 by Khairuddin Djawad, MD, PhD; Siswanto Wahab, MD, PhD; Olivia Wibisono, MD; Christina Avanti, PhD; Astridani R. Putranti, PhD; Agussalim Bukhari, MD, PhD; and Ilham J. Patellongi, MD, PhD
by Khairuddin Djawad, MD, PhD; Siswanto Wahab, MD, PhD; Olivia Wibisono, MD; Christina Avanti, PhD; Astridani R. Putranti, PhD; Agussalim Bukhari, MD, PhD; and Ilham J. Patellongi, MD, PhD
Drs. Djawad, Wahab, Wibisono, Bukhari, and Patellongi are with Faculty of Medicine at Hasanuddin University in Makassar, Indonesia. Drs. Djawad and Wibisono are also with the Dr. Wahidin Sudirohusodo Hospital in Makassar, Indonesia. Drs. Avanti and Putranti are with the Faculty of Pharmacy at the University of Surabaya (UBAYA), in Surabaya, Indonesia.
J Clin Aesthet Dermatol. 2020;13(10):E59–E65
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this study.
Abstract: Background. Exposing the skin to ultraviolet B (UVB) radiation triggers inflammation, with erythema as the most prominent acute clinical manifestation. Xanthones, secondary metabolites of the mangosteen pericarp, have been shown to possess anti-inflammatory and antioxidant properties. With the increasing evidence of harmful effects of chemical sunscreens to marine organisms, it is necessary to develop environmentally friendly sunscreen options.
Objective. We sought to assess the protective effect of a nano-liposomal mangosteen pericarp extract (MPE) cream against UVB-induced erythema.
Methods. Thirty-one healthy subjects with Fitzpatrick Skin Types III or IV were enrolled.Six sites on the back of each volunteer were used for testing; Sites 1 to 4 were exposed to UVB at a dosage of two minimal erythema dose. Before UVB exposure, the test materials, including 5%, 10%, or 20% MPE cream, or a base cream without MPE, were applied to each site. Sites 5 and 6 served as the positive (UVB only, no treatment) and negative (no treatment, no UVB) control groups. The lightness index (?L*) and erythema index (?a*) were assessed before and 24 hours after treatment using Chromameter.
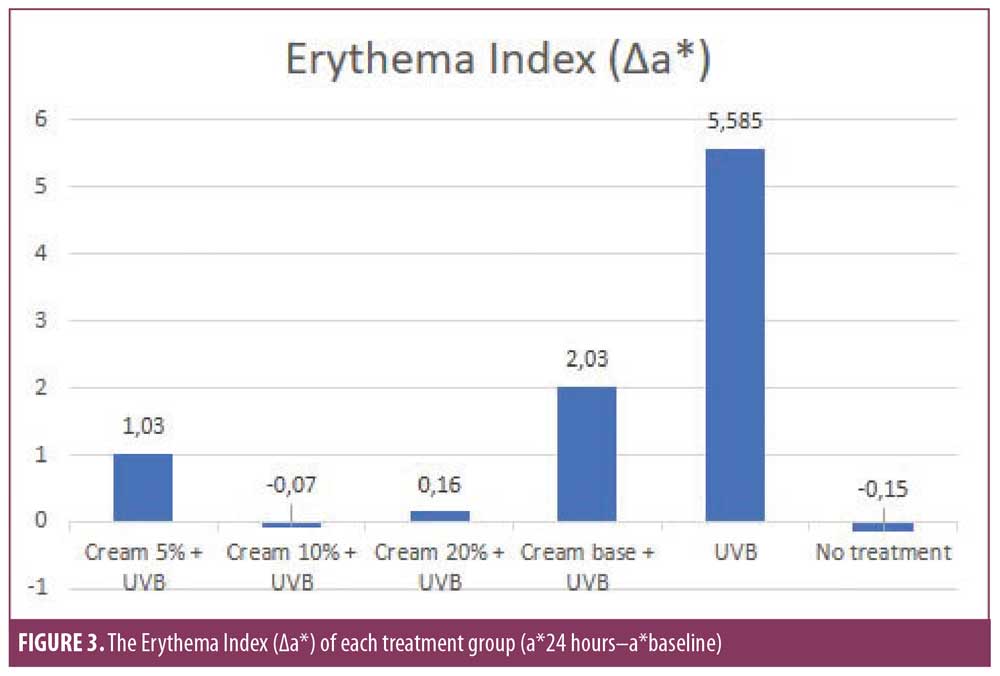
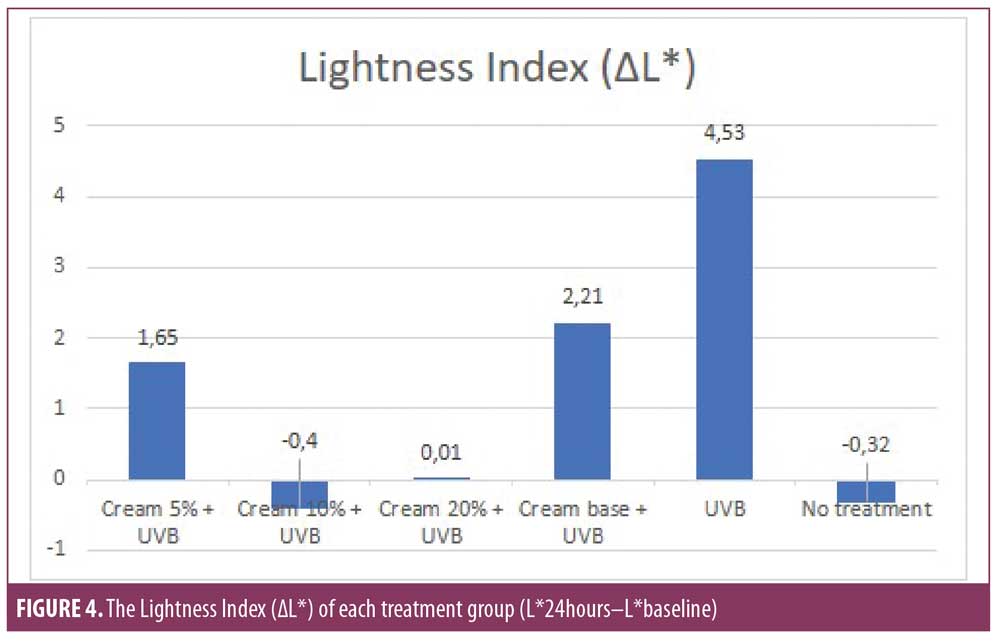
Results. On all treated sites, there were only slight decrease of ?L* and increase of ?a*. The ?L* of the 5%, 10%, and 20% cream groups were 1.65, -0.4, and 0.01, respectively, which were significantly different from UVB group (4.53). The ?a* of the 5%, 10%, and 20% cream (1.03, -0.07; 0.16, respectively) was significantly different from the base cream (2.03) and UVB group (5.585).
Conclusion. The 10% and 20% nano-liposomal cream of MPE appeared to exert protective effects against UVB-induced erythema on human skin.
Keywords: Chromameter, erythema, mangosteen pericarp extract, sunscreen, ultraviolet B
The skin occupies a strategic location between the harmful external environment and the biochemically active internal environment. Ultraviolet (UV) radiation is an external environmental factor that can harm the skin. Excessive sun exposure can cause erythema, edema, sunburn, hyperplasia, premature aging, melanoma, and non-melanoma skin malignancies.1 Erythema is the most visually acute prominent aspect of the sunburn response. The inflammatory process manifests as erythema and edema due to increased vasodilation and vascular permeability.2 Erythema appears in a diphasic pattern, usually seen 3 to 5 hours after exposure, then reaches maximum intensity after 12 to 24 hours and generally subsides within 72 hours. Various biochemical processes at the cellular level occur, including increased blood flow, endothelial cell activation, and increased levels of inflammatory mediators.3 The absorbed UVB radiation activates the enzyme phospholipase and releases arachidonic acid from cell membrane phospholipids. Arachidonic acid is then converted to prostaglandin (PGE) and 12-hydroxyeicosatetraenoic acid (12-HETE) by the enzymes cyclooxygenase (COX) and lipoxygenase (LOX).2 UVB exposure triggers inflammation through direct induction of epidermal keratinocytes to produce proinflammatory cytokines, including TNF-?, IL-1?, and COX-2.4
Mangosteen (Garcinia mangostana L.), is mainly cultivated in Indonesia, Malaysia, the Philippines, and Thailand.5 Secondary metabolites of mangosteen pericarp, called xanthones, have broad pharmacological properties, including anti-inflammatory, antineoplastic, antioxidant, antiproliferation, anticancer, antimalarial, antibacterial, antiobesity, hepatoprotective, neuroprotective, and cardioprotective.6,7 A total of 68 organic xanthone compounds have been isolated from all parts of the mangosteen.8 The highest xanthones concentration, reaching 78, has been isolated from the mangosteen pericarp, namely ?-mangostin.9,10 Studies have shown ?-mangostin can inhibit the production of inflammatory mediators and decrease levels of mRNA expression from TNF-?, IL-1?, and IL-6.4 Also, MPE has been shown to inhibit COX enzymes in the arachidonic acid pathway.11
Although current evidence suggests that the use of sunscreens does not yield any adverse health effects, the potentially harmful environmental effects of sunscreen agents are still of concern. Currently, there are issued statements in certain areas of the United States prohibiting sunscreens containing oxybenzone and octinoxate, since these materials have been shown to have a fatal impact on the maintenance of coral reefs, which play an important role in supporting the preservation of fish resources and marine organisms.12 Therefore, it is necessary to develop safer natural ingredients as sunscreen agents, such as from plant extracts that contain photoprotective and antioxidant effects.13
This double-blind, randomized, controlled study was designed to clinically assess the protective effect of a nano-liposomal MPE cream against UVB-induced erythema on human skin.
Methods
Subjects. Thirty-one healthy subjects with Fitzpatrick Skin Types III or IV were enrolled in the study. All participants followed the study’s specific protocol and signed an informed consent form. The subjects were 20 to 45 years of age with no history of photodermatosis or skin cancer and were not taking any photosensitizing or anti-inflammatory medications. This study complied with the ethical guidelines and was approved by the Health Research Ethics Commission Committee of Hasanuddin University (process No. 681/UN4.6.4.5.31/PP36/2019).
Materials. Mangosteen pericarp liquid extract was made at the laboratory of Phytochemistry and Pharmacognosy, Faculty of Pharmacy at Hasanuddin University. The MPE creams were prepared at the Laboratory of Pharmaceutical Technology, Faculty of Pharmacy at the University of Surabaya (UBAYA), based on the ingredients listed in Table 1. Four formulas were created using the same cream base; the first formula comprised the cream base without active ingredients, the second formula contained 5% mangosteen pericarp liquid extract, the third formula contained 10% mangosteen pericarp liquid extract, and the fourth formula contained 20% mangosteen pericarp liquid extract.
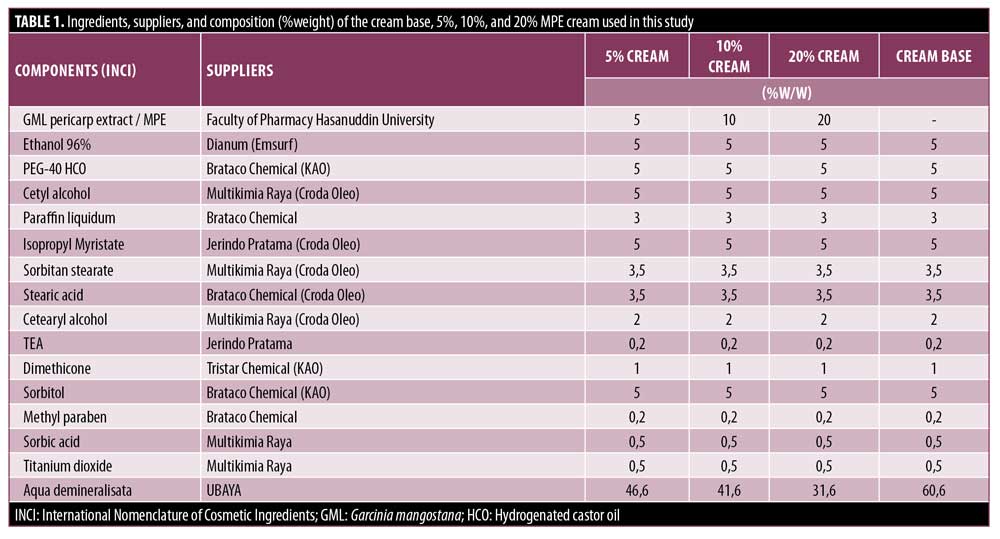
Preparation of skin cream. The cream was formulated by melting the oil phase (cetyl alcohol, sorbitan stearate, Cetearyl alcohol, isopropyl myristate, paraffin liquidum, stearic acid) at 65 °C. The water phase (aqua, sorbitol, methylparaben, sorbic aciwd, and TEA) was heated at 65 °C. Then, the water phase was put into the oil phase and stirred with Ultraturrax at a speed of 11.000rpm for three minutes, then the stirring speed was reduced to 3.000rpm and stirred for another five minutes. Stirring was continued for five minutes, then dimethicone and titanium dioxide were added, then stirring continued for five minutes to form a homogeneous emulsion. MPE was dissolved in 96 ethanol in a mortar, then added PEG-40 HCO, crushed and stirred until a homogeneous mixture was formed. The extract mixture was then put into the emulsion while still stirring at 3.000rpm for three minutes, then cooled while still stirring.
Accelerated stability study. To obtain stability data of the MPE cream at all formulas, an accelerated stability study was conducted for six months using the climatic chamber with a temperature of 25 to 28 °C and relative humidity of 60 to 70 percent. Parameters that were measured include organoleptics, pH, and viscosity. Data were recorded every month.
Solar simulator. Psora-Comb Dermalight 80 (Dr. K. Honle GmbH, Munich, Germany) was used as a UVB emitting source. It emits a continuous spectrum of UVB radiation, with peaks of 313nm, placed at a fixed distance of 3cm using its comb attachment.
Determination of the Minimal Erythema Dose (MED). Once the skin type was defined using Chromameter CR-400 (Konica Minolta, Inc., Tokyo, Japan) and Individual Typology Angle (ITA), the determination of the MED was performed at the first visit. Daavlin MED dose patch (Daavlin Company; Bryan, Ohio) was used on the back of each, which comprises six 1.9×1.9 cm squares (3.61 cm2 area). The MED is defined as the lowest dose that induces erythema in an individual.14 The MED is determined by exposing six squares to gradually increasing amounts of UVB radiation (200, 400, 600, 800, 1000, 1200mJ/cm2) and examining the UV-exposed areas 24 hours later.15 The area with the least erythema observed by the investigators is considered the MED subjectively, while objectively we used a 2.5-point difference in the a* value before and after irradiation to indicate a significant difference in erythema.16 Thus, in this study, the determination of the MED was performed subjectively and objectively.
Treatment protocol. This study was conducted in our dermatology and venereology outpatient clinic in September 2019. Before each irradiation, the following materials were applied to sites 1 to 4, respectively: 5% MPE cream, 10% MPE cream, 20% MPE cream, and cream base. No product was applied to Site 5, which served as the positive control, and neither UVB nor MPE cream was applied to Site 6, which served as the negative control. A standardized dose of 2.77mg/cm2 MPE cream was calculated, in which 10mg of MPE cream was applied to each site. All test product applications were performed by other volunteers using latex gloves to maintain the double-blind study. The MPE cream was applied and left for 20 minutes before irradiation. A summary of the treatment sites is given in Table 2.
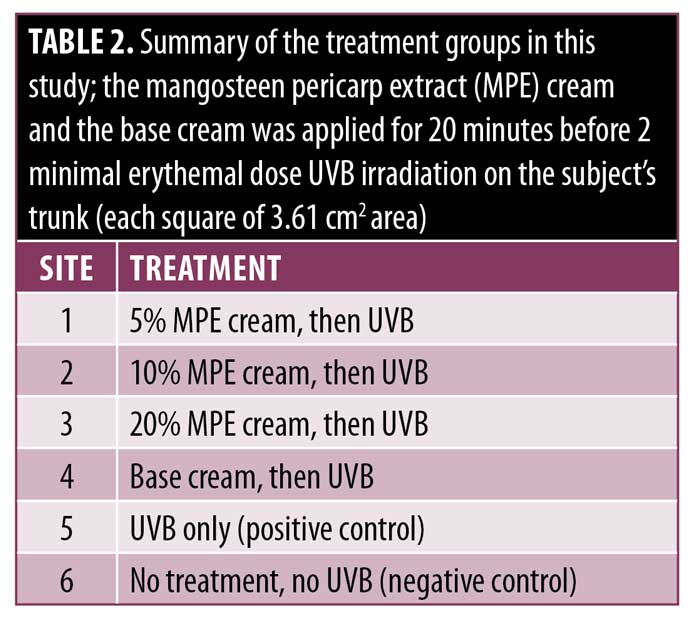
We chose to evaluate the creams on the back of each participant, as it proved to be the test site with the smallest fluctuations of photometric measurements.17 Six squares of the Daavlin MED dose patch was used on the back of each subject with designation as Sites 1 to 6. All subjects received the equivalent of 2 MED in each site. This dose is considered sufficient to induce defined erythema that can be separated from the background readings of untreated.17,18 Irradiation was performed at the second visit, then chromameter measurements were performed after 24 hours to measure the erythema and lightness index.
Chromameter measurement. Before and 24 hours after exposure to UVB, the L* and a* values on the test sites were measured using a Chromameter CR-400 (Konica Minolta, Inc., Tokyo, Japan). The values were measured three times on each test site, and the mean values were determined. The differences (?) in median L* and a* values before and 24 hours after irradiation were calculated. Based on the Commission Internationale de l’Eclairage (CIE) system, the output values are expressed in three dimensions (L*, a*, b*), where a* represents erythema and L* is to assess lightness. The erythema index (?a*) and lightness index (?L*) was assessed by a difference (?) in baseline and 24 hours after treatment.
Statistical analysis. The data were analyzed using SPSS 25.0 software (SPSS Inc., Chicago, Illinois). Nonparametric statistical methods (Kruskal Wallis and Mann-Whitney test) were used with P-value <0.05 was considered statistically significant.
Results
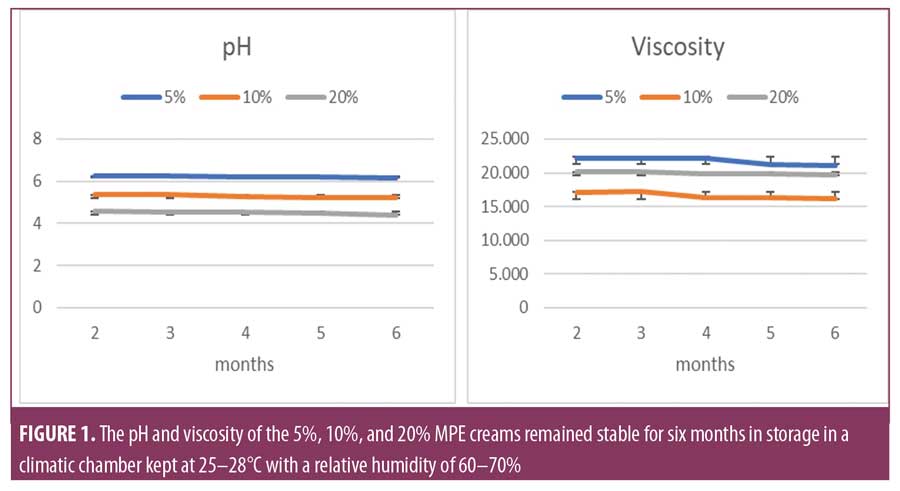
Cream stability. A difference in the pH of the three creams is caused by the differences in extract concentration. The pH of the cream decreases as the concentration of the MPE increases. Over a period of six months in storage, no significant pH differences were observed in each of the creams. Likewise, the cream viscosity did not show a significant difference over six months of storage (Figure 1). Changes in viscosity are also influenced by differences in extract concentration. The lowest viscosity was observed in the 10 percent MPE cream and the highest was observed in the five percent MPE cream. The pH and viscosity in the 5 percent, 10 percent, and 20 percent MPE creams were found to be stable while in storage for six months in a climatic chamber at 25 to 28 °C and a relative humidity of 60 to 70 percent.
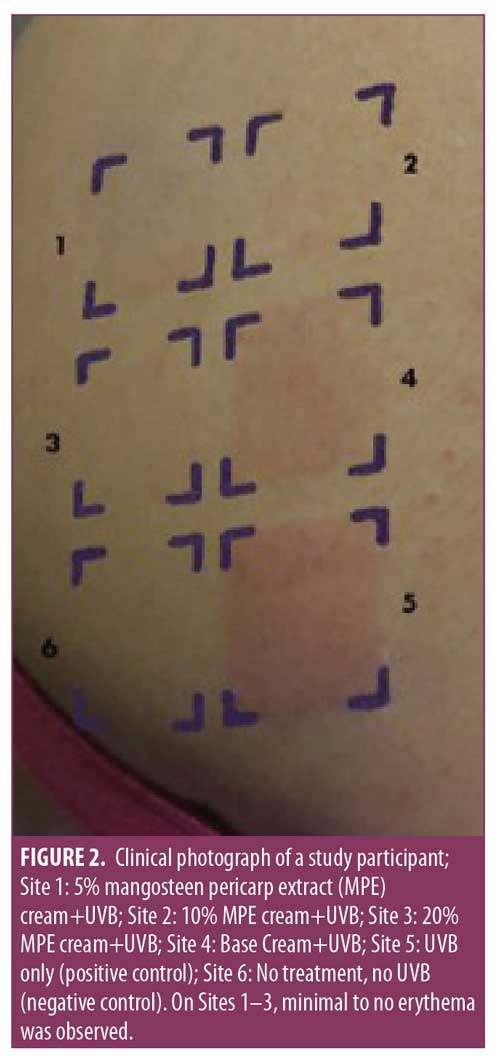
Clinical photographs. After UVB irradiation, some irradiated sites developed some degrees of erythema, as shown in Figure 2. Obvious erythema was seen on Site 5 (UVB) and Site 4 (cream base + UVB), suggesting the cream base alone provided minimal protection from UVB.
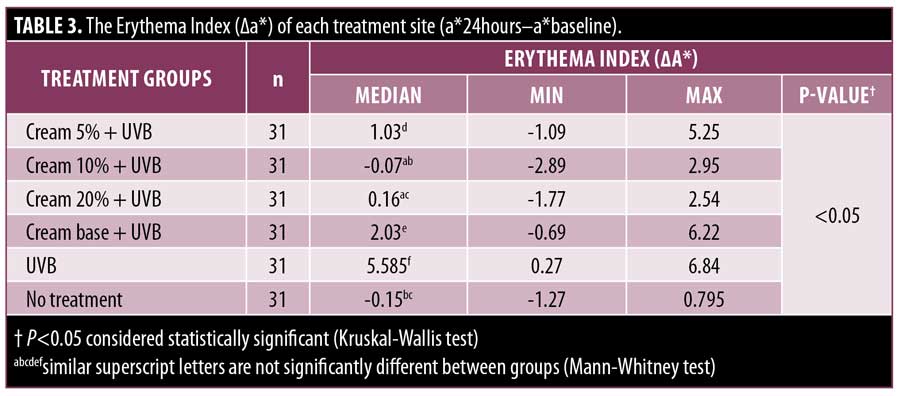
The erythema index. All subjects showed significant differences in the erythema index in the UVB and no treatment group (Figure 3). This is consistent with a statistically significant P-value (p<0.05) between both treatment groups with ?a* values of 5.585 and -0.15, respectively (Table 3). Based on this observation, 2 MED exposure to UVB was shown to cause significant erythema. Overall, the administration of MPE cream was found to reduce the erythema index. This is consistent with the statistically significant difference in the erythema index among the six treatment groups (p<0.05). The erythema index (?a*) of the 5%, 10%, 20% cream (1,03; -0,07; 0,16) differed significantly (p<0.05) to base cream (2.03) and to UVB group (5.585). However, the erythema index of the base cream was also significantly different from the UVB group (p<0.05), indicating a minimal protective effect against UVB. Based on the concentration ratio, the lowest erythema index was found in the 10% cream group followed by the 20% cream group and the 5% cream group. Statistical data supports the superiority of the 10% and 20% creams over the 5% cream, whereas the 10% and 20% creams did not differ significantly (p=0.198). The 10% cream group and the 20% cream group were not significantly different when compared to the no treatment group, with values of ?a* -0.07 (p=1.000) and 0.16 (p=0.210), respectively. This finding indicates that the 10% cream and 20% cream exhibit a substantial protective effect against UVB-induced erythema equivalent to the no treatment group.
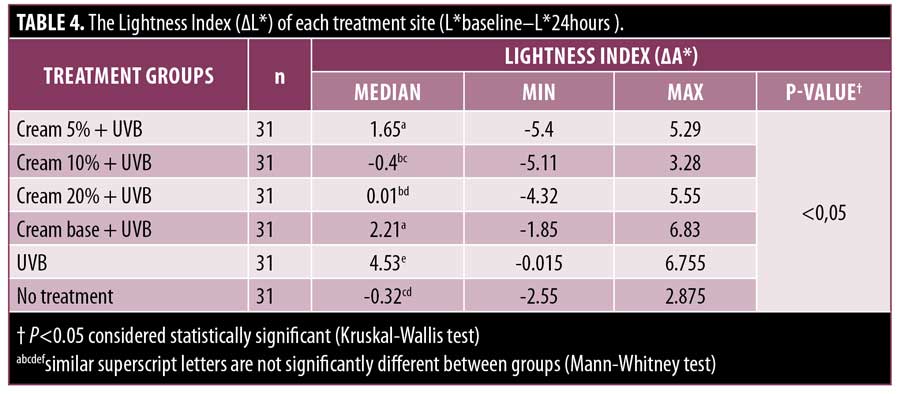
The lightness index. The lightness index in the UVB and no treatment group showed a significant difference (p<0.05) as shown in Table 4, with each L* value of 4.53 and -0.32, respectively, demonstrating 2 MED UVB exposure can significantly influence the lightness of the skin’s color (Figure 4). Overall, the administration of MPE cream was found to reduce the lightness index (?L*). This is consistent with the statistically significant difference between the six treatment groups (p<0.05). The lightness index of the 5%, 10%, and 20% cream groups differ significantly to UVB group (p<0.05), with each value ?L* 1.65; -0.4; 0.01, respectively. Moreover, the 10% and 20% cream groups were significantly different from base creams (p<0.05), but not 5% cream (p=0.314). These findings indicate that the base cream also exerts some protective effect against UVB-induced erythema. Although the 10% and 20% cream groups showed better protection, the base cream and the 5% cream group showed a similar protective effect. Based on three concentrations, the 10% cream was found to be more superior, followed by the 20% cream and the 5% cream group, though the 10% cream and the 20% cream were not significantly different (p=0.451). When compared to the no treatment group, the 10% and 20% creams did not differ significantly, with a P-value of 0.746 and 0.410, respectively. These findings exhibit the protective ability of MPE cream that was found to be quite similar to the no treatment group based on the lightness index evaluation.
Discussion
The Fitzpatrick Skin Type I to VI is the most useful clinical scale in assessing skin sensitivity to UV radiation, with lesser skin types more susceptible to sunburn and at a higher risk of developing skin cancer.19 This study was conducted on 31 subjects with Fitzpatrick Skin Types III and IV, which constitute the majority of the skin color of the Indonesian population. The sensitivity of an individual’s skin to UV radiation can be measured by visual assessment of a MED. In general, MED increases with skin type.20 Most studies have used twice the MED to induce defined erythema.17,18
The peak intensity of UVB radiation occurring during the day can have an acute impact on human skin, leading to edema, erythema, or sunburn before pigmentation begins.21 In this study, the erythema index and skin lightness index were observed by assessing the difference in the a* value and the L* value at the baseline to 12 hours post-UVB exposure. The higher a* value indicates the increasingly erythematous state of the skin, while the higher L* value indicates brighter skin.
Erythema response is influenced by the strength of the energy exposure and the presence or absence of protection. Erythema is usually seen 3 to 5 hours after UVB exposure and reaches maximum intensity 12 to 24 hours after exposure. Various biochemical courses at the cellular level occur as an initial response of the skin to UV radiation, which includes vasodilation of blood vessels, activation of endothelial cells, and increased levels of inflammatory mediators.3,22
Excessive exposure to UVB radiation in the skin increases COX-2 protein receptors and the formation of reactive oxygen species (ROS), which triggers the expression of MMP1 that degrades collagen.23 UVB exposure also results in massive neutrophil infiltration, in which the neutrophil will produce cytokines and chemokines that also activate MMP.24 Erythema results from rapid degranulation of mast cells followed by TNF-? release and vasodilation of blood vessels.22 Therefore, the inflammatory process and erythema can be limited by reducing the formation of ROS, mast cells, and neutrophils or other inflammatory mediators.25
The biochemical composition of MPE has been reported to contain flavonoids, which play an important role in counteracting free radicals, and xanthones, which have various antibacterial, antifungal, and anti-inflammatory properties.26 Of all the xanthones that have been identified, ?-mangostin has the highest level of xanthones isolated from mangosteen pericarp.9,10
High antioxidant activity on mangosteen pericarp xanthones has been reported by various studies. Yoshikawa et al27 found that methanol extract from mangosteen pericarp showed DPPH radical scavenging activity. Leong and Shui28 compared the total antioxidant capacity using ABTS and DPPH tests, which describe a high antioxidant activity of mangosteen extract. In a similar study, Garcia et al29 studied antioxidant capacity through the measurement of lipoperoxidation and hydroxyl radical scavenging activity, which reported that MPE had the highest antioxidant activity. Moongkarndi et al30 also reported mangosteen extract significantly reduced intracellular ROS production through measurement of 2.7-DCFH-DA. Jung et al31 also reported a high capacity of scavenging free radicals by ?-mangostin, gartanin, ?-mangostin, garcinone B, garcimangone B, 1-isomangostin and garcinone D. Also, a study by Chomnawang et al32 measured antioxidant activity by inhibiting DPPH radical formation, which showed that mangosteen ethanol extract had significant antioxidant activity. Data from these various studies show the high antioxidant activity of MPE.7
In addition to being an antioxidant, various studies have also reported the role of ?-mangostin in inhibiting inflammatory mediators.33 Studies show that ?-mangostin can lower the expression of inflammatory mediators induced by lipopolysaccharides such as TNF-? and IL-6.34,35 Inhibition of activation of MAPK, NF-?B, AP-1 and diminishing of pro-inflammatory cytokine gene expression was also observed in other studies of ?-mangostin treatment.36,37 Another study examined the inhibitory effect of ?-mangostin on the secretion of pro-inflammatory mediators, where ?-mangostin inhibits the secretion of IL-8 and TNF-?.33 Other studies also evaluate the inhibition of ?- and ?-mangostin against NO and PGE2.38,39 This is consistent with other studies that reported a dose-dependent ?-mangostin inhibiting PGE2 release.40 Similar studies report suppression of histamine release by ?-, ?- and ?-mangostin which is observed in rat basophilic leukemia cells.41 This effect is associated with decreased COX-2 mRNA and protein expression, and activation of NF-?B. Other studies observed intraperitoneal and oral administration of ?-mangostin, 1-isomangostin, or triacetate mangostin and reported an anti-inflammatory activity in several mouse inflammatory models.42 The in-vivo anti-inflammatory activity of ?-mangostin has been confirmed in edema models of the soles of rat feet.38,43 Administration of ? and ?-mangostin orally also shows anti-inflammatory activity in ovalbumin-induced asthma (OVA) mice.44 The topical administration of MPE gel has been reported to reduce periodontal inflammation.45 Data from these studies show the anti-inflammatory roles of MPE through its bioactive content.
Antioxidant and anti-inflammatory effects are the main mechanism of the photoprotective activity of herbal extracts. Phenolic and flavonoid organic compounds are the main source of natural antioxidant compounds that can provide oxidative protection against free radicals including ROS. In UV-damaged skin, where ROS is formed, ROS reacts negatively with DNA, protein, and fatty acids which can induce carcinogenic processes and inflammatory responses.46
Several in-vitro studies have reported a fraction of the MPE compound having UV filter ability. This is based on a spectrophotometric assessment of the ability of these compounds to absorb light.46 A study in Sri Lanka reported in-vitro research on the sunscreen activity of MPE with the highest concentration in the methanol fraction reaching an SPF value of 33.80.47 This shows that the observed solar filtering effect is natural, intrinsic and has high potential to be developed into an inexpensive and efficacious and environmentally friendly sunscreen. Two other studies from Indonesia also reported the same thing. The first study reported the results of in-vitro testing of dichloromethane and butanol fractions of MPE that has a high UV-protection ability by reaching an SPF value >50.48 The second study reported the ethanol fraction of MPE showed sunscreen activity that reached an SPF value >30 at a concentration of 100ppm.49
Conclusion
In this study, the base cream group was found to protect against erythema and affect skin brightness 12 hours after UVB exposure. The base cream used contains titanium dioxide (TiO?), which is a snow-white inorganic filter compound that is insoluble in water. TiO?, with a size of more than 200nm, can protect the skin physically from all UV wavelengths.50 However, the 10% and 20% MPE cream demonstrate a higher protective effect against UVB and was statistically significant. This study involved subjects with limited skin types; hence, additional research in other skin types would be beneficial in understanding the efficacy and tolerability.
References
- Tobin DJ. Introduction to skin aging. J Tissue Viability. 2017;26(1):37–46.
- Buckman S, Gresham A, Hale P, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19(5):723–729.
- Kochevar I, Taylor C, Krutmann J. Fundamentals of cutaneous photobiology and photoimmunology. Fitzpatrick’s dermatology in general medicine. 2008;1:1031–1048.
- Im AR, Kim YM, Chin YW, et al. Protective effects of compounds from Garcinia mangostana L. (mangosteen) against UVB damage in HaCaT cells and hairless mice. Int J Mol Med. 2017;40(6): 1941–1949.
- Gondokesumo ME, Pardjianto B, Sumitro SB, et al. Microstructural Characterization of the Garcinia mangostana Fruit at Different Maturity Level. J Nat Med. 2018;18(2):63–70.
- Chen G, Li Y, Wang W, et al. Bioactivity and pharmacological properties of ?-mangostin from the mangosteen fruit: a review. Expert Opin Ther Pat. 2018;28(5):415–427.
- Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, et al. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol. 2008;46(10):3227–3239.
- Obolskiy D, Pischel I, Siriwatanametanon N, et al. Garcinia mangostana L.: a phytochemical and pharmacological review. Phytother Res. 2009;23(8):1047–1065.
- Kurose H, Shibata MA, Iinuma M, et al. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with ?-mangostin extracted from mangosteen pericarp. J Biomed Biotechnol. 2012;2012:672428.
- Walker EB. HPLC analysis of selected xanthones in mangosteen fruit. J Sep Sci. 2007;30(9):1229–1234.
- Marzaimi IN, Aizat WM. Current Review on Mangosteen Usages in Antiinflammation and Other Related Disorders. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases: Elsevier; 2019. 273–289.
- American Academy of Dermatology Association statement on sunscreen access [press release]. ROSEMONT Ill: American Academy of Dermatology Association. Updated May 4, 2018.
- Seok JK, Kwak JY, Choi GW, et al. Scutellaria radix Extract as a Natural UV Protectant for Human Skin. Phytother Res. 2016;30(3):374–379.
- Runger T. Cutaneous Photobiology. In: Kang S, Amagai M, Bruckner A, Enk A, Mcmichael A, Orringer J, et al., editors. Fitzpatrick’s Dermatology. 9th ed: McGraw-Hill Education; 2019. 272.
- Jaleel T, Pollack B, Elmets C. Phototherapy. In: Kang S, Amagai M, Bruckner A, et al., editors. Fitzpatrick’s Dermatology. 9th ed: McGraw-Hill Education; 2019. 3640.
- Heckman CJ, Chandler R, Kloss JD, et al. Minimal Erythema Dose (MED) testing. J Vis Exp. 2013;(75):e50175. 2013.
- Jocher A, Kessler S, Hornstein S, et al. The UV erythema test as a model to investigate the anti-inflammatory potency of topical preparations–reevaluation and optimization of the method. Skin Pharmacol Physiol. 2005;18(5):234–240.
- Hughes-Formella BJ, Bohnsack K, Rippke F, et al. Anti-inflammatory effect of hamamelis lotion in a UVB erythema test. Dermatology. 1998;196(3):316–322.
- Del Bino S, Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol. 2013;169 Suppl 3:33-40.
- Harrison GI, Young AR. Ultraviolet radiation-induced erythema in human skin. Methods. 2002;28(1): 14–19.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100–S109.
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–568.
- Marwaha V, Chen Y-H, Helms E, et al. T-oligo treatment decreases constitutive and UVB-induced COX-2 levels through p53-and NF?B-dependent repression of the COX-2 promoter. J Biol Chem. 2005;280(37):32379–32388.
- Han YP, Tuan TL, Wu H, et l. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114(Pt 1):131–139.
- Che DN, Xie GH, Cho BO, et al. Protective effects of grape stem extract against UVB-induced damage in C57BL mice skin. J Photochem Photobiol B. 2017;173:551–559.
- Pothitirat W, Chomnawang MT, Supabphol R, et al. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia. 2009;80(7):442–447.
- Yoshikawa M, Harada E, Miki A, et al. Antioxidant constituents from the fruit hulls of mangosteen (Garcinia mangostana L.) originating in Vietnam. Journal of the Pharmaceutical Society of Japan. 1994;114(2):129–133.
- Leong L, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76(1):69–75.
- Garcia VV, Magpantay TO, Escobin LD. Antioxidant potential of selected Philippine vegetables and fruits. Philippine Agricultural Scientist. 2005;88(1):78.
- Moongkarndi P, Kosem N, Luanratana O, et al. Antiproliferative activity of Thai medicinal plant extracts on human breast adenocarcinoma cell line. Fitoterapia. 2004;75(3-4):375–377.
- Jung H-A, Su B-N, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54(6):2077–2082.
- Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. 2007;78(6):401–408.
- Gutierrez-Orozco F, Chitchumroonchokchai C, Lesinski GB, et al. ?-Mangostin: anti-inflammatory activity and metabolism by human cells. J Agric Food Chem. 2013;61(16):3891–3900.
- Bumrungpert A, Kalpravidh RW, Chuang C-C, et al. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J Nutr. 2010;140(4):842–847.
- Liu S-H, Lee L-T, Hu N-Y, et al. Effects of alpha-mangostin on the expression of anti-inflammatory genes in U937 cells. Chin Med. 2012;7(1):19.
- Bumrungpert A, Kalpravidh RW, Chitchumroonchokchai C, Chuang C-C, West T, et al. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J Nutr. 2009;139(6):1185–1191.
- Bumrungpert A, Kalpravidh RW, Suksamrarn S, et al. Bioaccessibility, biotransformation, and transport of ?-mangostin from Garcinia mangostana (Mangosteen) using simulated digestion and Caco-2 human intestinal cells. Molecular Nutrition & Food Research. 2009;53(S1):S54–S61.
- Chen L-G, Yang L-L, Wang C-C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46(2):688–693.
- Tewtrakul S, Wattanapiromsakul C, Mahabusarakam W. Effects of compounds from Garcinia mangostana on inflammatory mediators in RAW264. 7 macrophage cells. J Ethnopharmacol. 2009;121(3):379–382.
- Nakatani K, Nakahata N, Arakawa T, et al. Inhibition of cyclooxygenase and prostaglandin E2 synthesis by ?-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem Pharmacol. 2002;63(1):73–79.
- Itoh T, Ohguchi K, Iinuma M, et al. Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation. Bioorg Med Chem. 2008;16(8):4500–4508.
- Shankaranarayan D, Gopalakrishnan Ct, Kameswaran L. Pharmacological profile of mangostin and its derivatives. Archives internationales de pharmacodynamie et de therapie. 1979;239(2): 257–269.
- Nakatani K, Yamakuni T, Kondo N, et al. ?-Mangostin inhibits inhibitor-?B kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol Pharmacol. 2004;66(3):667–674.
- Jang H-Y, Kwon O-K, Oh S-R, et al. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem Toxicol. 2012;50(11):4042–4050.
- Rassameemasmaung S, Sirikulsathean A, Amornchat C, et al. Topical application of Garcinia mangostana L. pericarp gel as an adjunct to periodontal treatment. Complement Ther Med. 2008;16(5):262–267.
- Buso P, Radice M, Baldisserotto A, et al. Guidelines for the Development of Herbal-Based Sunscreen. Herbal Medicine: IntechOpen; 2017.
- Ratnasooriya W, Pathirana R, Gamage R, et al. Sunscreen Activity of Pericarp of Fruit of Sri Lankan Garcinia mangostana L.(Mangosteen) in vitro. IMP. 2017;3.
- Susanti M, Putra DP. In-vitro UV protection activity of Garcinia mangostana. Pharmaceutical Journal of Indonesia. 2012;13(2):61–64.
- Iwo MI, Soemardji AA, Hanafi M. Sunscreen activity of ?-mangostin from the pericarps of Garcinia mangostana Linn. J Appl Pharm Sci. 2013;3(6):70–73.
- Chung J. Photoprotection. In: Kang S, Amagai M, Bruckner A, et al., editors. Fitzpatrick’s Dermatology. 9th ed: McGraw-Hill Education; 2019. 3623.

