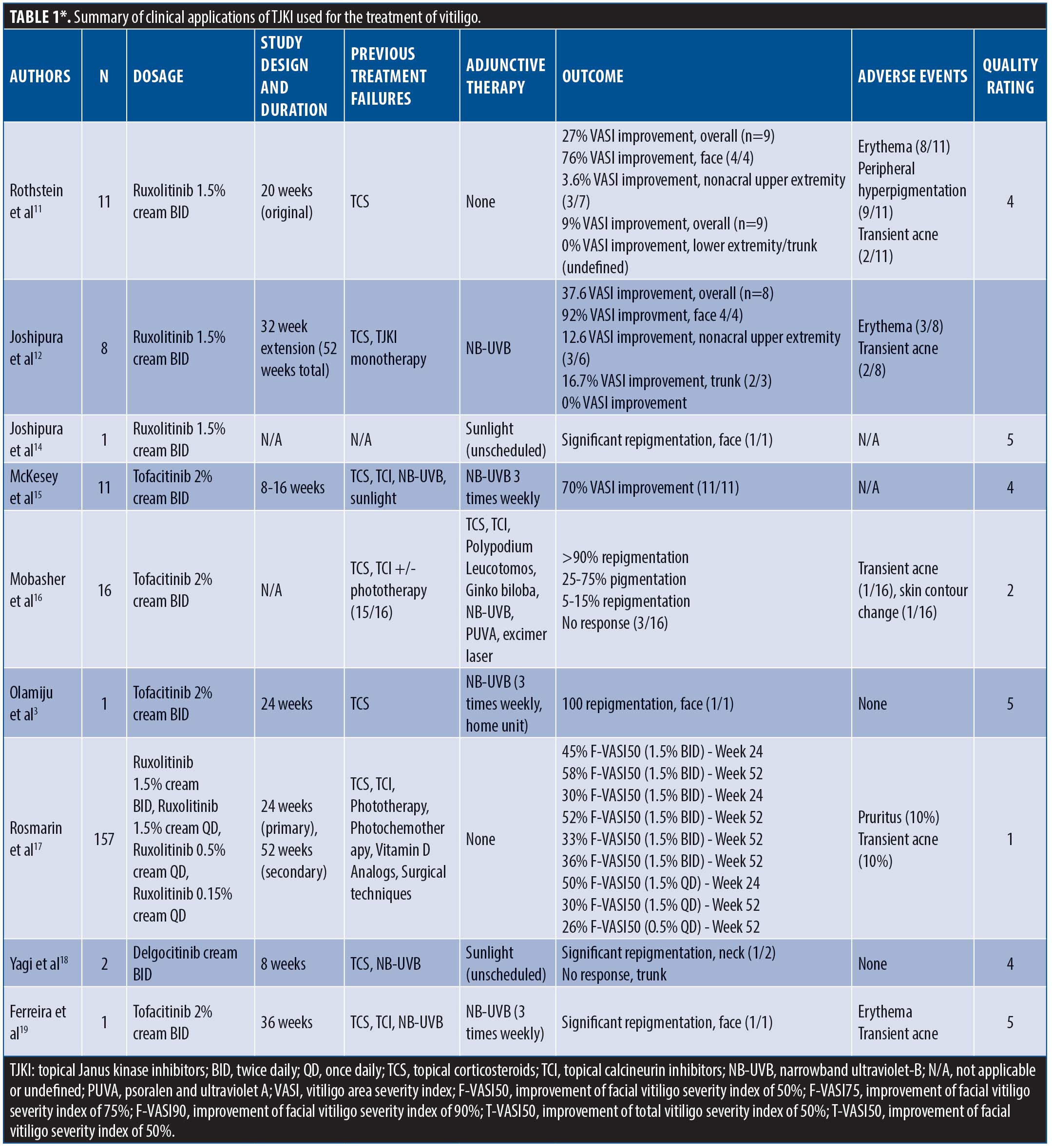
*The original version of Table 1, which appeared in the print edition of this issue, contained four errors; the current version that appears here reflects updates to the table to correct those errors.
Limitations. Our review is limited by a lack of standardization in terms of grading disease severity and treatment response. Additionally, the identification of only one RCT in our search means the majority of selected reports have a moderate-to-low level of evidence. The majority of reviewed studies had small sample sizes, diminishing the power of this review. Our search criteria and database sourcing may also have been insufficient to identify all publications on the topic.
Conclusion
TJKI offer an attractive new treatment option for the management of vitiligo. Preliminary reports suggest that they are more effective than TCI and at least equal in efficacy to TC. While still not offering a monotherapy for vitiligo, TJKI serve as a durable, safe, and well-tolerated alternative for vitiligo cases failing other topical regimens. Reported synergism with NB-UVB therapy supports a multimodal approach to treatment, and patients with predominantly facial vitiligo are likely to benefit most. As we await additional research and clinical trials in use of TJKI for vitiligo and other inflammatory and immunologic dermatoses, we hope this review serves as a foundation for practical clinical applications for this emerging drug class.
References
- Zubair R, Hamzavi IH. Phototherapy for vitiligo. Dermatol Clin. 2020;38(1):55–62.
- Relke N, Gooderham M. The use of Janus kinase inhibitors in vitiligo: a review of the literature. J Cutan Med Surg. 2019;23:298–306.
- Olamiju B, Craiglow BG. Tofacitinib cream plus narrowband ultraviolet B phototherapy for segmental vitiligo in a child. Pediatr Dermatol. 2020;37(4):754–755.
- Karagaiah P, Valle Y, Sigova J, Zerbinati N, et al. Emerging drugs for the treatment of vitiligo. Expert Opin Emerg Drugs. 2020;25(1):7–24.
- Asawanonda P, Taylor C. Wood’s light in dermatology. Int J Dermatol. 1999;38:801–807.
- Rashighi M, Agarwal P, Richmond J, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Trans Med. 2014;6(223):223ra23.
- Strassner J, Harris J. Understanding mechanisms of autoimmunity through translational research in vitiligo. Curr Opin Immunol. 2016;43:81–88.
- Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342.
- Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 1 – general considerations and applications in vitiligo and alopecia areata. Actas Dermosifiliogr (Engl Ed). 2020;S0001–7310(20)30554–8.
- Tegtmeyer K, Ravi M, Zhao J, et al. Off-label studies on the use of ruxolitinib in dermatology. Dermatitis. 2021;32(3):164–172.
- Rothstein B, Joshipura D, Saraiya A, et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol. 2017;76(6):1054–1060.
- Joshipura D, Alomran A, Zancanaro P, Rosmarin D. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib: a 32-week open-label extension study with optional narrow-band ultraviolet B. J Am Acad Dermatol. 2018;78(6):1205–1207.
- Harris J, Rashighi M, Nguyen N, Jabbari A. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata. J Am Acad Dermatol. 2016;74(2):
370–371. - Joshipura D, Plotnikova N, Goldminz A, et al. Importance of light in the treatment of vitiligo with JAK-inhibitors. J Dermatolog Treat. 2018 Feb;29(1):98–99.
- McKesey J, Pandya A. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81(2):646–648.
- Mobasher P, Guerra R, Li S, et al. Open-label pilot study of tafacitinib 2% for the treatment of refractory vitiligo. Br J Dermatol. 2020;182(4):1047–1049.
- Rosmarin D, Pandya A, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomized, controlled, Phase 2 trial. Lancet. 2020;396:110–120.
- Yagi K, Ishida Y, Otsuka A, Kabashima K. Two cases of vitiligo vulgaris treated with topical Janus kinase inhibitor delgocitinib. [published online ahead of print, 2021 Mar 5]. Australas J Dermatol. 2021;10.
- Ferreira S, Ferreira R, Neto A, et al. Topical tofacitinib: a Janus kinase inhibitor for the treatment of vitiligo in an adolescent patient. Case Rep Dermatol. 2021;13:190–194.
- Mohammed G, Gomaa A, Al-Dhubaibi MS. Highlights in pathogenesis of vitiligo. World J Clin Cases. 2015;3(3):221–230.
- Regazzetti C, Joly F, Marty C, et al. Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol. 2015;135:3105–3114.
- Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation – a target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–47.
- Hosking AM, Juhasz M, Mesinkovska NA. Topical Janus kinase inhibitors: a review of applications in dermatology. J Am Acad Dermatol. 2018;79(3):535–544.
- Di Lernia V, Bardazzi F. Profile of tofacitinib citrate and its potential in the treatment of moderate-to-severe chronic plaque psoriasis. Drug Des Devel Ther. 2016;10:533–539.
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
- Njoo MD, Spuls PI, Bos JD. Nonsurgical repigmentation therapies in vitiligo: meta-analysis of the literature. Arch Dermatol. 1998;134(12):1532–1540.
- Lee JH, Kwon HS, Jung HM, et al. Treatment outcomes of topical calcineurin inhibitor therapy for patients with vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:929–38.
- Passeron T. Medical and maintenance treatments for vitiligo. Dermatol Clin. 2017;35(2):163–170.
- Searle T, Al-Niaimi F, Ali FR. Vitiligo: an update on systemic treatments. Clin Exp Dermatol. 2021;46(2):248-258.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–744.
- Phan K, Phan S, Shumack S, Gupta M. Repigmentation in vitiligo using Janus kinase (JAK) inhibitors with phototherapy: systemic review and meta-analysis. J Dermatolog Treat. 2020:1–5.
- Esmat S, Hegazy R, Shalaby S, et al. Phototherapy and combination therapies for vitiligo. Dermatol Clin. 2017;35(2):171–192.
- Narla S, Oska S, Lyons AB, Lim HW, et al. Association of myalgias with compounded topical Janus kinase inhibitor use in vitiligo. JAAD Case Rep. 2020;6(7):637–639.
- Liu L, Craiglow B, King B. Tofacitinib 2% ointment, a topical Janus kinase inhibitor, for the treatment of alopecia areata: a pilot study of 10 patients. J Am Acad Dermatol. 2018;78(2):403–404.e1.
- Sideris N, Vakirlis E, Tsentemeidou A, et al. Under development JAK inhibitors for dermatologic diseases. Mediterr J Rheumatol. 2020;31(Suppl 1):137–144.
- Incyte announces positive results from Phase 3 True-V program evaluating ruxolitinib cream in patients with vitiligo. Global Vitiligo Foundation website. Published May 17, 2021. https://globalvitiligofoundation.org/positive-results-from-phase-3-clinical-trial. Accessed May 21, 2021.
- ClinicalTrials.gov. Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). Bethesda, MD: National Library of Medicine; 2019.
- ClinicalTrials.gov. Topical ruxolitinib evaluation in vitiligo study 2 (TRuE-V2). Bethesda, MD: National Library of Medicine; 2019.

