J Clin Aesthet Dermatol. 2024;17(3):34–41.
J Clin Aesthet Dermatol. 2024;17(3):34–41.
by Toral Vaidya, MD, MPH; Lauren Hoffman, MD; and Anne Chapas, MD
Dr. Vaidya is with the Weill Cornell Department of Dermatology in New York, New York. Drs. Hoffman and Chapas are with UnionDerm in New York, New York.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the contents of this article.
ABSTRACT: Objective. Acne vulgaris is a common skin condition treated with various medications targeting different aspects of its pathogenesis. Though increasing in popularity, the United States Food and Drug Administration (FDA) does not evaluate the safety or efficacy of dietary supplements marketed for the treatment of acne, calling into question the veracity of their labels. This review aimed to assess the safety and effectiveness of ingredients in popular acne supplements.
Methods. A comprehensive review was conducted on 13 popular supplements marketed for acne, found through a Google search. Their ingredients, prices, ratings, and existing literature on efficacy and safety were analyzed. A literature review was performed regarding the most common ingredients contained in these supplements.
Results. The most common ingredients in acne supplements were probiotics, diindolylmethane (DIM), vitamin A, vitamin B complex, and zinc. Despite the increasing popularity of dietary supplements, including those for skin health and acne, the absence of FDA regulation and evidence-based data raises concerns about their safety and efficacy. The safety of acne supplement ingredients raises significant worries, with reported cases of thrombotic events and adverse effects, even during pregnancy. The lack of standardized labeling and clear dosing information further complicates the understanding and potential risks of these supplements. Additionally, there is a potential for interactions with other medications, yet this information is often not provided on the product labels.
Limitations. A Google search was used to identify popular acne supplements. Search engine algorithms determine the ranking and presentation of results based on various factors, such as popularity, keywords, as well as user preferences and location, thus posing a potential sampling bias.
Conclusion. It is crucial to exercise caution and prioritize evidence-based information when counseling patients regarding the use of acne supplements.
Keywords: Acne vulgaris, supplements, skincare
Acne vulgaris is a common chronic inflammatory disorder of the skin, particularly in young adults and adolescents. While its pathogenesis is complex, it is widely accepted that the pathophysiology involves a combination of hyperkeratinization, excess sebum production stimulated by androgens, and microbial colonization of pilosebaceous units by Propionibacterium acnes.1 Treatments for acne usually target one or more of these steps in its pathogenesis and can be administered orally or topically. Common examples of treatment include retinoids, antibiotics, antibacterial agents, and comedolytic agents.
In addition to these traditional treatments, there are many over-the-counter dietary supplements that claim to target acne. In a survey distributed to acne patients at a dermatology clinic, respondents reported that they believed acne supplements could help with acne,2 suggesting that people may be turning to supplements for adjunctive treatment. However, dietary supplements are not evaluated with the same rigor and standard as medications by the United States Food and Drug Administration (FDA).3 As a result, the safety and efficacy of individual supplements is often unknown. Furthermore, supplement labels can be misleading and may even contain inaccurate information. For example, Zamil et al,4 found that some acne supplements containing Vitamin A exceeded the daily level of intake that meets nutritional needs, with some supplements failing to include a pregnancy warning and posing a teratogenic risk.
The objective of this review was to investigate the efficacy and safety of specific ingredients listed in popular acne supplements.
Methods
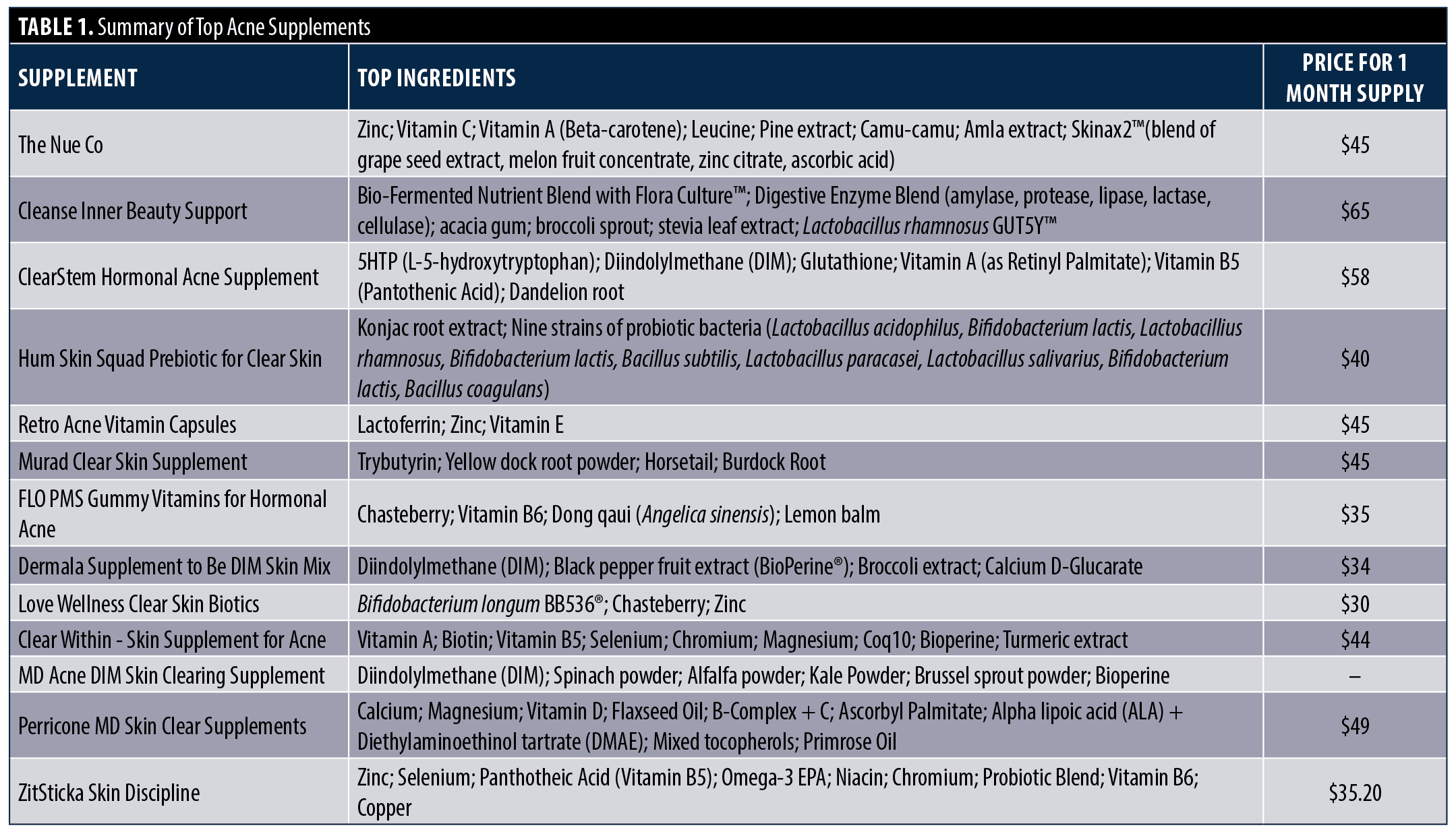
A Google search was performed using the key term “acne supplement” with default search settings and ranking items based on relevance. The top 13 dietary supplements specifically indicated for the treatment of acne were identified between March 1, 2023, and April 1, 2023, and were reviewed based on price, ratings, and ingredient profiles (Table 1). For each supplement, the highlighted ingredients were reviewed and categorized. A literature search was performed of the most listed ingredients regarding treatment efficacy and safety profiles.
Results
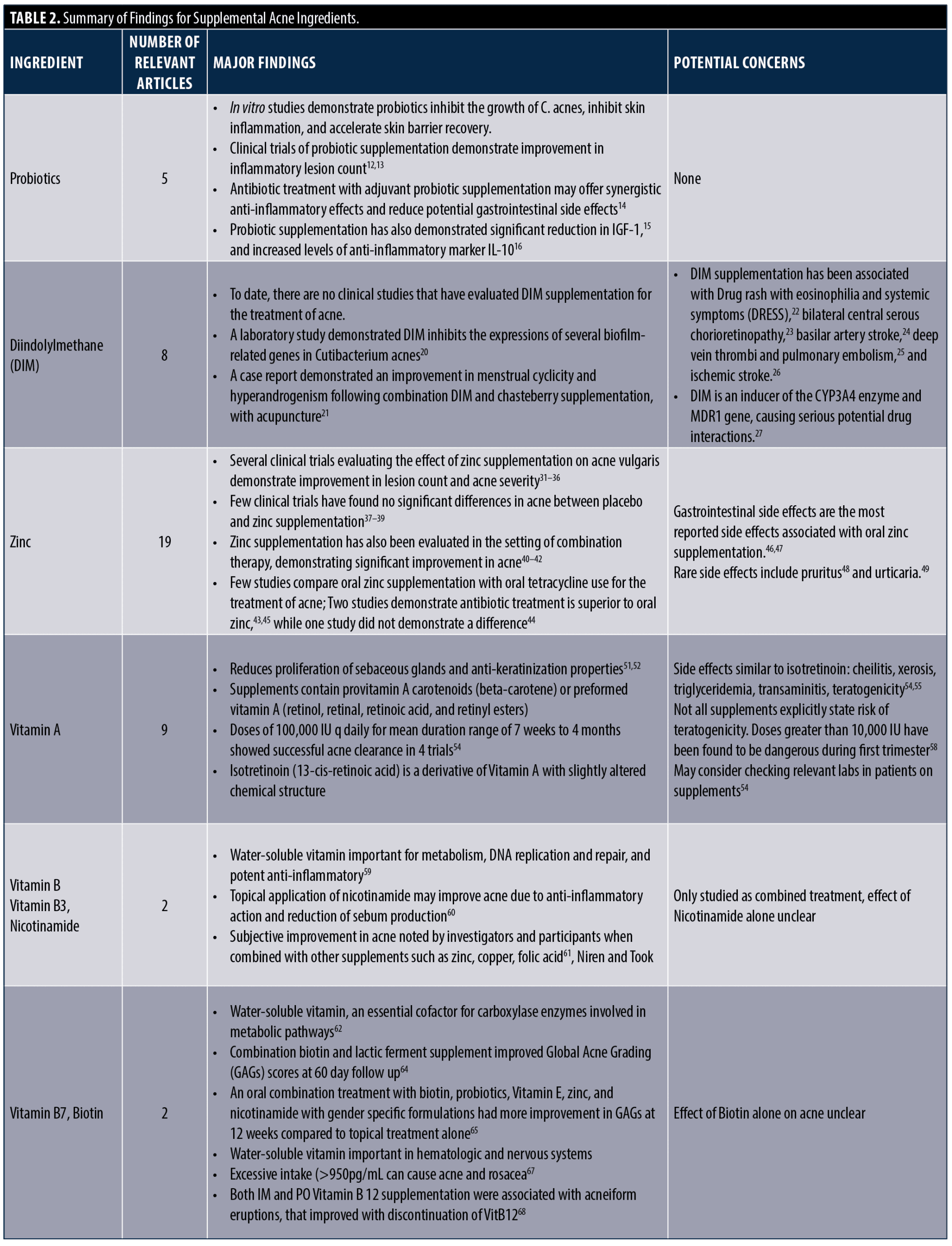
The most common ingredients contained in most popular supplements marketed for treating acne vulgaris were identified, including probiotics, diindolylmethane (DIM), vitamin A, vitamin B complex, and zinc. A full list of ingredients found in the most popular supplements are listed in Table 1. A summary of supplement evidence is listed in Table 2.
Probiotics. There is growing evidence to support the use of probiotics in the treatment of acne. The formation of acne depends on multifactorial causes, including follicular hyperkeratinization, excess sebum production, P. acnes colonization, and inflammation.5 Probiotics supplementation is thought to impact several factors that play a pathophysiological role in the formation of acne. In vitro studies have demonstrated the ability of probiotic strains to inhibit the growth of acne-causing bacteria through various mechanisms including promoting an anti-inflammatory response, improving skin barrier function, and forming ceramides.
In vitro studies have demonstrated the ability of probiotic strains such as Streptococcus6 and Lactococcus7,8 to inhibit the growth of C. acnes; the probiotic Bifidobacterium has shown antimicrobial properties against both C. acnes and S. aureus.9 In vitro studies involving Lactobacillus have also demonstrated the ability of probiotics to inhibit skin inflammation as well as accelerate skin barrier function recovery.10 In addition, strains of Streptococcus thermophilus have been shown to increase the production of ceramides, which not only promote skin hydration, but also have antimicrobial activity against C. acnes.11
Trials investigating the effects of probiotics in acne patients are limited. The first clinical trial was performed by Siver et al in 1961.12 In this study, a mixture of Lactobacillus acidophilus and Lactobacillus bulgaricus administered orally to 300 patients with acne (treatment for eight days, followed by two weeks of washout followed by two more weeks of treatment) demonstrated varying levels of improvement in 80 percent of patients; effects were more evident in cases of inflammatory acne. A 2010 study by Kim et al13 investigating the effects of lactoferrin-enriched fermented milk in 36 patients demonstrated a significant reduction in the number of inflammatory lesions, total number of lesions, degree of acne severity, amount of sebum, free fatty acids, and triglycerides.
A 2013 study by Jung et al14 evaluated the impact of a probiotic mixture of Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum strains on acne, minocycline alone, and combination treatment with probiotics and minocycline. At Week 8, patients in the combination treatment group showed significantly greater improvement in total number of lesions compared to the other study groups, suggesting that antibiotic treatment with adjuvant probiotics may offer synergistic anti-inflammatory effects and reduce potential gastrointestinal adverse effects of prolonged antibiotic therapy.
Studies have also demonstrated that probiotic supplementation in patients with acne is associated with a reduction in inflammatory markers, and an increase in anti-inflammatory markers. A 2016 study by Fabbrocini et al15 involved a double-blind, placebo-controlled, randomized clinical trial in 20 adult subjects to assess the efficacy of the oral probiotic Lactobacillus rhamnosus on acne and expression of insulin-like growth factor 1 (IGF-1) and forkhead box protein O1 (FoxO1). Patients in the probiotic arm of the study showed a statistically significant reduction in the expression of the IGF-1, a statistically significant increase in the FOXO1 gene, which mediates inhibitor action on insulin and IGF-1, and an improvement in acne.15 Because dysregulation of insulin signaling has been implicated in the pathogenesis of adult acne, it is thought that supplementation with the probiotic strain Lactobacillus rhamnosus SP1 (LSP1) normalizes skin expression of genes involved in insulin signaling and subsequently improves the appearance of adult acne. A study in 2019 by Rahmayani et al. investigated the effects of a probiotic mixture containing strains of Bifidobacterium and Lactobacillus on interleukin (IL)-10 levels in 33 patients with acne. An increase in levels of the anti-inflammatory IL-10 was observed after treatment with the probiotic mixture.16
DIM. DIM is a metabolite of indole-3-carbinol (I3C), which is a compound found in cruciferous vegetables such as broccoli, cauliflower, and cabbage. In vitro and animal studies suggest DIM has anti-inflammatory17 and chemoprotective effects;18 however, studies in humans are quite limited. Recently, DIM supplements have gained popularity for the treatment of acne, due to their ability to modulate estrogen metabolism.19 DIM has been shown to inhibit aromatase, which converts testosterone to estrogen.19
To date, there are no clinical studies that have evaluated DIM supplementation for the treatment of acne. A laboratory study demonstrated DIM inhibits the expressions of several biofilm-related genes in Cutibacterium acnes.20 A case report by Alois et al. demonstrated an improvement in menstrual cyclicity, as well as clinical and biochemical hyperandrogenism following 10-month supplementation with a botanical supplement containing Vitex agnus-castus (chasteberry) and DIM, in addition to acupuncture.21
The safety of DIM supplementation remains a concern. DIM has been associated with drug rash with eosinophilia and systemic symptoms (DRESS), a severe and potentially fatal drug reaction. In a case report by Le at al,22 a 36-year-old female patient developed DRESS four weeks following ingestion of a supplement consisting of DIM complex with diindolylmethane; drug causality was established through a drug patch test.22 Another report demonstrates a case of bilateral central serous chorioretinopathy following excessive daily consumption of DIM for two months.23 In this case, the patient was instructed to discontinue DIM and reported that visual improvement began two weeks after, with resolution to baseline after eight weeks.23
There have been several case reports linking DIM use to thrombotic events, including basilar artery stroke in a healthy female patient,24 and deep vein thrombi and pulmonary embolism in a healthy, middle-aged male patient.25 The doses of DIM were unknown in both cases. An ischemic stroke has been reported in a healthy young female patient who ingested 200mg DIM daily for a few months.26 Increasing evidence supports the impact of DIM on increasing hypercoagulable states; however, to date, there are no studies assessing potential negative effects of DIM in healthy individuals. Further studies are needed to elucidate any potential effects of this supplement.
Of note, DIM is an inducer of CYP3A4 and MDR1; altered levels of these enzymes can significantly affect the therapeutic response of a variety of co-administered drugs and can cause serious drug interactions.27
Zinc. Zinc supplementation has been proposed to be an alternative for treating inflammatory skin conditions, including acne. Zinc demonstrates anti-inflammatory properties through various mechanisms: decreasing neutrophil chemotaxis,28 inhibiting T helper-17 cell activity,29 and downregulating the expression of Toll-like receptor 2 (TLR-2) from keratinocytes.30 There is conflicting data in the literature regarding the use of oral zinc supplementation in the treatment of acne.
A study by Drono et al31 describing a multicenter, double-blind, controlled trial that included 66 patients demonstrated statistically significant reduction of inflammatory acne lesion count after two months of oral zinc gluconate administration. A study by Meynadier et al32 in 2000 demonstrated a significant improvement in superficial inflammatory acne lesion count after treatment of oral zinc gluconate with both standard regimens (100mg capsules, 2 capsules for 13 weeks) and loading dose regimens (60mg for 3 weeks, followed by 30mg for 4 weeks, followed by 15 mg for 6 weeks).32 A double-blind randomized, controlled trial (RCT) by Goransson et al33 which included 59 subjects demonstrated a significant improvement in total acne lesion count and acne severity index scores after six weeks of zinc supplementation, compared to placebo.33 A double blind placebo-controlled trial by Verma et al34 demonstrated a significant decrease in acne papule count, infiltrate count, and cyst count after 12 weeks of oral zinc supplementation, compared to placebo. A double-blind RCT by Liden et al35 that included 54 patients demonstrated a statistically significant improvement in lesion count and acne severity score in patients treated with oral zinc sulfate compared to placebo, after six weeks of treatment. A case report by Kobayashi et al36 of a patient with acne conglobata and cystic acne demonstrated a regression of nodules after four weeks of zinc supplementation.
Several studies have found no significant differences between oral zinc supplementation and control groups in the treatment of acne as well. A double blind RCT by Orris et al37 that included 30 male subjects with moderate acne demonstrated no statistically significant differences in papule, pustule, or comedone count in groups treated with oral zinc versus lactose placebo for eight weeks.37 A double blind RCT by Weimar et al38 demonstrated no significant difference in pustule count and acne severity index between oral zinc sulfate and placebo groups after 12 weeks of treatment. Similarly, a double blind RCT by Weissman et al39 demonstrated no significant difference in inflammatory lesion and infiltrate count after 12 weeks of zinc supplementation, compared to placebo.
Zinc has been evaluated in combination therapy for the treatment of acne as well. In a 2017 RCT by Chan et al,40 lactoferrin in combination with zinc and vitamin E demonstrated a significant reduction in comedones, inflammatory lesions, and sebum production after 10 weeks of treatment. A multicenter, open-label prospective study by Shalita et al41 evaluating the effect of a supplement NicAzel (nicotinamide, azelaic acid, zinc oxide, pyridoxine, copper, and folic acid) found a significant reduction in lesion count when given in addition to the patients’ acne regimen. An observational study by Sardana et al42 demonstrated that treatment with APC complex, a methionine-based zinc complex with antioxidants, resulted in a significant improvement in global acne count, pustules, papules, and closed comedone count; there was no statistically significant difference in nodule and open comedone count.
There are also a few studies in the literature comparing oral zinc supplementation and oral tetracycline use. A double blind RCT by Cunliffe et al43 comparing zinc sulfate versus tetracycline in patients with moderate to severe acne demonstrated that oral zinc sulfate was statistically inferior to tetracycline regarding improvement in comedone, papule, pustule, and nodule count. A double-blind study by Michaelsson et al44 of 40 patients with moderate to severe acne demonstrated no significant difference between papule, pustule, and comedone count in subjects treated with oral zinc sulfate versus oxytetracycline for 12 weeks. A multicenter, double blind RCT that included 332 patients with inflammatory acne demonstrated that minocycline hydrochloride treatment was superior to oral zinc gluconate regarding improvement in superficial inflammatory count and comedone count after three months of use.45
Gastrointestinal side effects are the most frequently reported side effects associated with oral zinc supplementation,46 the most common among them being nausea reported in up to 25 percent of patients.47 Other reported side effects include abdominal pain, vomiting, and diarrhea. Rare side effects include pruritus48 and urticaria.42
Vitamin A. Vitamin A is a lipid-soluble vitamin also referred to as retinoic acid. A total of three supplements that included vitamin A were identified in our review. The forms of vitamin A contained in these supplements, if reported, included beta-carotene (450mcg) and retinyl palmatate (900mcg to 6,100mcg). Vitamin A is important for vision, cellular differentiation, embryonic development, epithelial barrier response, and immune response.49 Certain isoforms reduce proliferation of sebaceous glands and have anti-keratinization properties, which makes vitamin A a useful tool for the treatment of acne and acne related disorders.50,51 Isotretinoin (13-cis-retinoic acid) is a derivative of vitamin A and differs in its chemical composition by altering a polar end group within the vitamin A molecule.51
Two forms of vitamin A are prevalent in our diet; provitamin A carotenoids (e.g., beta-carotene) and preformed vitamin A (e.g., retinol, retinal, retinoic acid, and retinyl esters). Both of these forms can be acquired from food sources. Preformed vitamin A is abundant in animal derived foods, whereas provitamin A carotenoids are abundant in darkly colored fruits and vegetables, oily fruits, and red palm oil.52 Vitamin A supplements are most commonly provitamin A, or a combination of the two forms.
Vitamin A supplements have long been evaluated for the treatment of acne management. Nine studies were evaluated in a review of oral vitamin A for acne management. Treatment doses ranged from 36,000 IU daily to 500,000 IU daily, with 100,000 IU daily showing successful acne treatment in four trials.53 Treatment lengths ranged from 1 to 7 months, with a mean duration of seven weeks to four months until visible clinical improvement was noted; relapse was noted in 33 percent of reviewed trials.53 The most frequent side effects were mucocutaneous, such as cheilitis and xerosis, with severity being dose dependent.53 Laboratory monitoring was performed in many of these studies and a transient rise in triglycerides and liver enzymes occurred in some patients but returned to baseline 2 to 3 weeks after stopping treatment, similar to that of isotretinoin.53 Given these findings, providers should consider monitoring for liver function tests and lipids during a treatment course with supplemental vitamin A.
Similar to isotretinoin, there is a risk of teratogenicity with vitamin A. Supplemental doses of vitamin A greater than 10,000 IU (3,000 mcg retinol activity equivalents [RAE]) have been found to be dangerous during the first trimester of pregnancy.54 A study by Zamil et al. characterizing over the counter vitamin A supplements found that at least three were teratogenic and four contained doses that exceed the recommended daily level of intake.4 The half-life of vitamin A is longer than that of isotretinoin (12 days versus 24–49 hours, respectively); as a result, females of child-bearing age should be advised to wait at least three months after treatment cessation before conceiving.55 The beta carotene form of vitamin A may be less teratogenic in high doses compared to preformed vitamin A, but more data is needed to confirm this suspicion.56 However, a recent animal study found that nano-encapsulated beta-carotene resulted in embryonic developmental defects, including craniofacial and eye birth defects.57 Onset of signs and symptoms of both acute and chronic vitamin A toxicity should also be monitored for, including fatigue, headache, emotional lability, bone pain, muscle pain, edema, lymphadenopathy, hepatosplenomegaly.53
Consumers should be cautious when researching supplements, as labeling discrepancies and incorrect labeling, specifically for vitamin A supplements, has been reported. Zamil et al4 found that some supplements did not list the dosing, different websites had conflicting dosage data, or percent daily value was not consistent with the calculated vitamin A dose in micrograms.4 Given that dietary supplements do not require FDA approval before going to market, consumers should remain cautious about the consumption of vitamin A supplements, especially those of childbearing age.
Vitamin B. The B vitamins found in acne supplements reported include B3(niacinamide), vitamin B5 (pantothenic Acid), B6 (pyridoxine), B7 (biotin), B9 (folate), B12 (methylcobalamin). Few studies have examined the safety and efficacy of these vitamins found in oral formulations for the treatment of acne.
Vitamin B3 (nicotinamide). Vitamin B3 (niacin or nicotinic acid) is a water-soluble vitamin that is important for metabolism, DNA replication and repair, along with providing potent anti-inflammatory properties.58 Nicotinamide is the amide form of nicotinic acid (niacin) and carries out the same functions; however, it does not have the vasodilatory, hypolipidemic, and gastrointestinal upset.58 Topical application of nicotinamide is postulated to improve acne vulgaris due to its anti-inflammatory action and reduction of sebum production.59
Niren and Took60 investigated a combination of oral nicotinamide with zinc, copper, and folic acid in 198 patients, and found that overall 79 percent reported improvement in disease as “better/much better” after eight weeks of treatment. Shalita et al41 found that patients with inflammatory acne showed significant improvement in acne severity and appearance when a combination of nicotinamide, azelaic acid, pyridoxine, copper, and folic acid was added to existing acne regimens at both four and eight week follow up. The role of nicotinamide alone cannot be discerned from these studies though, as the individual products of each combination product were not tested separately.
Vitamin B7 (biotin). Vitamin B7, is a water- soluble vitamin that is an essential cofactor for carboxylase enzymes involved in metabolic pathways.61 It can be synthesized by the gut or found in protein-rich foods such as egg yolks, nuts, milk, and grains.62 There are no established guidelines for daily biotin allowances, however, the Food and Nutrition Board of the Institute of Medicine recommends 30mg/day for adults.61 In an Italian single center interventional study, patients treated with a combined biotin and lactic ferment supplement had improved global acne grading scores (GAGs) at 60-day follow up.63 Tolino et al,64 tested an oral supplement with two distinct formulations for men and women compared to a topical salicylic based keratolytic treatment for the treatment of acne. The supplement contained biotin, probiotics, vitamin E, zinc, and nicotinamide, with the men’s formulation containing beta sitosterol and Boswellia serrata and the women’s formulation containing myo-inositol and folic acid. The patients treated with oral supplementation, regardless of sex, had significantly more improvement in GAGs at Week 12 compared to a topical acne treatment alone.64
Vitamin B12. Vitamin B12 (cobalamin) is a water-soluble vitamin important in the hematologic and nervous system. It is only synthesized by bacteria, so it must be consumed by humans through dietary sources such as animal products, cheese, and milk.65 Excess vitamin B12 intake (>950pg/mL) can cause acne and rosacea.66 Both intramuscular and oral vitamin B12 therapy have been reported to be associated with an acneiform eruption that appeared within one week to five months after initiation of treatment and was characterized by papules and pustules on the face, neck, shoulders, chest, and upper portion of back; In all cases, spontaneous remission was seen upon discontinuation of vitamin B12 therapy.68 One proposed mechanism for this phenomenon is supported by the finding of increased porphyrin production from P. acnes cultures in the presence of vitamin B12 supplementation, which has been shown to induce inflammation in acne.67
Discussion
Up to 76 percent of adults in the United States take dietary supplements daily.68 Despite their popularity and growing market value, the majority of supplements lack evidence-based data regarding safety and efficacy.69 The popularity of dietary supplements, particularly those marketed for beauty purposes, has been on the rise in recent years. In recent years, “beauty” or “skincare” supplements have become increasingly popular on social media and in retail stores; in fact, the beauty supplement market is estimated to reach $6.8 billion in 2024.70 The lack of FDA regulation and oversight in the supplement industry has led to concerns regarding product safety and efficacy. As such, purchasing a supplement marketed for the improvement of acne can pose risks. In addition, the absence of FDA approval also means that manufacturers are not required to provide evidence regarding efficacy of their products. The sheer number of available supplements marketed for skin health, the lack of evidence-based efficacy data, and reports of serious side effects pose challenges to consumers. It is imperative to fully understand the benefits and risk profiles of these supplements and provide patients with an evidence-based approach regarding their use.
One of the most significant concerns regarding dietary acne supplements is product safety. Consumers may mistakenly assume that a product is safe because it is over the counter or marketed as “natural.” However, there is growing evidence highlighting the potential harms associated with ingredients contained in common acne supplements. For example, several thrombotic events have been reported in otherwise healthy patients following DIM supplementation24, 25 as well as a case of DRESS.22 The safety of these products in pregnancy is also concerning. Unlike prescription medications, supplements are not required to carry pregnancy categories; Those that pose a risk for teratogenicity may not even be labeled.4 The majority of the ingredients reviewed in this article have little to no data regarding safety in pregnancy; in fact, there is supporting evidence regarding potential harms related to ingesting acne supplements. For example, Zamil et al4 found that at least three vitamin A supplements were teratogenic, and four contain doses that exceed the recommended daily level of intake.
The lack of regulation and standardization of supplement product labeling also poses additional risks. Supplement labels are often difficult to interpret, or do not clearly list specific dosages. For example, Zamil et al4 found that some supplements did not list the dosing, different websites had conflicting dosage data, or percent daily value was not consistent with the calculated vitamin A dose in micrograms. Dietary supplements have the potential to interact with foods, other supplements, or prescription medications as well. However, this information is not required to be included on product labels. For example, DIM is an inducer of the CYP3A4 system, which can increase the metabolism of other co-administered drugs and has the potential to cause serious drug interactions.27
Conclusion
The popularity of dietary supplements, particularly those marketed for treating acne, has been on the rise in recent years. As dermatologists, it is important to understand safety and efficacy of the most common ingredients contained in these products. Given the lack of FDA regulation and oversight, absence of standardized labeling, and potential interactions with substances contribute to challenges faced by consumers. To ensure informed decision-making, it is essential to prioritize an evidence-based approach and educate individuals about the potential risks associated with the use of acne supplements, emphasizing the importance of thorough research and consultation with healthcare professionals.
References
- Toyoda M, Morohashi M. Pathogenesis of acne. Med Electron Microsc. 2001 Mar;34(1):29–40.
- Nguyen QG, Markus R, Katta R. Diet and acne: an exploratory survey study of patient beliefs. Dermatol Pract Concept. 2016 Apr 30;6(2):21–27.
- Blendon RJ, DesRoches CM, Benson JM, et al. Americans’ views on the use and regulation of dietary supplements. Arch Intern Med. 2001 Mar 26;161(6):805–810.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of Birth Defects From Vitamin A “Acne Supplements” Sold Online. Dermatol Pract Concept. 2021 May 20;11(3):e2021075.
- Kober MM, Bowe WP. The effect of probiotics on immune regulation, acne, and photoaging. Int J Womens Dermatol. 2015 Apr 6;1(2):85–89.
- Bowe WP, Filip JC, DiRienzo JM, et al. Inhibition of propionibacterium acnes by bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. J Drugs Dermatol. 2006 Oct;5(9):868–870.
- Oh S, Kim SH, Ko Y, et al. Effect of bacteriocin produced by Lactococcus sp. HY 449 on skin-inflammatory bacteria. Food Chem Toxicol. 2006 Aug;44(8):1184–1190.
- Deidda F, Amoruso A, Nicola S, et al. New Approach in Acne Therapy: A Specific