 J Clin Aesthet Dermatol. 2020;13(8):36–39
J Clin Aesthet Dermatol. 2020;13(8):36–39
by Suparuj Lueangarun, MD, MSc; Punyaphat Sirithanabadeekul, MD; Prapalpitch Wongwicharn, MD; Chutimon Namboonlue, MD; Sarun Pacharapakornpong, MD; Premjit Juntongjin, MD; and Therdpong Tempark, MD
Drs. Lueangarun, Sirithanabadeekul, Wongwicharn, Namboonlue, Pacharapakornpong, and Juntongjin are with the Division of Dermatology at the Chulabhorn International College of Medicine of Thammasat University in Pathumthani, Thailand. Dr. Tempark is with the Department of Pediatrics, Faculty of Medicine at Chulalongkorn University in Bangkok, Thailand.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Despite being an effective treatment for melasma, there have been limited reports on the long-term efficacy of intradermal tranexamic acid (TA) injection.
Objective: This study sought to evaluate the 48-week efficacy of a 4mg/mL intradermal TA injection for the treatment of melasma.
Methods: Five female patients with melasma participated in the 48-week follow-up after receiving 4-mg/mL intradermal TA injections on the face every two weeks for seven sessions and a sunscreen prescription. Assessments were performed at baseline and Weeks 4, 8, 12, 16, and 48 using the modified Melasma Area Severity Index (mMASI) score, melanin index, and patient satisfaction score. Safety and adverse effects were also evaluated.
Results: The mean (standard deviation) age of patients was 53.6 (8.14) years and Fitzpatrick Skin Type IV (60%) and Fitzpatrick Skin Type V (40%) were observed. The mean (standard deviation) duration of melasma was 7.6 (2.51) years and 60 percent of participants reported a family history of melasma. There was a significant decrease in mMASI score and melanin index at 16 weeks, without a statistically significant improvement of mMASI score at 48 weeks. Melasma recurrence was observed in 60 percent of the participants, with higher mMASI scores recorded, but the severity remained less than at baseline. The patient satisfaction score was lower from Week 16 to Week 48. Interestingly, a statistically significant decrease in the melanin index was observed up to Week 48, with no serious adverse effects.
Conclusion: The 4-mg/mL intradermal TA injection yields significant efficacy at Week 16; however, melasma recurrence occurred during the 48-week follow-up. In addition to tranexamic acid injections, maintenance therapy and sun protection should be considered for patients with melasma.
Keywords: Melasma, melasma pathogenesis, melasma treatment, tranexamic acid, intradermal microinjection
Melasma is a common acquired pigmentary disorder of facial hyperpigmentation, with characteristic presentations that include symmetrically distributed, hyperpigmented macules and patches at frequently sun-exposed areas of the skin, especially on the face.1 There are several treatment modalities available for melasma, such as whitening agents, chemical peeling, laser and light therapy.2 However, these treatments can yield adverse effects such as mottled hypopigmentation, irritation, acneiform eruptions, and rebound hyperpigmentation.3
Tranexamic acid (trans-4-aminomethylcyclohexane-carboxylic acid; TA) is a plasmin inhibitor, with the synthetic derivative of amino acid lysine that works by reversibly blocking lysine binding sites on plasminogen molecules to inhibit the plasminogen activator (PA) from converting plasminogen to plasmin.4 The main mechanism of the hypopigmentation effects of TA is due to its antiplasmin activity, with a structural similarity relative to tyrosine that can inhibit tyrosinase competitively.5,6 Whereas, plasmin transforms the vascular endothelial growth factor (VEGF) into a diffusing form, which demonstrates a crucial role of TA following histological examination in the reduction of erythema and vascularities as well as the number of mast cells in the dermis.7,8
In clinical studies, localized intradermal TA injection can be efficacious for melasma treatment,9–12 with a significant decrease in pigmentation,13 little systemic absorption, and no systemic side effects. However, the efficacy of intradermal TA injections in previous studies have been evaluated for only a short period of time, while recurrence after cessation of treatment seems very common.3 This study aimed to evaluate the efficacy and safety profile of long-term 48-week follow-up of intradermal TA injection for the treatment of melasma.
Methods
This prospective clinical trial was conducted at Benjakiti Park Hospital from May 2017 to November 2018 and included five female patients, aged 26 to 60 years, with bilateral symmetric melasma. Exclusion criteria included oral contraceptive pill use, pregnancy and lactation within 12 months prior to the study, a history of coagulation and thrombotic disorder, use of anticoagulants, allergy to TA, treatment for melasma within six months prior to the study, and history of herpes simplex lesions on the face. This study was approved by Thammasat University’s ethical review board in accordance with the Declaration of Helsinki. All patients signed a written informed consent form.
Intervention. Patient demographic data, including age, sex, disease duration, and family history of melasma, and skin phenotype, were recorded. Patients were asked to apply topical anesthetic cream (EMLA®, 2.5% lidocaine and 2.5% prilocaine; Astra Pharmaceuticals, Wayne, Pennsylvania) on the face for 45 minutes under occlusion, then wash it off before treatment with 4-mg/mL intradermal TA injection. Treatment sessions took place every two weeks for a total of seven sessions. The 30-gauge needles were injected intradermally into the melasma lesion at a 1-cm intervals (about 0.05 mL/1 cm2),11 approximately 3mL on each cheek, with an ice pack applied to relieve pain. TA (Transamin®; Daiichi Sankyo Co., Ltd., Tokyo, Japan) was prepared under sterile conditions and drawn in a 100-U/mL insulin syringe, then diluted with normal saline up to 1mL for the 4mg/mL of concentrated TA.10 All patients were prescribed a broad-spectrum sunscreen with a sun protection factor of 50 for the 48-week study period.
The follow-up period included assessments at baseline and Weeks 4, 8, 12, 16, and 48 using the modified Melasma Area and Severity Index (mMASI score)14 applied by two blinded dermatologists. A Biometric camera (Antera 3D®, Miravex Ltd., Dublin, Ireland) was used to assess the melanin index. Patient satisfaction and adverse side effects were also evaluated at each follow-up visit.
Statistical analysis. All outcome measurements were reported as means and standard deviations (SDs). The mMASI score and melanin index were analyzed using the Wilcoxon signed-rank test. A p-value of less than 0.05 was considered to indicate statistical significance. The statistician was blinded to data in the study.
Results
Demographic data. There were five female patients, with a mean (SD) age of 53.6 (8.14) years and Fitzpatrick Skin Types IV (60%), and V (40%). The mean (SD) duration of melasma was 7.6 (2.51) years and 60 percent of patients had a family history of melasma and prior treatment with topical medication. None of the patients reported any underlying diseases. Precipitating factors included sunlight exposure (100%) and pregnancy (20%).
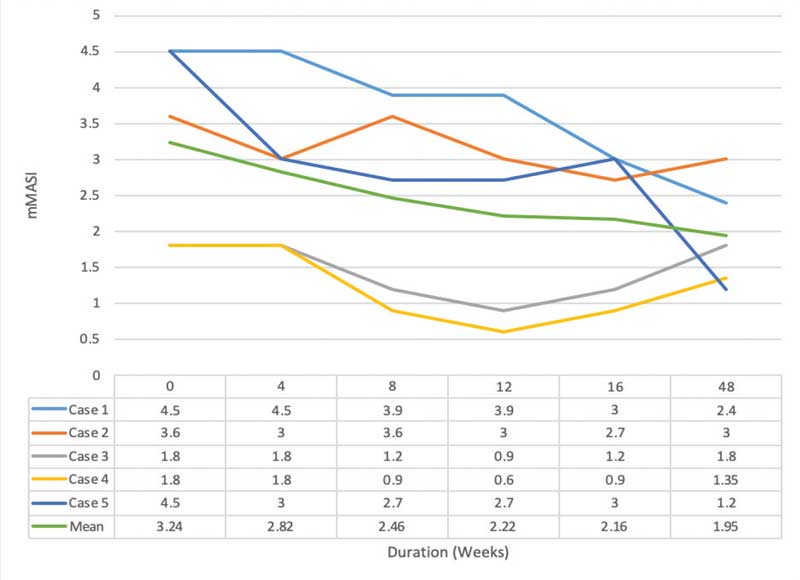
Efficacy assessment. There was statistical significance for mMASI scores of 24±1.36, 2.82±1.11 (p=0.18), 2.46±1.36 (p=0.07), 2.22±1.42 (31.48% reduction, p= 0.04*), 2.16±1.02 (33.33% reduction p= 0.04*), and 1.95±0.75 (p=0.07) points at baseline and Weeks 4, 8, 12*, 16*, and 48, respectively.
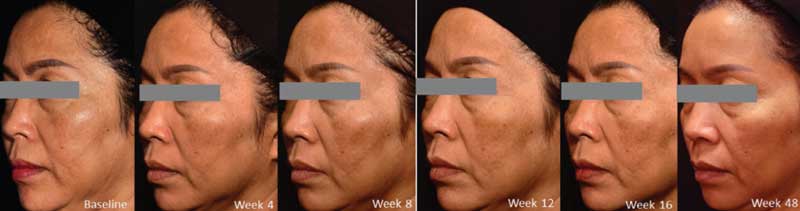
Intradermal TA injection yielded a statistically significant improvement at Week 12 and Week 4 after the last treatment. However, a decrease in the mMASI score was observed at Week 48, without statistical significance. Three patients showed recurrence of melasma with an increase in mMASI score, albeit to a lesser degree than at baseline (Figures 1 and 2).
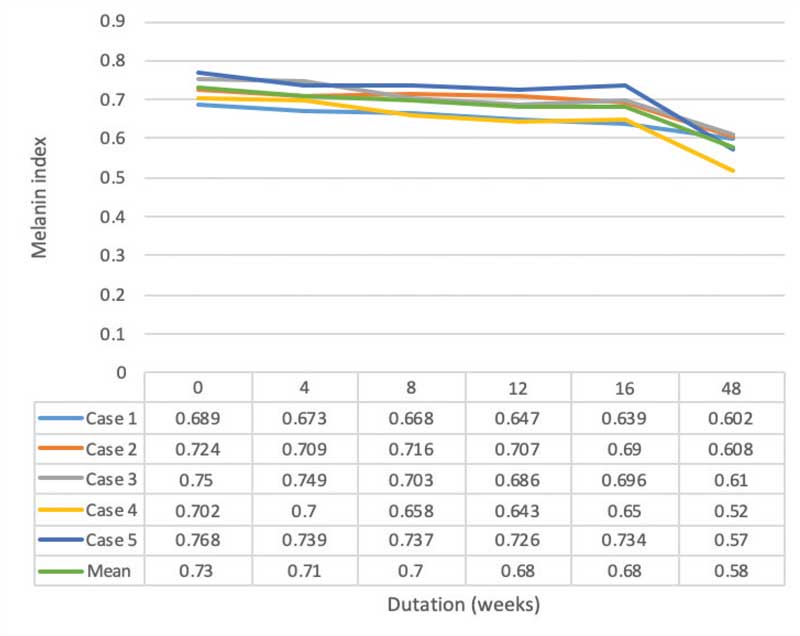
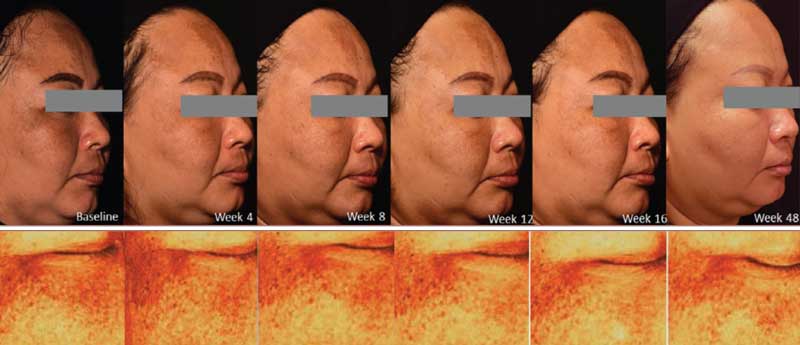
The melanin index showed a statistical significance for 0.73±0.03, 0.71±0.03 (p=0.04), 0.70±0.03 (p=0.04), 0.68±0.04 (p=0.04), 0.68±0.04 (p=0.04), 0.58±0.04 (p=0.04) points at baseline and Weeks 4, 8, 12, 16, and 48, respectively. Intradermal TA injection yielded a statistically significant improvement at all follow-up periods. All participants demonstrated an improvement in the melanin index at Week 48 when compared to baseline (Figures 3 and 4).
Moreover, there was a decrease in the patient satisfaction score, where 0 points=worse and 10 points=excellent, at Week 4 after the last treatment (Week 16), particularly from 6.90±2.01 points to 4.80±2.75 points at Week 48 (p=0.042).
Adverse effects. Local side effects, such as erythema and swelling, were observed and disappeared within 1 to 2 hours after injection. Other local side effects, including pain, irritation, and bruising were minimal, with patient tolerance until the completion of study. There were no significant systemic side effects, such as gastrointestinal discomfort, headache, hypomenorrhea, or spotting menstruation.15
Discussion
To the best of our knowledge, this is the first study to evaluate the long-term 48-week efficacy and safety of intradermal 4-mg/mL TA injections performed every two weeks for seven sessions for the treatment of melasma, using both subjective (mMASI and patient satisfaction scores) and objective (biometric camera) assessments. There was a significant decrease in mMASI score at Week 16 for 33.33 percent compared to baseline (p=0.04).
Recently, there have been several reports on the efficacy and side effects of localized intradermal TA injections the treatment of melasma using different concentrations of TA, including 4 mg/mL, 20 mg/mL, and 100 mg/mL, with the frequency ranging from weekly to monthly and the treatment duration from eight to 12 weeks.10–12,19,20 In our study, we chose to use 4-mg/mL intradermal TA injection referred to by previous research.11 For safety reasons, the average dose of intradermal TA injection in our patients was only 22mg (range: 12–32 mg), much less than the antifibrinolytic dose of TA.15 Thus, no significant systemic side effects related to TA were noted.
Following a pilot study of 4-mg/mL intradermal TA injection,11 a decrease of 42.74 percent in mMASI score was observed after 12 weeks of treatment. Moreover, the melanin level significantly decreased at Week 16 for 6.85 percent relative to baseline (p=0.04). In addition, Saki et al12 observed improvements of 5.6 percent and 6.44 percent at Week 8 and Month 3 of follow-up, respectively, in the melanin level following treatment of melasma with 20-mg/mL intradermal TA injections.
However, in our study, the mMASI score showed no statistically significant improvement at Week 48 in 39.81 percent relative to baseline (p=0.07). Meanwhile, three (60%) patients showed recurrence of melasma with an increase in the mMASI score, albeit still remaining improved compared to baseline. Patient satisfaction scores also significantly decreased from Week 16 to Week 48.
Interestingly, there was a statistically significant decrease in the melanin index up until Week 48 (0.58±0.04; 20.55% relative to baseline; p=0.04). The prolonged pigmentation improvement from melanin index evaluation could have resulted from the use of sunscreen and better sun-protection behaviors.16–18
In prior reports, a significant improvement in mMASI score was observed at Week 8 and Week 12 from baseline, corresponding to our study reporting a significant mMASI score improvement at Week 12 and Week 16.9–11,19 Meanwhile, a significant improvement of melanin level at Week 4 has been previously demonstrated,12 similar to our study, in which we observed a significant melanin index improvement after four weeks of treatment compared to baseline.
In addition, most studies evaluated the efficacy of intradermal TA injection in melasma treatment after 12, 20, and 24 weeks.10–12,19 Meanwhile, the gradual recurrence of melasma following the 4-mg/mL intradermal injection was commonly observed at Week 24, but remained less severe than the initial hyperpigmentation.11 Pazyar et al9 detected a melasma recurrence rate of 54.5 percent at Week 24, comparable to in our study, which showed a recurrence rate of 60 percent at Week 16. In particular, with our long-term 48-week follow-up period, the recurrence of melasma was 60 percent, but was less severe than the initial hyperpigmentation.
Despite many treatment modalities for melasma, none have been demonstrated to be consistently satisfactory. Thus, melasma treatment becomes a challenge for dermatologists, as no gold-standard exists and recurrence is common.1
After treatments with TA, including oral, topical application, localized intradermal injection, localized microneedling, and other combined modalities, such as intense pulse light or neodymium-doped yttrium aluminum garnet (Nd:YAG) laser,21 the initial efficacy and recurrence of melasma were commonly observed.
A study from Bala et al22 reported efficacy of oral TA in Asian skin, even at a low dose of 500mg daily over a short period of 8 to 12 weeks. The authors concluded oral TA could be a safe and convenient therapeutic option to administer with minimal, reversible, and mild side effects. Nonetheless, patients should be cautiously screened for contraindications and thromboembolic risk factors prior to the commencement of therapy. Additionally, multiple studies have also demonstrated that melasma tends to recur following the cessation of oral TA treatment.23,24
For topical treatment, triple combination (containing fluocinolone acetonide, hydroquinone, and tretinoin) therapy also showed relapse as early as 1 to 2 weeks after treatment cessation.25 Even with maintenance treatment with triple combination therapy, Arellano et al18 reported relapse in 47 percent of patients after six months, particularly among those with severe melasma at baseline, with a median relapse time of 190 days. Meanwhile, treatment with a 1064-nm Q-switched Nd:YAG laser and a 12-week follow up after the last laser session noted partial recurrence of melasma in all cases.26 After treatment with copper bromide lasers, Lee et al27 recorded recurrence of melasma after six months in 30 percent of patients. Thus, the high prevalence of melasma recurrence with previous intradermal, oral TA, topical, and laser treatment modalities were compatible with the findings of the our 48-week follow-up study. Despite melasma recurrence, our study found that the mMASI score was still better than at baseline, while the melanin index also showed a significant improvement with patient satisfaction.
Considering this, localized intradermal TA injection could be efficacious for melasma treatment. Nonetheless, it could be recommended as a combination treatment paired with other modalities, such as topical and laser therapies, or maintenance therapy to decrease the relapse rate of melasma.
Limitations. Our study was limited by the small number of patients and lack of a control group.
Conclusion
Localized 4-mg/mL intradermal TA injection yielded significant efficacy at Week 16 after treatment, but a 60-percent recurrence rate of melasma was observed at Week 48. However, the mMASI score and melanin index were still better than at baseline, with patient satisfaction and no serious side effects. Hence, localized intradermal TA injection might be a promising therapeutic option as monotherapy or a combination treatment for melasma due to its efficacy and safety profile. However, the recurrence of melasma is expected during the follow-up period from 16 to 48 weeks. Thus, the inclusion of maintenance therapy and sun protection are strongly recommended to improve efficacy and decrease the recurrence of melasma.
Acknowledgments
The authors would like to extend special thanks to Dr. Voraphol Vejjabhinanta for the initiation and Ms. Sunattee Kessung for her assistance in editing and revising this manuscript.
References
- Sheth VM, Pandya AG. Melasma: a comprehensive update: part I. J Am Acad Dermatol. 2011;65(4):689–697.
- Rivas S, Pandya AG. Treatment of melasma with topical agents, peels and lasers: an evidence-based review. Am J Clin Dermatol. 2013;14(5):359–376.
- Sheth VM, Pandya AG. Melasma: a comprehensive update: part II. J Am Acad Dermatol. 2011;65(4):699–714.
- Perper M, Eber AE, Fayne R, et al. Tranexamic acid in the treatment of melasma: a review of the literature. Am J Clin Dermatol. 2017;18(3): 373–381.
- Ando H, Matsui MS, Ichihashi M. Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders. Int J Mol Sci. 2010;11(6):2566–2575.
- Li D, Shi Y, Li M, et al. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010;20(3):289–292.
- Na JI, Choi SY, Yang SH, et al. Effect of tranexamic acid on melasma: a clinical trial with histological evaluation. J Eur Acad Dermatol Venereol. 2013;27(8):1035–1039.
- Kim EH, Kim YC, Lee ES, et al. The vascular characteristics of melasma. J Dermatol Sci. 2007;46(2):111–116.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115–122.
- Budamakuntla L, Loganathan E, Suresh DH, et al. A randomised, open-label, comparative Study of tranexamic acid microinjections and tranexamic acid with microneedling in patients with melasma. J Cutan Aesthet Surg. 2013;6(3):139–143.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32(5):626–631.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29(4):405–410.
- Sirithanabadeekul P, Srieakpanit R. Intradermal tranexamic acid injections to prevent post-inflammatory hyperpigmentation after solar lentigo removal with a Q-switched 532-nm Nd:YAG laser. J Cosmet Laser Ther. 2018;20(7–8):398–404.
- Majid I, Haq I, Imran S, et al. Proposing melasma severity index: a new, more practical, office-based scoring system for assessing the severity of melasma. Indian J Dermatol. 2016;61(1):39–44.
- Krivokuca I, Lammers JW. Recurrent pulmonary embolism associated with a hemostatic drug: tranexamic acid. Clin Appl Thromb Hemost. 2011;17(1):106–107.
- Tempark T, Lueangarun S, Chatproedprai S, et al. Sun protection behavior and knowledge of patients attending laser clinic to prevent adverse events of laser: a cross-sectional, single-center, tertiary care study. Photodermatol Photoimmunol Photomed. 2018;34(6):374–386.
- Jutley GS, Rajaratnam R, Halpern J, et al. Systematic review of randomized controlled trials on interventions for melasma: an abridged Cochrane review. J Am Acad Dermatol. 2014;70(2):369–373.
- Arellano I, Cestari T, Ocampo-Candiani J, et al. Preventing melasma recurrence: prescribing a maintenance regimen with an effective triple combination cream based on long-standing clinical severity. J Eur Acad Dermatol Venereol. 2012;26(5):611–618.
- Steiner D, Feola C, Bialeski N. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatology. 2009;1:4.
- Tehranchinia Z, Saghi B, Rahimi H. Evaluation of therapeutic efficacy and safety of tranexamic acid local infiltration in combination with topical 4% hydroquinone cream compared to topical 4% hydroquinone cream alone in patients with melasma: a split-face study. Dermatol Res Pract. 2018;2018:8350317.
- Laothaworn V, Juntongjin P. Topical 3% tranexamic acid enhances the efficacy of 1064-nm Q-switched neodymium-doped yttrium aluminum garnet laser in the treatment of melasma. J Cosmet Laser Ther. 2018;20(6):320–325.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44(6): 814–825.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75(2):385–392.
- Tan AWM, Sen P, Chua SH, et al. Oral tranexamic acid lightens refractory melasma. Australas J Dermatol. 2017;58(3):e105–e108.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111(1):40–48.
- Wattanakrai P, Mornchan R, Eimpunth S. Low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1,064 nm) laser for the treatment of facial melasma in Asians. Dermatol Surg. 2010;36(1):76–87.
- Lee HI, Lim YY, Kim BJ, et al. Clinicopathologic efficacy of copper bromide plus/yellow laser (578 nm with 511 nm) for treatment of melasma in Asian patients. Dermatol Surg. 2010;36(6): 885–893.

