 J Clin Aesthet Dermatol. 2020;13(10)
J Clin Aesthet Dermatol. 2020;13(10)
by Neal Bhatia, MD; Leon H. Kircik MD; Ava Shamban, MD; Varsha Bhatt, PhD; Radhakrishnan Pillai, PhD; and Eric Guenin, PharmD, PhD, MPH
Dr. Bhatia is with Therapeutics Clinical Research in San Diego, California. Dr. Kircik is with Indiana University School of Medicine in Indianapolis, IN, Physicians Skin Care, PLLC, in Louisville, Kentucky, and Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Shamban is with AVA MD Santa Monica, Medical and Cosmetic Dermatology in Santa Monica, California. Drs. Bhatt and Pillai are with Bausch Health US, LLC, in Petaluma, California. Dr. Guenin is with Ortho Dermatologics in Bridgewater, New Jersey.
FUNDING: This study was funded by Ortho Dermatologics.
DISCLOSURES: Bausch Health US, LLC is an affiliate of Bausch Health Companies Inc. Ortho Dermatologics is a division of Bausch Health US, LLC. Neal Bhatia has received honoraria and investigator grants from Bausch Health. Leon Kircik has acted as an investigator, advisor, speaker, and consultant for Ortho Dermatologics. Ava Shamban has received grants/research support and/or consulting from Galderma, Merz, Endo, Brickell, B10, Revance, Teoxane, and Allergan. Varsha Bhatt and Radhakrishnan Pillai are employees of Bausch Health US, LLC and may hold stock and/or stock options in its parent company. Eric Guenin is an employee of Ortho Dermatologics and may hold stock and/or stock options in its parent company. Bausch Health US, LLC is an affiliate of Bausch Health Companies Inc. Ortho Dermatologics is a division of Bausch Health US, LLC.
ABSTRACT: Objective: This study was conducted to assess compatibility of tretinoin 0.05% acne lotion with foundation makeup.
Design: This was a single-center, evaluator-blinded, randomized, controlled clinical trial. Participants were randomized to apply tretinoin 0.05% lotion to either the right or left side of the face before applying full-face foundation makeup.
Setting: Participants were enrolled at a single center in the United States.
Participants: Female individuals aged 18 to 50 years who used facial foundation makeup at least 5 days per week were included.
Measurements: Investigator-assessed grading for foundation coverage and participant evaluations of makeup appearance were conducted at post-makeup application (Post-Makeup) and Hour 6 (6H) timepoints. Antera 3D® images were taken for skin texture roughness analysis and tolerability evaluations were performed at baseline, post-tretinoin application, Post-Makeup, and 6H timepoints.
Results: A total of 30 participants were enrolled and 29 completed the study. There were no significant differences between tretinoin treated and untreated sides for any outcomes of investigator-assessed grading of foundation (percentage coverage, blotchiness, overall coverage, skin tone evenness, visual smoothness). There was a small but statistically significant worsening in percent coverage at 6H versus Post-Makeup on the untreated side, but not the treated side. As rated by participants, even/full coverage and skin smoothness were significantly better on the tretinoin-lotion treated versus the untreated side. Three-dimensional imaging showed there were no significant differences in skin roughness between the treated and untreated sides. Participants reported overall satisfaction with the tretinoin lotion-treated side.
Conclusion: Tretinoin 0.05% lotion did not interfere with facial makeup application or wearability and was well tolerated.
Keywords: Retinoid, tretinoin, acne, lotion, makeup, foundation
Acne vulgaris affects nearly 1 in 10 people worldwide.1 While it occurs more frequently in adolescents, prevalence among adults is increasing, and it is more common in adult women than men.2–4 Adult acne has a negative impact on quality of life as well as self-esteem,5–7 with a greater negative impact on adult women and older patients.8,9 Furthermore, compared with men, acne seems to have a more negative impact on women’s feelings regarding their appearance.9 In a survey of women with facial acne, three quarters reported feeling self-conscious about their appearance around others.5
To address the lack of confidence in their appearance, 58 percent of women with acne reported wearing makeup and almost half reported wearing “a lot of makeup” to cover up acne most or all of the time.5 The use of makeup in patients with acne has been shown to improve quality of life,10,11 but many dermatologists discourage makeup use due to the belief that it will exacerbate acne symptoms.10 Studies have shown, however, that makeup use combined with acne treatment does not interfere with treatment efficacy.10,11
Topical treatments, such as retinoids, are a mainstay of acne treatment12 and are recommended for women who are not pregnant or breast feeding.13 Current gel, cream, and foam formulations of topical retinoids such as tretinoin are effective, but their use is associated with significant cutaneous irritation and drying,14 which can lead to poor adherence.15 Furthermore, traditional semisolid formulations can feel greasy or sticky to the touch.16 Because of the need for a less irritating, lower-dose treatment in an aesthetically pleasing preparation, a new lotion formulation of tretinoin was developed using polymeric emulsion technology.
This novel tretinoin 0.05% lotion formulation utilizes a polymeric honeycomb matrix, which allows for efficient and uniform delivery of micronized tretinoin and moisturizing/hydrating ingredients into the skin. The use of moisturizers and humectants in this lotion formulation have been shown to enhance skin hydration and skin comfort without feeling greasy. Furthermore, compared with other tretinoin formulations, tretinoin 0.05% lotion appears to have greater efficacy against inflammatory lesions with low rates of dryness, pain, and erythema.17
Tretinoin 0.05% lotion was approved in the United States in 2018 (Altreno®, Ortho Dermatologics, Bridgewater, NJ) for the treatment of acne vulgaris in patients nine years of age and older18 and has demonstrated efficacy in patients with moderate-to-severe acne.19 In addition, tretinoin 0.05% lotion was well tolerated,19 and reports of dryness, burning, and erythema were lower than with other tretinoin formulations.20,21
While tretinoin is efficacious and well tolerated for the treatment of acne, the effects of topical tretinoin on the application and appearance of facial makeup is unknown. Here we investigate the compatibility of tretinoin 0.05% lotion with foundation makeup in adult female participants.
Methods
This was a single-center, evaluator-blinded, randomized, controlled study. Female participants between 18 and 50 years of age who were frequent users of foundation and facial makeup (at least 5 days per week) were eligible. Participants were instructed to bring their current foundation (used for at least 30 days with no irritation), any products used under foundation (e.g., toner and/or moisturizer), and any applicators (e.g., sponge) to the baseline visit for documentation and in-clinic use. Participants were also instructed to remove all makeup at least 30 minutes prior to the baseline visit and to apply no other topical products to the face or eye area. Institutional Review Board reviewed and approved the study protocol and written informed consent was obtained from each participant.
Prior to tretinoin lotion application, participants washed their faces with mild facial cleanser, rinsed with warm water, and patted dry. Products typically used under foundation makeup (e.g., toner and/or moisturizer) were then applied to the whole face. Participants applied tretinoin 0.05% lotion to either the right or left side of their face, according to a predetermined randomization; the opposite side of the face remained untreated with tretinoin. After 15 minutes (±5 minutes), imaging and tolerability assessments were conducted. Participants then applied their own foundation makeup in the clinic to the entire face, applying to the untreated side first to avoid cross-contamination.
Three timepoint evaluations were conducted at Visit 1: baseline (BL), post-tretinoin lotion application (Post-Tretinoin), and post-makeup application (Post-Makeup); one timepoint evaluation was conducted at Visit 2: six hours (±1 hour) Post-Makeup (6H). Investigator-assessed clinical grading for foundation coverage, comprising percentage coverage, blotchiness, overall coverage (overall appearance of foundation), skin tone (color) evenness, and visual smoothness, were assessed using a 10-point modified Griffiths scale22 where 0=none (best possible condition); 1–3=mild; 4–6=moderate; and 7–9=severe (worst possible condition). Participant-assessed makeup appearance and satisfaction was conducted by asking participants to rate each side of their face for the following eight items: natural-looking, even/full coverage, even skin tone, smooth skin, satisfaction with test materials, ease of application, confidence wearing foundation, and willingness to add the treatment to everyday routine. Ratings were based on a four-point scale where 1=strongly disagree and 4=strongly agree.
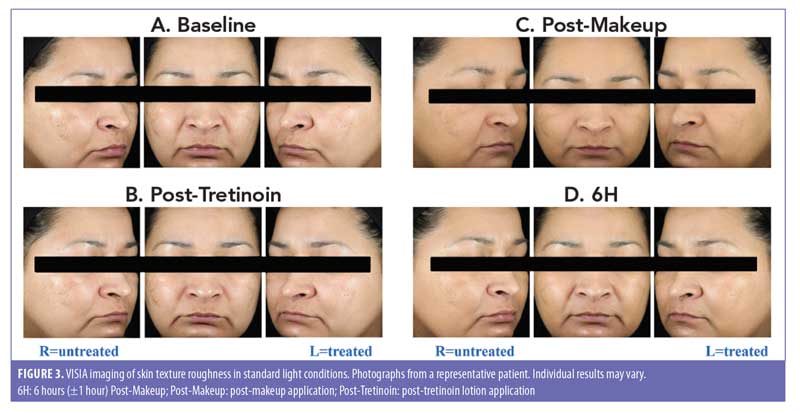
For analysis of skin texture roughness, Antera 3D® imaging (Miravex Limited, Dublin, Ireland) was performed at BL, Post-Tretinoin, Post-Makeup, and 6H for each participant. Antera 3D imaging uses multi-directional illumination and computer-aided reconstruction of the skin surface to reconstruct the surface in three dimensions; the field of view is 56x56mm with lateral resolution of 0.1mm and vertical resolution 0.01mm. Images of the left, center, and right sides of each participant’s face were taken at Post-Tretinoin, Post-Makeup, and 6H using the VISIA CR® photo station (Canfield Imaging Systems, Fairfield, NJ) with a Canon Mark II digital SLR camera (Canon Incorporated, Tokyo, Japan). Images were obtained under standard and cross-polarized lighting conditions.
Tolerability analyses were scored on a four-point scale where 0=none and 3=severe; investigator assessments of tolerability included erythema, edema, dryness, and peeling and participant assessments included burning, stinging, and itching.
A paired t test was used to compare the treated and untreated sides of the face.
Results
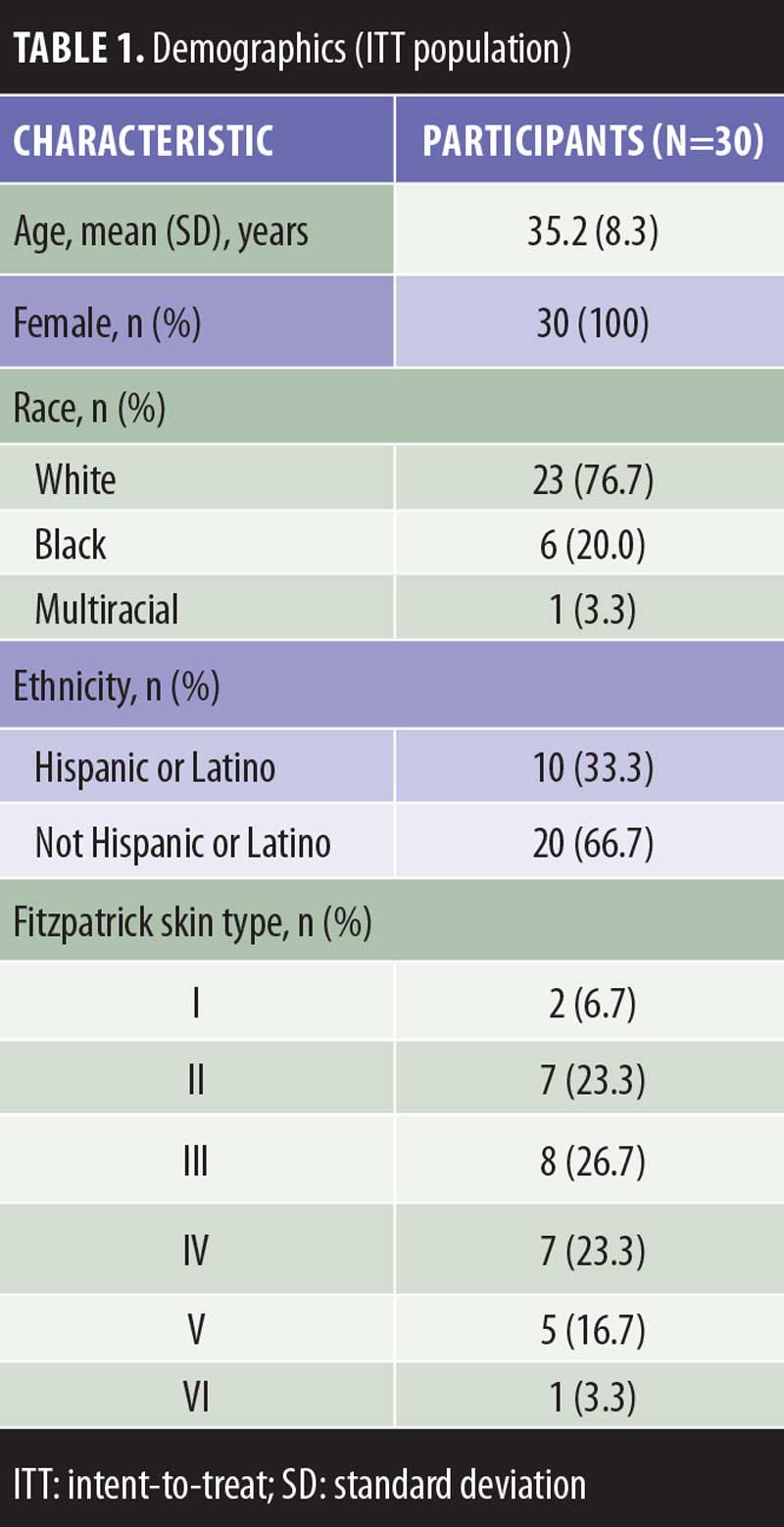
Demographics and Disposition. Thirty participants enrolled in the study and were included in the intent-to-treat population; of these, 29 completed the study (per protocol population) and one participant discontinued per the sponsor’s decision due to assessments being completed outside of the specified time frames. The mean age was 35 years, six participants (20.0%) had a Fitzpatrick skin type of V or greater, 23 participants (76.7%) were white, and 10 participants (33.3%) were Hispanic or Latino (Table 1).
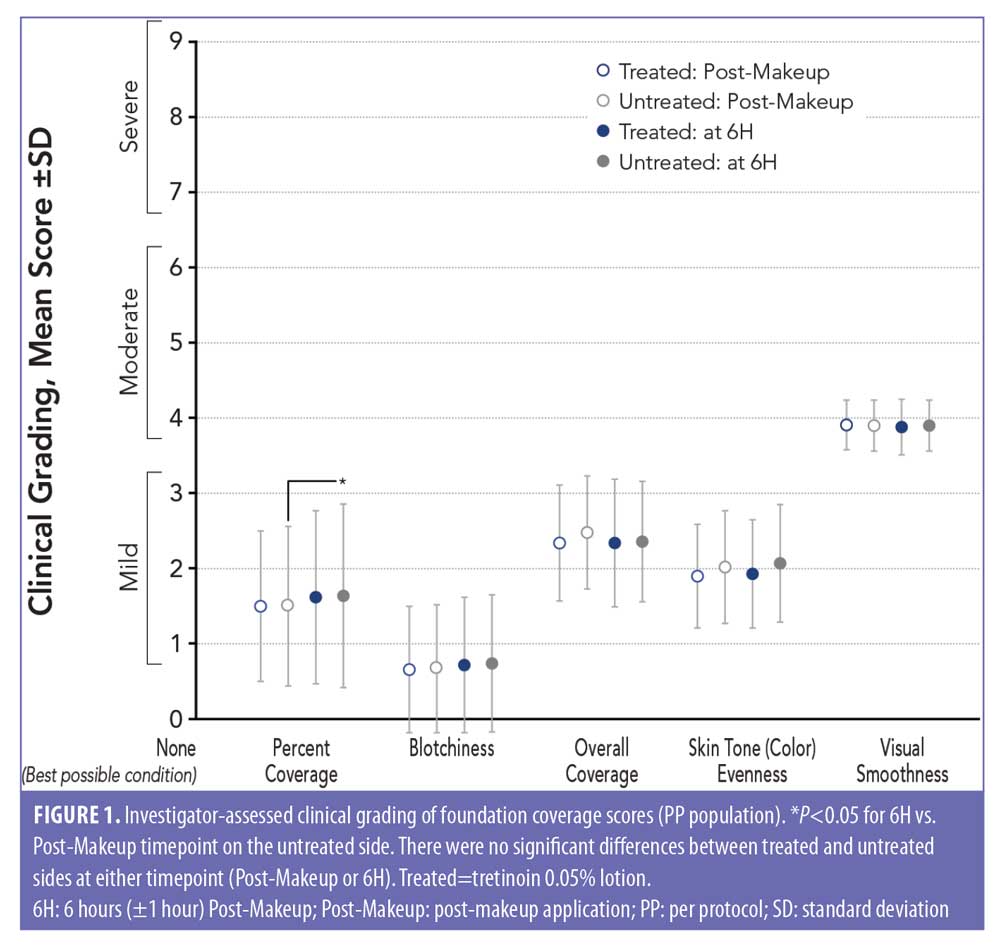
Investigator-assessed clinical grading (foundation coverage). Analysis of the investigator-assessed clinical grading of foundation coverage showed no significant differences between the tretinoin lotion-treated and untreated sides for any outcome (percent coverage, blotchiness, overall appearance of makeup, evenness, or visual smoothness) at either timepoint (6H and Post-Makeup; Figure 1). There was a small but statistically significant worsening in percent coverage at 6H versus Post-Makeup on the untreated side (P<0.05) but not on the tretinoin-treated side.
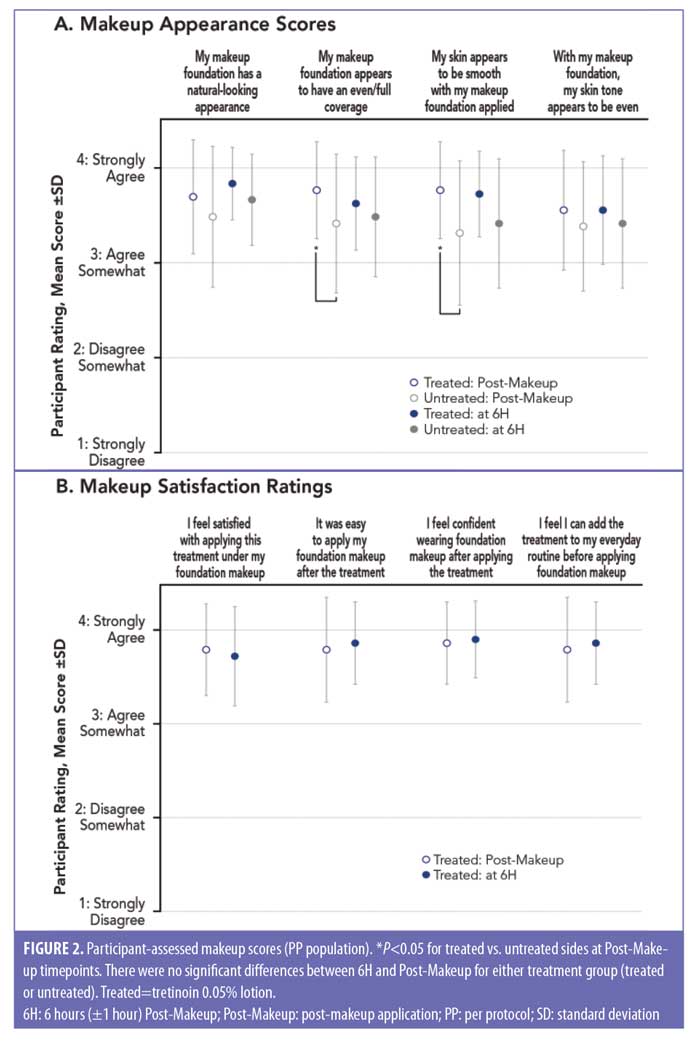
Participant-assessed makeup scores. Mean participant grading scores for even/full coverage and skin smoothness were significantly higher (more favorable) on the tretinoin-treated side than on the untreated side immediately after makeup application (Post-Makeup: P<0.05, both; Figure 2A). No significant differences in makeup appearance between the treated and untreated sides were found at 6H (Figure 2A). Participants generally indicated overall satisfaction and confidence with the tretinoin treated side at Post-Makeup and at 6H with mean scores between 3 and 4 (3=agree somewhat; 4=strongly agree; Figure 2B). Participants also indicated that foundation makeup was easy to apply after tretinoin lotion treatment (mean [SD]: 3.79 [±0.56] at Post-Makeup and 3.86 [±0.44] at 6H).
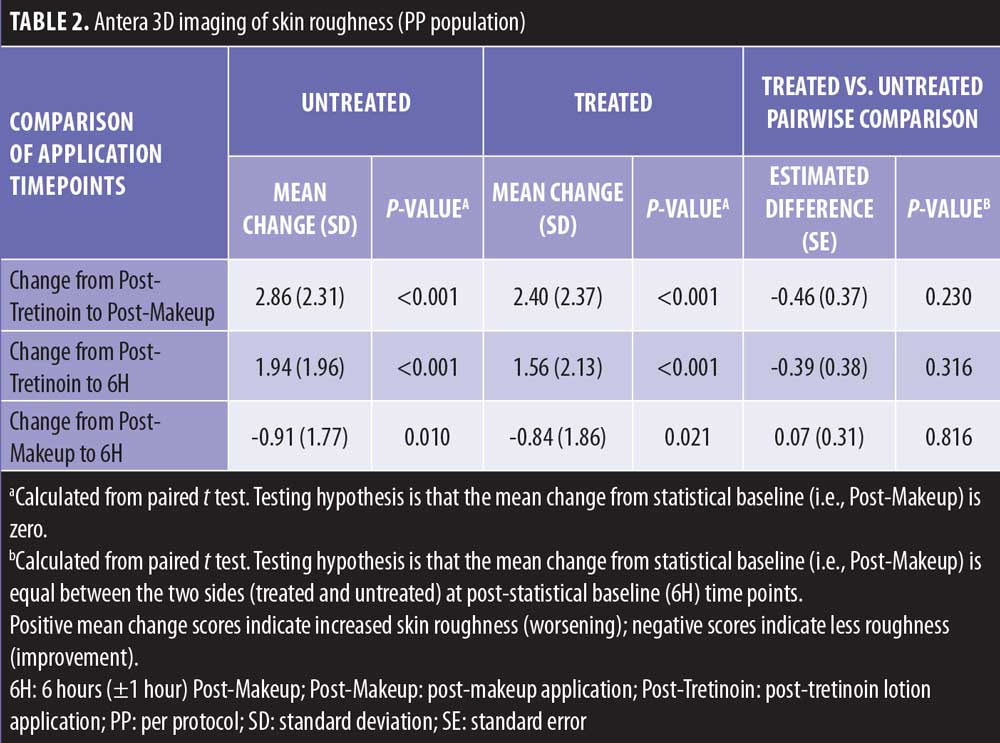
Imaging. Antera 3D imaging between multiple timepoints were assessed. As expected, there was a significant worsening in skin roughness (higher scores) at Post-Makeup compared with Post-Tretinoin for both the treated and untreated sides (P<0.001 both; Table 2); this significant worsening was maintained at 6H versus Post-Tretinoin for both treated and untreated sides (P<0.001, both). At 6H versus Post-Makeup, the images showed a significant improvement in skin roughness for both the treated and untreated sides (P<0.05, both). Together, these data suggest that skin roughness increases following makeup application, likely due to pigments/small particles in the makeup product that wear off slightly over time but is not impacted by tretinoin lotion application. This was confirmed in a statistical pairwise comparison of the treated and untreated sides, in which the change in skin roughness from Post-Tretinoin to either Post-Makeup or 6H were not significantly different between the tretinoin-treated and untreated sides (Table 2). Similar results were observed for change in skin roughness from Post-Makeup to 6H. Photographs using VISIA imaging are shown in Figures 3A–D.

Tolerability. Most cutaneous tolerability outcomes had a rating of “none” (0) from all 30 participants at Post-Makeup and at 6H for both the tretinoin-treated and untreated sides; no outcome was rated as “severe” (3) by any participant. For edema, peeling, and stinging, all participants rated tolerability outcomes as none at both timepoints. One participant (3.3%) indicated moderate burning (2) on the tretinoin-treated side at 6H (Table 3).
Discussion
Acne has a substantial negative impact on self-esteem and can lead to impaired psychological and physical well-being.7,23 As acne is more prevalent among women than men,2–4 and using makeup to conceal the appearance of acne and other facial skin conditions can improve quality of life,10,11 it is of interest whether acne medications are compatible with foundation makeup.
Topical acne treatments that are intended to be used underneath facial makeup must be able to be evenly distributed on the skin to prevent clumping and streaking.24 The polymeric technology utilized in this formulation of tretinoin 0.05% lotion was designed to deliver the active ingredient, solvents, emollients, and humectants in a honeycomb matrix to provide uniform delivery of ingredients.17
The present study showed that this formulation of tretinoin 0.05% lotion did not interfere with full facial foundation makeup application or wearability, and tretinoin use was compatible for up to six hours after application of foundation makeup. Makeup application between the tretinoin treated and untreated sides was not negatively affected in terms of percent and overall coverage, blotchiness, evenness of skin tone, and smoothness at either the Post-Makeup or 6H timepoints. Though analysis of clinical grading showed a statistically significant worsening for percent coverage on the untreated side of the face at 6H compared with Post-Makeup, this change was not clinically relevant. Furthermore, there was no negative impact on skin roughness due to tretinoin lotion application based upon Antera 3D imaging results that showed no significant difference between the treated and untreated sides.
Participants also reported satisfaction and confidence with makeup applied to the tretinoin-treated side. Interestingly, mean scores were significantly more favorable on the tretinoin-treated side versus the untreated side for two questions related to makeup and skin appearance: “my makeup foundation appears to have an even full coverage” and my “skin appears to be smooth with my makeup foundation applied.” This favorable response following tretinoin treatment might be due to the non-greasy and aesthetically pleasing lotion formulation, which is highly spreadable and moisturizing.
In addition to compatibility, tretinoin 0.05% lotion was well tolerated. Consistent with the two 12-week Phase 3 studies of tretinoin in patients with moderate-to-severe acne,19 most cutaneous tolerability outcomes had a rating of “none” from all 30 participants and no outcome was rated as “severe.” As retinoids have the highest discontinuation rate among acne treatments due to dryness, peeling, and irritation,14,15 this new lower dose lotion formulation might improve adherence by reducing these adverse effects.
There are several limitations to this study that should be considered. The number of participants included in this study was relatively small, therefore generalizations should be made with caution. In addition, this study was conducted over the course of one day, and thus the long-term compatibility of tretinoin 0.05% lotion and facial makeup is not known. Furthermore, as it was not required for the participants in this study to have acne, results might differ in participants who do have acne.
Conclusion
This study shows that tretinoin 0.05% lotion did not negatively impact clinical grading of foundation makeup, including coverage and blotchiness, compared with no treatment. Furthermore, tretinoin lotion appeared beneficial on the participant-assessed foundation coverage and skin smoothness immediately after foundation application. Tretinoin lotion was also compatible for up to six hours post-foundation makeup application and was well tolerated.
Acknowledgements
The study was funded by Ortho Dermatologics. Medical writing support was provided by Jacqueline Benjamin, PhD, and Emily H. Seidman, MSc, CMPP (Prescott Medical Communications Group, Chicago, IL) with financial support from Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC.
References
- Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(1):3–12.
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41(4):577–580.
- Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78(3):335–341.
- Skroza N, Tolino E, Mambrin A, et al. Adult acne versus adolescent acne: A retrospective study of 1,167 patients. J Clin Aesthet Dermatol. 2018;11(1):21–25.
- Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7(2):22–30.
- Gorelick J, Daniels SR, Kawata AK, et al. Acne-related quality of life among female adults of different races/ethnicities. J Dermatol Nurses Assoc. 2015;7(3):154–162.
- Gallitano SM, Berson DS. How acne bumps cause the blues: The influence of acne vulgaris on self-esteem. Int J Womens Dermatol. 2018;4(1):12–17.
- Tan JK, Li Y, Fung K, Gupta AK, et al. Divergence of demographic factors associated with clinical severity compared with quality of life impact in acne. J Cutan Med Surg. 2008;12(5):235–242.
- Hassan J, Grogan S, Clark-Carter D, et al. The individual health burden of acne: appearance-related distress in male and female adolescents and adults with back, chest and facial acne. Br J Health Psychol. 2009;14(8):1105–1118.
- Hayashi N, Imori M, Yanagisawa M, et al. Make-up improves the quality of life of acne patients without aggravating acne eruptions during treatments. Eur J Dermatol. 2005;15(4):284–287.
- Matsuoka Y, Yoneda K, Sadahira C, et al. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol. 2006;33(11):745–752.
- Leyden J, Stein-Gold L, Weiss J. Why topical retinoids are mainstay of therapy for acne. Dermatol Ther (Heidelb). 2017;7(3):293–304.
- Tan AU, Schlosser BJ, Paller AS. A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol. 2018;4(2):56–71.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e933.
- Dikicier B. Topical treatment of acne vulgaris: efficiency, side effects, and adherence rate. J Int Med Res. 2019;47(7):2987–2992.
- Tanghetti EA, Stein Gold L, Del Rosso JQ, et al. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J Dermatolog Treat. 2019:1–8.
- Kircik LH, Draelos ZD, Berson DS. Polymeric emulsion technology applied to tretinoin. J Drugs Dermatol. 2019;18(4):s148–154.
- Altreno® (tretinoin 0.05% lotion). US prescribing information. Bausch Health US, LLC, Bridgewater, NJ; 2019.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: Assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17(10):1084–1091.
- Harper JC, Roberts WE, Zeichner JA, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of safety and tolerability in subgroups. J Dermatolog Treat. 2019:1–8.
- Webster G, Cargill DI, Quiring J, et al. A combined analysis of 2 randomized clinical studies of tretinoin gel 0.05% for the treatment of acne. Cutis. 2009;83(3):146–154.
- Griffiths CE, Wang TS, Hamilton TA, et al. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128(3):347–351.
- Davern J, O’Donnell AT. Stigma predicts health-related quality of life impairment, psychological distress, and somatic symptoms in acne sufferers. PLoS One. 2018;13(9):e0205009.
- Bhatia N, Pillai R. Randomized, observer-blind, split-face compatibility study with clindamycin phosphate 1.2%/benzoyl peroxide 3.75% gel and facial foundation makeup. J Clin Aesthet Dermatol. 2015;8(9):25–32.

