J Clin Aesthet Dermatol. 2024;17(3):24–33.
J Clin Aesthet Dermatol. 2024;17(3):24–33.
by Faraz Yousefian, DO; Ciaran Smythe, DO; Haowei Han, DO; Boni E Elewski, MD; and Mark Nestor MD, PhD
Drs. Yousefian, Smythe, Han and Nestor are with the Center for Clinical and Cosmetic Research in Aventura, Florida. Dr. Elewski is with the Department of Dermatology at the University of Alabama at Birmingham School of Medicine in Birmingham, Alabama. Additionally, Dr. Nestor is with the University of Miami’s Miller School of Medicine in Miami, Florida.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Onychomycosis is a fungal infection of the nail unit that affects a large patient population globally. Onychomycosis, or tinea unguium, has a benign chronic clinical course; however, it can cause complications in certain patient populations suffering from diabetes and peripheral vascular disease. As nails grow slowly, onychomycosis requires a lengthy treatment plan, and choosing appropriate treatments can be challenging. There are a variety of treatment modalities available for patients including topical, oral, laser, light therapy, procedures such as avulsion and matrixectomy, supplements, over-the-counter medication, and plasma therapy that can be used as monotherapy or in combination for patient satisfaction.
Objective. We sought to review treatment options for onychomycosis, taking into consideration the efficacy, side effect profiles, practicality of treatment (adherence), and costs to help healthcare providers offer ethically appropriate treatment regimens to their patients.
Methods. A literature search was conducted using electronic databases (PubMed, Embase, Medline, CINAHL, EBSCO) and textbooks, in addition to the clinical experiences of the authors and other practitioners in treating onychomycosis, and a summary of the findings are presented here.
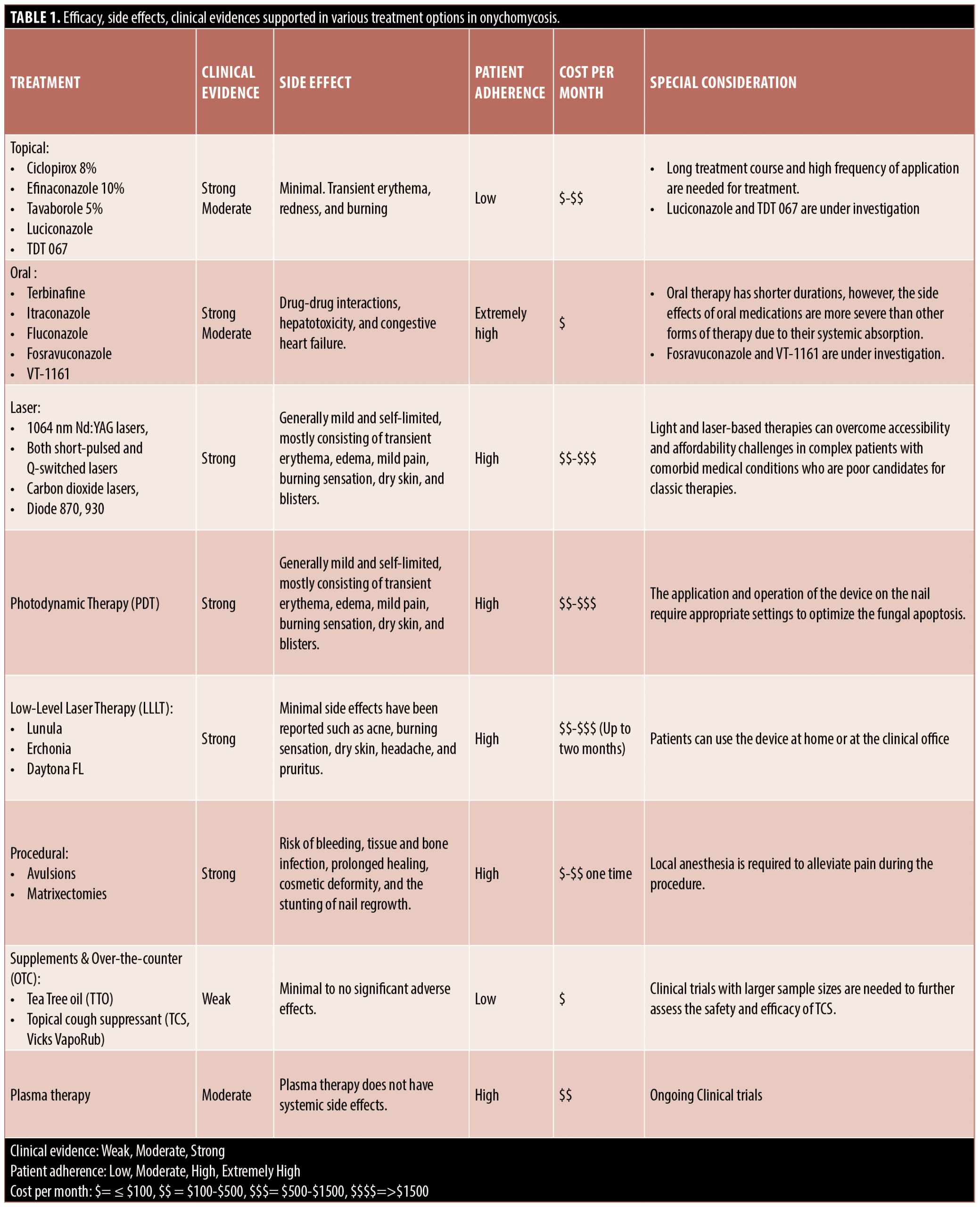
Results. Although topical (efinaconazole, tavaborole, ciclopirox), oral (terbinafine, itraconazole), and laser (1064nm Nd:YAG lasers, both short-pulsed and Q-switched lasers, carbon dioxide lasers, and the diode 870, 930nm) are the current Food and Drug Administration (FDA)-approved treatments for onychomycosis, they are just a fraction of available treatment options. New and emerging therapies including new topical and oral medications, combination therapy, photodynamic light therapy, procedural, supplements, over-the-counter medication, and plasma therapy are discussed in our review.
Discussion. Onychomycosis has high reinfection and recurrence rates, and the treatment remains challenging as treatment selection involves ethical, evidence-based decision-making and consideration of each individual patient’s needs, adherence, budget, the extent of quality of life discomfort, and aesthetic goals, independent of potential financial benefits to the clinicians.
Keywords: Onychomycosis, ethics, review, therapies, treatments
Introduction
Onychomycosis is the fungal infection of the nail unit caused by dermatophytes, non-dermatophyte molds, and yeast.1 It is characterized by nail dystrophy, discoloration, and thickening with a prevalence of 50 percent among patients over 70 years of age.1 Onychomycosis can be acquired or predisposed by various genetic factors.1 Toenails are more commonly affected than fingernails due to slower growth, the moist environment caused by occlusive footwear, and lower blood flow to the area. A nail unit serves many functions, including protection of the digits, grasping, and scratching. Sporadically, the failure to treat onychomycosis can lead to permanent damage to the nail plates and the surrounding anatomical attachments, pain, transmission to others, the local spread of infection (paronychia) and/or spread to other body parts, mobility disability in the elderly, and cellulitis due to secondary bacterial and other organisms.2 The cosmetic appearance of a nail can be impaired by onychomycosis and a variety of cutaneous and systemic diseases and can have a psychological impact on those affected by chronic nail changes.
Since most fungal infections of the nail unit are non-life-threatening, chronic, and superficial, treatment depends on clinician recommendations and patient expectations.1 For the majority of patients, onychomycosis can not only be a health issue, but also a cosmetic issue, and it can have a psychological burden on patients and their family members.3 In the midst of the disease benign course, healthcare professionals should educate patients that onychomycosis is still an infection and it can spread to other body parts and their family members. However, it is challenging to treat this chronic condition with its high recurrence in the midst of limited medical, light-based, nutraceutical, and surgical options for several months of treatments in the majority of patients without severe symptoms or complications.3 Onychomycosis has a reinfection rate of 20 to 25 percent after successful treatment and has a recurrence rate of 6.5 to 53 percent3; therefore, healthcare providers should identify the ethical consequences, financial burden, adherence, and side effects involved in each therapeutic modality before selecting and justifying treatment in patients.
Predisposing factors for onychomycosis can be categorized into three groups: genetic and nonmodifiable risk factors, medical conditions, and physical/environmental factors.4 Predisposing genetic and non-modifiable risk factors include older age, male sex, and having a parent or child with onychomycosis. Genetic predisposition is noted in distal subungual onychomycosis, which can be inherited in an autosomal dominant pattern, HLA-DR53 and HLA-DR6 may confer protection against the development of onychomycosis in a Mexican Mestizo population,5 Intercellular Adhesion Molecule 1 (ICAM-1), deficiency can give rise to chronic nail candidiasis, and single nucleotide polymorphism in the dectin-1 gene leads to decreased dectine-1 protein to detect fungal β-glucans.4,6 Predisposing medical conditions include diabetes mellitus, immunosuppression, human immunodeficiency virus (HIV), tinea pedis, psoriasis, peripheral arterial disease, and venous insufficiency.4 Lastly, environmental factors such as smoking, athletic activity, nail trauma, obesity, and the prevalence of opportunistic fungal pathogens in the environment can play a role.4 Moreover, nail tools at pedicures have been reported as a source of fungal transmission in nail polishes for individuals.7
Onychomycosis can be caused by dermatophytes (Trichophyton rubrum and Trichophyton mentagrophytes), non-dermatophytic molds (Scopulariopsis brevicaulis, Fusarium species, Aspergillus species, Scytalidium dimidiatum, and Acremonium species), and yeasts (Candida).1 Dermatophyte infections constitute 80 to 90 percent of onychomycosis and are more responsive to antifungal treatment when compared to non-dermatophytic molds.8 These organisms can alkalize the human nail plate pH, which leads to the clinical findings due to the inactivation of several of the skin’s defense mechanisms.9
After fungi adhesion to the stratum corneum and invasion of the keratin layers, various patterns of infection may result. Onychomycosis can be classified into five subtypes based on the pattern of nail unit involvement: distal lateral subungual onychomycosis (DLSO), superficial onychomycosis (SO), proximal subungual onychomycosis (PSO), endonyx onychomycosis (EO), and total dystrophic onychomycosis (TDO).4 In addition, patients may present with mixed patterns of onychomycosis (MPO), most commonly with DLSO and SO.10
Diagnosis of onychomycosis can be complex and challenging but is needed prior to treatment. Empiric oral antifungal treatment of onychomycosis has shown medication resistance in patients.11,12 An accurate diagnosis can be made based on the patient’s medical and social history. A patient’s prior history of onychomycosis, and risk factors such as the use of gyms, pedicures, and military service, are integral to distinguishing onychomycosis from nail psoriasis. A variety of other conditions can cause nail dystrophy, including lichen planus, alopecia areata, eczema, and contact dermatitis, which should be taken into consideration while making a diagnosis.8 Periodic acid–Schiff (PAS) stain is the gold standard for diagnosis to examine the presence or absence of fungal elements, while fungal culture and polymerase chain reaction (PCR) analysis identify the fungus.1 KOH tests also can affirm the diagnosis but do not differentiate fungus live from and it cannot specify pathogen organism type.
The most common endpoints used in onychomycosis studies are a clinical cure and a mycological cure. A clinical cure is defined as a normal nail without any evidence of fungal infection, which is the most important outcome for patients. The mycological cure is defined as having a negative culture and negative direct microscopy. A complete cure is the combination of mycological cure plus a normal nail which physicians commonly use to assess the efficacy of treatment.13
Topical Therapy
Background and efficacy. Topical treatments offer the benefits of low systemic exposure and minimal side effects.14 However, the number of Food and Drug Administration (FDA)-approved topical antifungal agents has been minimal due to the difficulty of medications penetrating the nail bed successfully.15 All topical medications discussed are recommended for mild to moderate disease and the recommended duration of treatment is 48 weeks.16 Until 2014, only one topical treatment, topical ciclopirox 8% lacquer was FDA-approved for the treatment of onychomycosis.17 Ciclopirox is a broad-spectrum antifungal medication that inhibits trivalent cations of fungal enzymes important for degrading toxic peroxides.18 In formulations for onychomycosis, ciclopirox acts as a hydro lacquer, forming a film over the nail and allowing for penetration of the nail plate.16 Ciclopirox has fairly low efficacy, with early studies demonstrating a mycologic cure rate of 34 percent versus 10 percent for placebo, and a complete cure rate of 5.5 to 8 percent.19 The medication is recommended for use in mild cases, as an adjuvant to other treatments, and in patients in whom oral therapy is contraindicated.19
In 2014, the FDA approved topical efinaconazole 10% and tavaborole 5% for the treatment of onychomycosis.16 These two medications are formulated as solutions, rather than lacquers, and have been shown to have superior penetration over ciclopirox and amorolfine (a topical medication not approved for use in the United States).20 Additionally, nail polish does not significantly affect the nail penetration of these solutions, enabling the concealment of onychomycosis during treatment.21,22 Efinaconazole works by blocking ergosterol synthesis via inhibition of sterol 14α-demethylase, while tavaborole is a boron-based solution that inhibits fungal cell protein synthesis.14,16 In a meta-analysis, efinaconazole showed the highest probability of cure after oral treatment and was comparable to itraconazole.23 Efinaconazole has demonstrated a mycologic cure rate of 54 percent and a complete cure rate of 17 percent.24
Some novel drugs are currently being tested but have not yet been approved by the FDA. In 2013, luciconazole was approved for the treatment of fungal infections of the skin and is now being investigated in the treatment of onychomycosis, with mycological and complete cure rates of 46 percent and 15 percent, respectively.25 TDT 067 is a spray formulation of terbinafine that has shown potent antifungal effects and may be promising for treating onychomycosis in the future.26 Lastly, ME-111 is a medication that inhibits fungal succinate dehydrogenase in the electron transport chain that is currently in Phase II clinical trials, and MOB-015 is a topical terbinafine formula that is currently in Phase III clinical trials.26
Due to the difficulty of clearing onychomycosis infection, recurrence is a concern, even when there is no clinical evidence of infection. Prophylactic treatment with topical medications once every 2 to 3 weeks in high-risk patients has been shown to decrease the time to recurrence by 200 days.3,27
Side effects. Side effects of topical medications are minimal, in large part due to the lack of systemic absorption. Side effects are generally limited to application site reactions like erythema, redness, and burning.16 Drug-drug interactions are not a concern due to little or no systemic absorption.
Ease of use. Adherence is a potential concern with topical treatment options due to the long treatment course and high frequency of application. Additionally, when applying these medications, ciclopirox needs to be removed once per week for better penetration of additional doses, whereas efinaconazole and tavaborole can be placed on top of existing treatment.26
Patient cost. Patient cost for topical therapies for onychomycosis range from less than $100 to $500 monthly.
Oral Therapy
Background and efficacy. Oral therapies are regarded as the gold standard for treating moderate to severe onychomycosis and provide the most effective therapy.16 There are three main oral agents for the treatment of onychomycosis: terbinafine, itraconazole, and fluconazole. Historically, griseofulvin and ketoconazole were also used to treat onychomycosis, but they are no longer recommended. Terbinafine is an allylamine antifungal that inhibits ergosterol synthesis, an essential component of fungal cell membranes, via the inhibition of squalene epoxidase enzyme.28 Itraconazole and fluconazole, both azole antifungals, also work by inhibiting the synthesis of ergosterol, but differ from terbinafine by inhibiting lanosterol 14α-demethylase.29
Oral therapy requires prolonged use due to limited bioavailability and limited capacity to maintain significant drug concentrations in the nail bed.16 Terbinafine is dosed (250mg daily) for six weeks for fingernail onychomycosis and 12 weeks for toenail onychomycosis.30 Pulsed dosing of terbinafine has been shown to be less effective than continuous dosing.31 Itraconazole is typically dosed in a pulsed fashion (1 week on of 400mg/day, followed by 3 weeks off), eight weeks for fingernail onychomycosis, and 12 weeks for toenail onychomycosis.32 Itraconazole can also be given in a continuous fashion of 200 mg/day for 12 weeks. Studies assessing cure rates of oral therapies show some variation. Several larger trials examining oral terbinafine have demonstrated cure rates from 70 to 81 percent.33–35 Studies evaluating oral itraconazole have shown a cure rate of 46 to 69 percent.33,34,36 Meta-analyses have shown that terbinafine 250mg daily and itraconazole 400mg pulsed dosing are superior to oral fluconazole and other topical treatments and that oral terbinafine is the most effective treatment, overall.23,37 A meta-analysis found that continuous terbinafine use is superior to pulsed terbinafine for achieving a mycological cure.31 Lastly, terbinafine has shown a broad range of efficacy in its ability to treat dermatophytes, non-dermatophyte molds, and mixed onychomycosis.23
There are currently some promising oral therapies under investigation that have not yet been approved. Fosravuconazole L-lysine ethanolate is a triazole drug that has demonstrated potent antifungal effects.38 In a Japanese study, fosravuconazole had mycological and complete cure rates of 82 percent and 59 percent, respectively, after a 12-week treatment course.38 Additionally, a novel tetrazole drug called VT-1161 taken for 10- or 22-week courses showed a mycological cure rate of 61 to 72 percent and a complete cure rate of 32 to 40 percent.38
Side effects. Side effects of oral medications are more severe than other forms of therapy due to their systemic absorption.16 These side effects include drug-drug interactions, hepatotoxicity, and congestive heart failure. A study with more than 25,000 patients on terbinafine 250mg/day showed an adverse event rate of 10.5 percent, with gastrointestinal disturbance (4.9%) and cutaneous reactions (2.9%) being the most common.39 In this same study, oral terbinafine was considered a possible or probable cause of 0.04% of the serious adverse events. A study with more than 15,000 patients on oral itraconazole therapy demonstrated that gastrointestinal disturbances, dizziness, pruritus, and headache are the most common side effects, with hepatotoxicity occurring rarely.40
Ease of use. Oral therapy has shorter durations than other types of treatment, including topical, device-based, and alternative treatments, making adherence more likely.16,23 Additionally, oral therapy is less time-consuming, versus the application of a lacquer or office visits for device-based therapies. Additionally, although onychomycosis has been shown to have detrimental effects on physical, functional, psychosocial, and emotional aspects of life, some may consider it more of a cosmetic inconvenience.41 Having a thorough discussion about the side effects of oral therapies may deter some patients from adhering to treatment due to the belief that the risks outweigh the benefits.
Patient cost. Patient cost for oral therapies for onychomycosis are $100 or less per month.
Classical laser therapy
Background and efficacy. For patients with onychomycosis, laser therapy has shown promising outcomes. The selective photodermophysis property of laser results in fungal cell death.42 Although, oral and topical FDA-approved antifungal treatments have been demonstrated to be the most efficacious in the treatment of onychomycosis, light and laser-based therapies can overcome accessibility and affordability challenges in complex patients with comorbid medical conditions who are poor candidates for classic therapies.
In order to have an effective laser treatment that penetrates the nail bed, the pulse duration should be shorter than the thermal relaxation time and a wavelength of 750-1300nm is traditionally used.43 Since 2012, the four FDA-approved laser systems (1064nm Nd:YAG lasers, both short-pulsed and Q-switched lasers, carbon dioxide lasers, and the diode 870, 930nm) have been utilized due to short treatment times and fewer numbers of treatments required.44 However, clinical studies have not shown exceedingly better outcomes than current topical and oral antifungal therapies for complete resolution of onychomycosis.45
Side effects. Laser procedures are considered to be safe and well-tolerated. Side effects are generally mild and self-limited, mostly consisting of transient erythema, edema, mild pain, burning sensation, dry skin, and blisters.
Ease of use. The application and operation of the device on the nail require appropriate settings to optimize the fungal apoptosis.
Patient cost. The cost of laser treatments for onychomycosis can range from $100 to $1,500 per month.
Photodynamic light therapy
Background and efficacy. Photodynamic light therapy (PDT) uses selective photodermophysis to destroy fungal cells.42 PDT utilizes light with the photosynthesizer (5-aminolevulinic acid (5-ALA), methyl aminolevulinate (MAL), porphyrins, aluminum-phthalocyanine chloride, methylene blue, toluidine blue, or rose bengal) to produce reactive oxygen species and free radicals to induce apoptosis and decrease the fungal burden.46–49 Clinical studies with PDT are limited; however, two studies found the effectiveness of methylene blue PDT in the treatment of onychomycosis in 20 patients.48,49 An 11-percent mycological cure rate has been reported with laser in comparison to 29 to 61 percent for topical and oral antifungal treatment.50
Side effects. PDT is considered to be a safe and well-tolerated procedure. Side effects are generally mild and self-limited, mostly consisting of transient erythema, edema, mild pain, burning sensation, dry skin, and blisters.
Ease of use. The application and operation of the device on the nail require appropriate settings to optimize the fungal apoptosis.
Patient cost. The cost of PDT for onychomycosis can range from $100 to $1,500 per month.
Low-level laser therapy
Background and efficacy. Low-level laser therapy (LLLT), also known as photobiomodulation, has been utilized in various medical conditions, including onychomycosis with various wavelengths and energies (400–1064nm, 1–1000 mW).51 In one study in renal fibrotic tissue, there was a proliferative activity in renal cortices with the 532nm laser, increased endothelial proliferation with the 635nm laser or MSC plus 405nm, and reductions of TGF beta with 532nm laser alone and when combined with mesenchymal stem cells (MSC).52 In addition, LLLT uses specific wavelengths to stimulate cytochrome c oxidase in mitochondria to increase ATP and cAMP production.53 It is an excellent alternative treatment for onychomycosis patients who are unwilling or unable to take oral antifungal medication. While treating onychomycosis with LLLT, topical cream or brief oral medication should be considered for tinea pedis.
The 635nm and 405nm dual-wavelength diode laser (Lunula® laser; Erchonia, Daytona Florida) is an FDA-approved device that utilizes LLLT technology and delivers a combined 23.5-25.5mW 405nm and 15.5-17.5mW 635nm wavelengths by a rotating line-generated laser beam.54 The 405nm wavelength appears to provide antimicrobial, antibacterial, and antifungal effects while the 635nm wavelength appears to enhance the immunological function of resident neutrophils and macrophages to degrade the infectious agent and induce tissue rejuvenation.55 The delivery system ensures that all infected tissue nails and toes are properly targeted and treated via both photon concentration and treatment surface maximization.
In onychomycosis treatment, the combined 405 and 635 produce peroxynitrite, which is an oxidant and nitrating agent, and react with lipids, DNA, and proteins, leading to oxidative injury, inducing cell apoptosis.56 In addition, peroxynitrite has powerful cytotoxic effects on fungal pathogens and specifically on dermatophytic fungi, candida, and bacteria, thus is extremely effective in the eradication of skin pathogens.56 The FDA approved this device via a retrospective analysis of a compilation of 54 great toenails with varying degrees of involvement which were treated with four sessions two weeks apart.54 Digital photographs were taken before treatment and six months after the last treatment. The photographs were evaluated by a blinded investigator using the GNU Image Manipulation Program (GIMP 2.8) validated software system.54 Sixty-seven percent (67%) of toenails met the individual success criteria of 3mm or more of clear nail growth at six months following treatment compared to baseline, exceeding the pre-established goal of 60 percent.54 The mean increase in mm of clear nails across the six-month evaluation period was 5.18mm (p<0.0001).54
In another study with 30 onychomycosis patients, the affected toenails were treated with Lunula® laser for 12-minute weekly sessions for one month, and an additional one session at 3, 5, 7, 9, and 11 months.57 The result of the study entailed a 70 percent clinical cure rate and 95 percent mycological cure rate with an overall 70 percent complete cure rate.57
Side effects. Minimal side effects of LLLT have been reported, such as acne, burning sensation, dry skin, headache, and pruritus.58
Ease of use. Treatment frequency of LLLT was not standardized across the literature, ranging from daily to several times per week. However, patients can use the device at home or at the clinical office.
Patient cost. The cost of LLLT for onychomycosis can range from $100 to $1,500 for up to two months.
Procedural treatments
Background and efficacy. Routine procedures, such as debridement and trimming of the infected nails, can be considered in patients with onychomycosis recurrence or several traditional treatment failures to decrease the fungal burden in nails. Debridement allows for medications to better penetrate the nail bed. Surgical procedures can eliminate the entire fungal infection in some instances of limited onychomycosis, and dermatophytoma. Monotherapy with surgical procedures has shown less efficacy when compared to its use as an adjuvant to oral and topical therapy. In 2009, Malay et al59 investigated the effects of debridement versus debridement with topical antifungal therapy in 55 patients with onychomycosis. The study showed the combination therapy resulted in a significant 76.7 percent mycological cure, however, there was no mycological cure with debridement alone.59
Although more invasive procedures, such as avulsions or matrixectomies, are not routine treatments they can be offered to patients with thick and painful nails despite multiple therapeutic failures.60 Patients should be aware of permanent nail plate loss with these procedures prior to treatment. The avulsion procedure can be quite time-consuming and difficult, but it may lessen the painful pressure on nail folds due to onychomycosis. In 1985, Baran et al61 studied the effects of combination therapy with partial nail avulsions with antifungal in 20 patients, which resulted in a 100-percent cure rate in the course of 6 to 18 months. Yet, the compression force from the avulsion procedure may cause nail bed shrinkage and nail in-growing distal edge. As an alternative option, a week-long nail plate occlusion with 40 percent urea application can be utilized as a chemical avulsion without any associated pain.44
In the absence of improvement with combination therapy, matrixectomy should be considered to remove the nail and the matrix. Sequentially, 89% phenol or 10% sodium hydroxide is applied 30 seconds 2 to 4 times to the matrix to prevent a recurrence.62
The nail procedures are usually co-administered with other treatments to optimize the clinical benefits. It has been reported there are higher clinical cure rates in patients with nail debridement and oral terbinafine, rather than oral terbinafine alone.63 In another study, debridement and ciclopirox resulted in 77 percent of mycological cure in comparison to the usage of ciclopirox alone.64
Side effects. The majority of these procedures require local anesthesia to alleviate pain during the procedure. There is a risk of bleeding, tissue and bone infection, prolonged healing, cosmetic deformity, and the stunting of nail regrowth. In matrixectomy, the loss of a nail unit in its entirety can be debilitating due to limited activity of daily living (ADL).
Ease of use. Debridement, avulsion, and matrixectomy procedures are common in outpatient podiatry practices. Local or regional anesthesia may be required for avulsion and matrixectomy.
Patient cost. The one-time patient cost of these procedures ranges from less than $100 to $500.
Supplements and over-the-counter (OTC) Treatments
Background and efficacy. Tea Tree oil (TTO). TTO is derived from Melaleuca alternifolia, predominantly found in Australia. In-vitro studies demonstrate that TTO has an inhibitory effect on Candida spp., Microsporum spp. and Trichophyton spp.65,66 Gas chromatography has identified over 100 active ingredients in TTO.67 Buck et al68 compared TTO 100% to clotrimazole 2%, both applied twice daily to patients with onychomycosis for six months in combination with debridement at 0, 1, 3, and 6 months. At Month 6, treatment with TTO yielded an 18-percent cure rate, while the clotrimazole yielded an 11-percent cure rate.68 Three-month follow-up data showed about 56-percent clinical cure rate for the TTO group. A placebo-controlled trial showed that 2% butenafine hydrochloride combined with 5% TTO in a cream were efficacious, with a clinical cure rate of 80 percent, and was well tolerated.69 Clinical trials with larger sample sizes are needed to further assess the safety and efficacy of TTO.
Topical cough suppressant (TCS, Vicks VapoRub). TCS consists of camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%. It can be applied topically on the chest and muscles to relieve cough and pain.70 In vitro study has identified several key ingredients of TCS (camphor, menthol, thymol, and eucalyptus oil) for inhibiting fungal growth.71 The same author also conducted a respective chart review in 85 patients who applied TCS once daily, of which 32 patients (38%) had complete clearance after 5 to 16 months of treatment.71 A pilot study recruited 18 participants who were instructed to apply TCS at least once daily and achieved a complete cure rate of 83 percent.72 Clinical trials with larger sample sizes are needed to further assess the safety and efficacy of TCS.
Side effects. These OTC treatments for onychomycosis have been shown to be well tolerated with minimal to no significant adverse effects.
Ease of use. Patients are able to use these treatments at home, increasing ease of use and adherence.
Patient cost. OTC treatments for onychomycosis cost less than $100 per month.
Combination therapy
Background and efficacy. A caveat for monotherapy includes antifungal resistance and low bioavailability in the nail.10 The combination of multiple therapies tends to offer better results owing to broad coverage and rapid recovery due to its action along multiple steps of the cell wall synthesis pathway.73,74 Combination therapy should be considered in recalcitrant diseases.10 Combination therapy may also reduce recurrences.75 Combination therapy can be administered in two ways: parallel (at the same time) and sequential (one after another).75 Parallel treatment is recommended for patients who are likely to fail monotherapy; whereas sequential may benefit patients who are poor responders.75
In vitro study has demonstrated that synergy is a viable antifungal option.73 Currently, oral and topical synergy is the best-studied regimen in the literature.10 Oral drugs accumulate in the nail bed, whereas topical medications penetrate the nail plate and may contribute to the prevention of recurrence.75 However, the literature is scarce on this approach and heterogeneity exists in terms of endpoint assessed and treatment regimen.10 A recent review article examined 30 clinical trials (15 trials on medication plus device and 15 trials on medication only). For medication-only trials, oral terbinafine plus topical amorolfine in addition to oral terbinafine and topical ciclopirox were the most frequently reported treatments. The superiority of dual therapies versus monotherapy was supported in 9 out of 15 studies. For instance, a randomized trial recruited over 200 patients with matrix involvement who were treated with amorolfine nail lacquer 5% once weekly plus oral terbinafine 250mg once daily compared with oral terbinafine alone. A significantly higher success rate was seen in the combination group at the 18-month follow-up. Furthermore, the dual therapies approach is more affordable than monotherapy.76
For the medication and device trials, almost all the studies (14/15) revealed that dual therapies are advantageous over monotherapy. Laser treatment and oral terbinafine were the most commonly reported procedures and medications, respectively.10 A comparison study assessing fractional 2940-nm Er: YAG laser and 5% amorolfine lacquer versus amorolfine alone found that dual therapies are superior in regards to onychomycosis severity index (OSI) and fungal culture.77 However, the beneficial effect of combination therapy is less obvious in nails with high OSI at baseline.77
The limitations of the aforementioned clinical trials include a short follow-up period, reproducibility, limited information on long-term recurrence, and small sample size, thus, cautious extrapolation is warranted.
Plasma therapy
Background and efficacy. Plasma therapy is a newly investigated, effective, non-invasive treatment for onychomycosis with an ongoing clinical trial study.78,79 Plasma therapy can be further categorized into thermal atmospheric plasma (TAP) and non-thermal atmospheric plasma (NTAP).4 NTAP is tolerated better due to the lack of heat-induced pain.4 NTAP tissue has been used for other medical applications for two decades.78,80 The broad-spectrum antifungal effect of NTAP is achieved via the generation of ozone, hydroxyl radicals, and nitric oxide. The passage of air by strong electrical current produces these antifungal components which have been shown to inactivate Trichophyton rubrum and Microsporum canis in vitro.4,78 NTAP therapy in onychomycosis has shown a 53.8 percent and 15.4 percent improvement in clinical cure rate and mycologic cure rate respectively
Side Effects. Plasma therapy does not have systemic side effects. NTAP therapy does not produce significant heat which leads to pain in comparison to TAP.78
Ease of use. Plasma therapy can be individualized based on various nail textures and curvatures.78 Several affected nails can receive spontaneous treatment in a controlled and repeatable fashion if needed.78
Patient cost. Patient cost of treatment for onychomycosis with plasma therapy can range from $100 to $500 monthly.
Ethical considerations in treating onychomycosis
The hallmark of medical ethics is to have a detailed and honest counsel with patients regarding treatment efficacy, side effect profiles, ease of use (need for adherence), and costs absolutely independent of the potential benefit to the clinician.81 Recommendations should be based on the clinician’s familiarity with certain remedies, the quality, and the quantity of current clinical evidence, instead of the monetary benefit to the clinician. Creating an algorithm for treating onychomycosis provides clinicians with a foundation to triage available therapies based on clinical factors, and an ethical framework encourages clinicians to tailor treatment regimens according to patient’s needs and expectations, including preferences, budget, and aesthetic goals. A subset of patients may choose to decline treatment due to indifference to the cosmetic appearance or concerns about the potential adverse effects of therapies. Patients should understand the significance of treatment adherence and motivation when treating their onychomycosis, as well as treatment practicality. Preferences are critical to adherence that is, many individuals will not apply a topical agent once or twice daily and/or do not have the time or the budget to undergo some of the more costly options. The clinician must recognize the risk/benefit balance of any treatment and clearly convey this to patients. Any treatment option requires long-term adherence, because recurrence is common. Patient education is a paramount part of ensuring optional results, otherwise, treatment cessation can cause loss of any achieved outcomes and induce medication resistance.
Consideration of efficacy. Onychomycosis treatment can be complex. Physicians and patients might use different criteria to consider a treatment efficacious for this disease that entails high reinfection and recurrence rates. Many patients seek treatment to prevent the spread of infection to themselves and their family members. Asymptomatic patients are usually disturbed by the cosmetic appearance of this condition and they are satisfied with the clinical cure and nail clearance results alone. However, physicians consider the complete cure which consists of the clinical cure and mycological cure to assess the efficacy of a treatment. Physicians associate a more efficacious treatment with higher clinical and mycological cures. However, it is important to note mycological cure rate can be 5 to 20 percent false-negative, and direct microscopy can be 53 to 94 percent sensitivity in patients with significant improvement in clinical cure improvement.82
All the onychomycosis treatments discussed in this review appear to have some efficacy, however, oral terbinafine and low-level laser therapy are considered more efficacious treatments due to higher clinical and mycological cure rates.
Consideration of side effect profiles. Many of the side effect concerns involve oral medication treatment regimens, as this is a common treatment method and patients must take the medications for several months. These include manageable, yet irritating side effects like gastrointestinal upset and cutaneous reactions. In addition, oral medications have the potential to cause liver toxicity, drug-drug interactions, and other more serious side effects.
Avulsions or matrixectomies can cause significant morbidity due to the procedures themselves and significant healing time. Short-term side effects like bleeding, pain, and infection should be considered along with long-term side effects like prolonged healing time in older patients and cosmetic deformity.
Low-level light therapy, topical medications, plasma therapy, and natural supplements offer very low side effect profiles. Overall, the risk of side effects alone should not deter physicians from treating onychomycosis which most are mild and manageable. In the case that more serious side effects arise, treatment can be ceased.
Consideration of ease of use (adherence). Patients’ medical comorbidities, availability, funding, and treatment goals are the factors that can affect their adherence with a particular treatment. As the majority of onychomycosis treatments require long-term treatment, it is vital for physicians to educate patients on the chronic nature of onychomycosis, which entails high reinfection and recurrence rates. Before prescribing any treatment, physicians must evaluate and determine if therapeutic intervention is needed and necessary for a patient. Physicians should consider the factors that affect patient adherence with a certain intervention if treatment is warranted.
Asymptomatic and low-risk patients may see any treatments as a burden to the quality of their lives, especially if they are not bothered by the cosmetic appearance of their nails. However, treatments for symptomatic and high-risk patients are warranted and encouraged. Patients may consider topical or oral treatment as more feasible to add to their daily routines. In others, they may prefer treatment modalities with less frequent treatment sessions, such as LLLT, despite being more expensive. Some patients may adhere better to non-oral treatments to avoid systematic side effects or drug interactions with medications they take for their comorbidities management.
Consideration of cost. When advising patients on appropriate onychomycosis treatment options, it is important to consider patients’ insurance and socioeconomic status. Onychomycosis is not a self-limited condition and requires a prolonged treatment course, and in some cases, further management is needed due to posttreatment recurrence. Physicians should discuss the nature of this chronic disease and associated expenses with each treatment modality to avoid the financial burden on patients and to increase patient adherence. Unnecessary costs of treatment to patients and the healthcare system can be prevented with only supervision if patients are not symptomatic and at high risk.
Overall, oral treatment is the least expensive of the treatments discussed here. Physicians should only consider combination therapies for patients who are able to afford multiple modality treatments. Supplements and over-the-counter treatments are not generally covered by insurance, and this increases the financial responsibilities to patients.
Lastly, it is important to remember and understand financial biases when offering in-office procedures. Laser, LLLT, PDT, and plasma therapy are more expensive procedures for patients that may potentially compensate physicians financially. Therefore, physicians should be mindful and conscious of their own gained interests when deciding these more costly treatments over less expensive options.
Patient and physician choice of treatment options. The physician and patient must first decide whether or not to treat onychomycosis. While it does not generally pose a significant risk to a patient’s physical health, onychomycosis is an infection and causes other dermatophytes, such as tinea cruris and corporis. It also can be cosmetically disfiguring and cause psychological distress.
For instance, oral therapies offer a decent cure rate at a low cost, but their systemic administration for several months increases the risk of side effects. On the other hand, topical therapies have a very low risk of side effects but are less effective than oral therapies, relatively expensive, and require long-term treatment. Combination therapies have proven to be the most effective, and given the treatment options available, would not significantly increase the risk of side effects. However, combination therapies increase the cost and decrease the ease of use.
As with the treatment of any disease, the decision should be collaborative between the physician and the patient. A thorough discussion about the efficacies, side effects, ease of use, and costs of the various treatment options should be discussed with the patients in order to achieve the best treatment outcome and patient satisfaction.
Conclusion
Onychomycosis is the most highly prevalent nail disorder reported in the clinical setting. Despite the chronic and benign clinical course, onychomycosis has high reinfection and recurrence rates even after months of treatment. This disease is not self-limited, and the management of onychomycosis can be complex. In order to lower the severity and recurrence rate of onychomycosis, individuals should be educated on hygiene behavior, nail tools sanitization, shoe-selection-induced nail trauma, sports participation, immunosuppression state, and early signs of infection relapse to prevent therapeutic failures and recurrence. Empiric antifungal treatment without a confirmatory diagnosis may lead to antifungal resistance plus unnecessary side effects, lack of symptomatic improvement, and increased risk of morbidity and mortality. Alternative treatment modalities such as devices may be the best options since antifungal resistance has increased in prevalence,
Physicians have an ethical responsibility to patients to educate them on onychomycosis and the available treatment modalities. Onychomycosis is a chronic illness with a high risk of posttreatment recurrence. The vast majority of patients are asymptomatic and are only disturbed by the cosmetic nature of the disease. Treatment should be saved for symptomatic and high-risk patients whose quality of life has been affected. Physicians and patients should work together to make informed decisions and select appropriate treatments. Prior to onychomycosis treatment, physicians should review the efficacy and side effects profile, and the financial consideration of each intervention for better patient compliance.
References
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: An Updated Review. Recent Pat Inflamm Allergy Drug Discov. 2020;14(1):32–45.
- Pariser DM Md. Efficacy and Safety of Onychomycosis Treatments: An Evidence-Based Overview. Semin Cutan Med Surg. 2015;34(3 Suppl):S46–S50.
- Tosti A, Elewski BE. Onychomycosis: Practical Approaches to Minimize Relapse and Recurrence. Skin Appendage Disord. 2016;2(1-2):83–87.
- Lipner SR, Scher RK. Onychomycosis: Treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80(4):853–867.
- Asz-Sigall D, López-García L, Vega-Memije ME, et al. HLA-DR6 association confers increased resistance to T. rubrum onychomycosis in Mexican Mestizos. Int J Dermatol. 2010;49(12):1406–1409.
- Zhou P, Xie Y, Yan Z, et al. Association between dectin-1 gene single nucleotide polymorphisms and fungal infection: a systemic review and meta-analysis. Biosci Rep. 2019;39(11).
- Klafke GB, Silva RA da, Pellegrin KT de, et al. Analysis of the role of nail polish in the transmission of onychomycosis. An Bras Dermatol. 2018;93(6):930–931.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11(3):415–429.
- Monod M, Méhul B. Recent Findings in Onychomycosis and Their Application for Appropriate Treatment. J Fungi (Basel). 2019;5(1).
- Falotico JM, Lapides R, Lipner SR. Combination Therapy Should Be Reserved as Second-Line Treatment of Onychomycosis: A Systematic Review of Onychomycosis Clinical Trials. J Fungi (Basel). 2022;8(3).
- Singh SK, Patwa DK, Tilak R, et al. In vitro susceptibility of dermatophytes to oral antifungal drugs and amphotericin B in Uttar Pradesh, India. Indian J Dermatol Venereol Leprol. 2019;85(4):388–392.
- Hay RJ. The Spread of Resistant Tinea and the Ingredients of a Perfect Storm. Dermatology. 2022;238(1):80–81.
- Kawa N, Lee KC, Anderson RR, et al. ONYCHOMYCOSIS: A Review of New and Emerging Topical and Device-based Treatments. J Clin Aesthet Dermatol. 2019;12(10):29–34.
- Jarratt M, Siu WJ, Yamakawa E, et al. Safety and pharmacokinetics of efinaconazole 10% solution in healthy volunteers and patients with severe onychomycosis. J Drugs Dermatol. 2013;12(9):1010–1016.
- Elkeeb R, Hui X, Murthy N, et al. Emerging topical onychomycosis therapies – quo vadis? Expert Opin Emerg Drugs. 2014;19(4):489–495.
- Gupta AK, Mays RR, Versteeg SG, et al. Update on current approaches to diagnosis and treatment of onychomycosis. Expert Rev Anti Infect Ther. 2018;16(12):929–938.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7(7):10–18.
- Subissi A, Monti D, Togni G, et al. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70(16):2133–2152.
- Gupta AK, Joseph WS. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J Am Podiatr Med Assoc. 2000;90(10):495–501.
- Coronado D, Merchant T, Chanda S, Zane LT. In vitro nail penetration and antifungal activity of tavaborole, a boron-based pharmaceutical. J Drugs Dermatol. 2015;14(6):609–614.
- Vlahovic T, MPharm TM, Chanda S, et al. In Vitro Nail Penetration of Tavaborole Topical Solution, 5%, Through Nail Polish on Ex Vivo Human Fingernails. J Drugs Dermatol. 2015;14(7):675–678.
- Zeichner JA, Stein Gold L, Korotzer A. Penetration of ((14)C)-Efinaconazole Topical Solution, 10%, Does Not Appear to be Influenced by Nail Polish. J Clin Aesthet Dermatol. 2014;7(9):34–36.
- Gupta AK, Daigle D, Foley KA. Network Meta-Analysis of Onychomycosis Treatments. Skin Appendage Disord. 2015;1(2):74–81.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68(4):600–608.
- Jones T, Tavakkol A. Safety and tolerability of luliconazole solution 10-percent in patients with moderate to severe distal subungual onychomycosis. Antimicrob Agents Chemother. 2013;57(6):2684–2689.
- Gupta AK, Stec N. Emerging drugs for the treatment of onychomycosis. Expert Opin Emerg Drugs. 2019;24(4):213–220.
- Shemer A, Gupta AK, Kamshov A, et al. Topical antifungal treatment prevents recurrence of toenail onychomycosis following cure. Dermatol Ther. 2017;30(5).
- McClellan KJ, Wiseman LR, Markham A. Terbinafine. An update of its use in superficial mycoses. Drugs. 1999;58(1):179–202.
- De Beule K, Van Gestel J. Pharmacology of itraconazole. Drugs. 2001;61 Suppl 1:27–37.
- Terbinafine hydrochloride label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27(3):267–272.
- Itraconazole label. Available at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020083s062lbl.pdf
- De Backer M, De Keyser P, De Vroey C, et al. A 12-week treatment for dermatophyte toe onychomycosis: terbinafine 250 mg/day vs. itraconazole 200 mg/day–a double-blind comparative trial. Br J Dermatol. 1996;134 Suppl 46:16–17: discussion 38.
- Bräutigam M, Nolting S, Schopf RE, et al. German randomized double-blind multicentre comparison of terbinafine and itraconazole for the treatment of toenail tinea infection. Br J Dermatol. 1996;134 Suppl 46:18–21: discussion 38.
- Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37(5 Pt 1):740–745.
- Elewski BE, Scher RK, Aly R, et al. Double-blind, randomized comparison of itraconazole capsules vs. placebo in the treatment of toenail onychomycosis. Cutis. 1997;59(4):217–220. pubmed/9104548
- Gupta AK, Foley KA, Mays RR, et al. Monotherapy for toenail onychomycosis: a systematic review and network meta-analysis. Br J Dermatol. 2020;182(2):287–299.
- Gupta AK, Katz HI, Shear NH. Drug interactions with itraconazole, fluconazole, and terbinafine and their management. J Am Acad Dermatol. 1999;41(2 Pt 1):237–249.
- Hall M, Monka C, Krupp P, et al. Safety of oral terbinafine: results of a postmarketing surveillance study in 25,884 patients. Arch Dermatol. 1997;133(10):1213–1219.
- Haria M, Bryson HM, Goa KL. Itraconazole. A reappraisal of its pharmacological properties and therapeutic use in the management of superficial fungal infections. Drugs. 1996;51(4):585–620.
- Elewski BE. Onychomycosis. Treatment, quality of life, and economic issues. Am J Clin Dermatol. 2000;1(1):19–26.
- Gupta AK, Chang P, Rosso JQ, et al. Hofstader SLR. PHARMACOLOGY AND THERAPEUTICS: Onychomycosis in children: Prevalence and management. Pediatr Dermatol. 2009;15(6):464–471.
- Gupta AK, Gupta G, Jain HC, et al. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30 000 patients visiting physicians’ offices. J Eur Acad Dermatol Venereol. 2016;30(9):1567–1572.
- Gupta AK, Stec N, Summerbell RC, et al. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020;34(9):1972–1990.
- Rodriguez DA. Efinaconazole Topical Solution, 10%, for the Treatment of Mild and Moderate Toenail Onychomycosis. J Clin Aesthet Dermatol. 2015;8(6):24–29.
- Piraccini BM, Alessandrini A. Onychomycosis: A Review. J Fungi (Basel). 2015;1(1):30–43.
- Christenson JK, Peterson GM, Naunton M, et al. Challenges and Opportunities in the Management of Onychomycosis. J Fungi (Basel). 2018;4(3).
- Morgado LF, Trávolo ARF, Muehlmann LA, et al. Photodynamic Therapy treatment of onychomycosis with Aluminium-Phthalocyanine Chloride nanoemulsions: A proof of concept clinical trial. J Photochem Photobiol B. 2017;173:266–270.
- Alberdi E, Gómez C. Efficiency of methylene blue-mediated photodynamic therapy vs intense pulsed light in the treatment of onychomycosis in the toenails. Photodermatol Photoimmunol Photomed. 2019;35(2):69–77.
- Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31(7):1111–1118.
- Hamblin MR. Photobiomodulation or low-level laser therapy. J Biophotonics. 2016;9(11-12):1122–1124.
- O’Connor M, Patil R, Yu J, et al. Mesenchymal Stem Cells Synergize with 635, 532, and 405 nm Laser Wavelengths in Renal Fibrosis: A Pilot Study. Photomed Laser Surg. 2016;34(11):556–563.
- Hashmi JT, Huang YY, Osmani BZ, et al. Role of low-level laser therapy in neurorehabilitation. PM R. 2010;2(12 Suppl 2):S292–S305.
- Zang K, Sullivan R, Shanks S. A Retrospective Study of Non-thermal Laser Therapy for the Treatment of Toenail Onychomycosis. J Clin Aesthet Dermatol. 2017;10(5):24–30.
- Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52.
- Buchczyk DP, Briviba K, Hartl FU, et al. Responses to peroxynitrite in yeast: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a sensitive intracellular target for nitration and enhancement of chaperone expression and ubiquitination. Biol Chem. 2000;381(2):121–126.
- Zang K, Sammons TM, Shanks S. Treatment of Toenail Onychomycosis using Laser PharmacologyTM. Int J Dermatol Clin Res. Published online January 29, 2021:001-006.
- Darwin E, Heyes A, Hirt PA, et al. Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med Sci. 2018;33(2):425–434.
- Malay DS, Yi S, Borowsky P, et al. Efficacy of debridement alone versus debridement combined with topical antifungal nail lacquer for the treatment of pedal onychomycosis: a randomized, controlled trial. J Foot Ankle Surg. 2009;48(3):294–308.
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown Toenail Management. Am Fam Physician. 2019;100(3):158–164.
- Baran R, Hay RJ. Partial surgical avulsion of the nail in onychomycosis. Clin Exp Dermatol. 1985;10(5):413–418.
- Ozdemir E, Bostanci S, Ekmekci P, et al. Chemical matricectomy with 10% sodium hydroxide for the treatment of ingrowing toenails. Dermatol Surg. 2004;30(1):26–31.
- Jennings MB, Pollak R, Harkless LB, et al. Treatment of toenail onychomycosis with oral terbinafine plus aggressive debridement: IRON-CLAD, a large, randomized, open-label, multicenter trial. J Am Podiatr Med Assoc. 2006;96(6):465–473.
- Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol. 2004;150(3):537–544.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13(4):377–383.
- Nenoff P, Haustein UF, Brandt W. Antifungal activity of the essential oil of Melaleuca alternifolia (tea tree oil) against pathogenic fungi in vitro. Skin Pharmacol. 1996;9(6):388–394.
- Brophy JJ, Davies NW, Southwell IA, et al. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37(5):1330–1335.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38(6):601–605.
- Syed TA, Qureshi ZA, Ali SM, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Trop Med Int Health. 1999;4(4):284–287.
- Vicks ® VapoRub ®. Accessed September 19, 2022. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=6b16b47e-9cbe-4e62-825b-f65416c900a1&type=display
- Ramsewak RS, Nair MG, Stommel M, Selanders L. In vitro antagonistic activity of monoterpenes and their mixtures against “toe nail fungus” pathogens. Phytother Res. 2003;17(4):376–379.
- Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24(1):69–74.
- Evans EGV. Drug synergies and the potential for combination therapy in onychomycosis. Br J Dermatol. 2003;149 Suppl 65:11–13.
- Gupta AK, Cernea M, Foley KA. Improving Cure Rates in Onychomycosis. J Cutan Med Surg. 2016;20(6):517–531.
- Olafsson JH, Sigurgeirsson B, Baran R. Combination therapy for onychomycosis. Br J Dermatol. 2003;149 Suppl 65:15–18.
- Baran R, Sigurgeirsson B, de Berker D, et al. A multicentre, randomized, controlled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol. 2007;157(1):149–157.
- Zhang J, Lu S, Huang H, et al. Combination therapy for onychomycosis using a fractional 2940-nm Er:YAG laser and 5 % amorolfine lacquer. Lasers Med Sci. 2016;31(7):1391–1396.
- Bulson JM, Liveris D, Derkatch I, et al. Non-thermal atmospheric plasma treatment of onychomycosis in an in vitro human nail model. Mycoses. 2020;63(2):225–232.
- Lipner SR, Friedman G, Scher RK. Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin Exp Dermatol. 2017;42(3):295–298.
- Laroussi M. Plasma Medicine: A Brief Introduction. Plasma. 2018;1(1):47–60.
- Stoff BK, Scully K, Housholder AL, et al. The American Academy of Dermatology (AAD) Ethics Pledge: I will put my patients’ welfare above all other interests, provide care that adheres to professional standards of practice, provide care for those in need, and foster collegiality through interaction with the medical community. J Am Acad Dermatol. 2016;75(2):445–448.
- The problems with our current definition of cure in onychomycosis. J Am Acad Dermatol. 2009;60(3):AB117.