 by Shilpi Sharma, MD, and Saurabh Agarwal, MD
by Shilpi Sharma, MD, and Saurabh Agarwal, MD
Dr. Sharma is Assistant Professor in the Department of Dermatology, Venereology, and Leprology at the Government Medical College Haldwani in Uttarakhand, India. Dr. Agarwal is Professor and Unit Head of the Department of Dermatology, Venereology, and Leprology at the Government Medical College Haldwani in Uttarakhand, India.
J Clin Aesthet Dermatol. 2020;13(8):40–44
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Introduction.Anogenital warts (AGWs) are one of the leading sexually transmitted infections in the world. This condition poses a number of challenges to dermatologists, including the reluctance of patients to consult a physician and the high likelihood of relapse. Individuals with AGWs suffer a substantial psychological morbidity. Intralesional immunotherapy with the measles, mumps, and rubella (MMR) vaccine has been reported to be an effective treatment for warts. However, literature on the efficacy of intralesional immunotherapy with the MMR vaccine for the treatment of anogenital warts is sparse.
Objective. We sought to determine the efficacy of the MMR vaccine for the treatment of anogenital warts at an outpatient dermatology department in Government Medical College Haldwani in India.
Methods. This was a hospital-based, longitudinal study the included 35 patients. In patients with genital warts, 0.5mL of the MMR vaccine after reconstitution with distilled water was injected intradermally into their single largest wart. Injections were given every three weeks until a maximum of three injections was achieved. Pre- and posttreatment photographs were assessed to compare the degree of reduction in the size and number of warts. The therapeutic response was evaluated as follows: No response (<50% reduction in the number of warts), Relative response (50%–99% reduction), complete response (100% reduction).
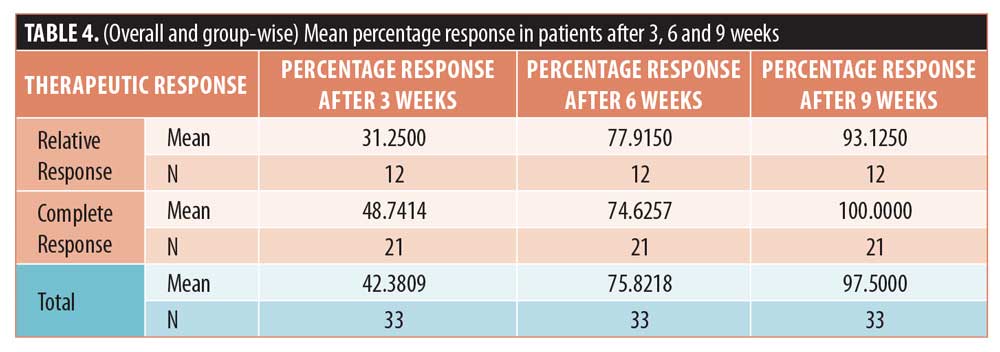
Results. On average, a 42.4-percent response was observed in the first three weeks after administering the MMR vaccine, which increased to 75.8 percent after the second vaccine at six weeks and nearly 98 percent after the last vaccine at nine weeks.
Conclusion. Our results suggest that intralesional immunotherapy with the MMR vaccine can serve as a safe and effective therapy for the treatment of AGWs.
Warts are common viral infections caused by more than 120 papillomaviruses. Of these, over 40 can cause the anogenital tract infections leading to external anogenital warts (AGWs) and anogenital cancers.1 Anogenital warts are one of the leading sexually transmitted infections in the world.2 This condition poses a number of challenges to dermatologists, including the reluctance of patients to consult a physician and the high likelihood of relapse. Individuals with AGWs can suffer from substantial negative psychological impacts. Treatment with lower side effects and low recurrence rate is desirable. Various treatment modalities are available for warts, including topical therapies, such as trichloroacetic acid, salicylic acid, podophyllotoxin, and 5-fluorouracil, radiocautery, cryotherapy, surgical excision, carbon dioxide laser, and immunotherapy.3
Recently, immunotherapy has gained popularity as an effective treatment for warts. Antigens, such as the measles, mumps, and rubella (MMR) vaccine, Mycobacterium w vaccine (MWV) Candida albicans and Bacillus Calmette-Guérin (BCG), have been used intralesionally for the treatment of warts, with variable responses.3 While intralesional immunotherapy with the MMR vaccine has been reported as an effective treatment for warts, the exact mechanism of action in the clearance of warts is not known.4 It seems to use the immune system’s capacity to mount a Type 1 helper T cell (TH1) mediated delayed-type hypersensitivity response to various antigens, including human papillomavirus (HPV).4 However, literature on the efficacy of intralesional immunotherapy with the MMR vaccine for the treatment of anogenital warts is sparse. We evaluated the efficacy of the MMR vaccine for the treatment of anogenital warts at an outpatient dermatology department at Government Medical College Haldwani in India.
Methods
This was a hospital-based, longitudinal study of 35 patients conducted in the Department of Dermatology, Venereology, and Leprosy at Government Medical College Haldwani in India following clearance from the Institutional Review Board.
Inclusion and exclusion criteria. Patients with genital warts who presented to our clinic from September 2018 to May 2019 and who were not using anti-wart treatment within the previous month were included in the study after providing informed consent. Exclusion criteria included prior hypersensitivity reaction to MMR antigen, pregnancy, lactation, presence of any active infections (e.g., herpes), tuberculosis, and immunosuppression (e.g., human immunodeficiency virus, patients taking immunosuppressives).
Baseline evaluation. Thirty-five patients met inclusion criteria and were recruited into the study. Written informed consent and detailed demographic and historical clinical data were obtained and recorded for each patient. Photographic documentation was performed for each patient.
Dosage and administration. The MMR vaccine (TRESIVAC®; Serum Institute of India Pvt. Ltd., Pune, India) used was a freeze-dried preparation of live attenuated strains of measles, mumps, and rubella viruses available in a single-dose vial of 0.5mL. In patients with genital warts , 0.5mL of the MMR vaccine after reconstitution with distilled water was injected intradermally into their single largest wart. Injections were administered every three weeks until a maximum of three injections was achieved.
Assessment methods. Pre- and post-intervention photographs were assessed to compare the degree of reduction in the size and number of warts. The therapeutic response was evaluated as follows: no response (<50% reduction in the number of warts), relative response (50%–99% reduction), and complete response (100% reduction). Adverse events, such as pain, swelling, and flu-like symptoms were noted following injection.
Statistical analysis. All the results have been generated using SPSS software v 22.0 and Microsoft Excel postanalysis on the clinical data. Independent t-tests and chi-square tests have been applied for analysis between various attributes at 95% confidence level.
Results
A total of 35 patients were included in the study. Of these, two patients did not complete the study or were lost to follow up, so they were excluded from further analysis. Hence, the total number of patients in the sample size was 33. The mean age of study participants was 24.0±7.6 years. All the patients had multiple lesions, ranging from 3 to 40, with the average number of lesions being 18. The maximum duration of disease among the patients was 16 weeks, with an average duration of 6.2±3.9 weeks (Table 1). Regarding sex distribution, 72.7 percent of the patients were men and 27.3 percent of the patients were women (Table 2).
On the first visit, an average of 18 lesions were observed, which decreased to 10 lesions after administration of the first MMR vaccine, five lesions after the second injection, and one lesion after the third injection. In the patients who showed complete response, around 14 lesions were observed on average, which decreased to six lesions after the first MMR vaccine and to three lesions after the second injection of MMR vaccines. However, in the patients who showed relative response, 24 lesions were observed on average, which decreased to 16 lesions after the first MMR vaccine, seven lesions after the second injections, and one lesion after the third (Table 3).
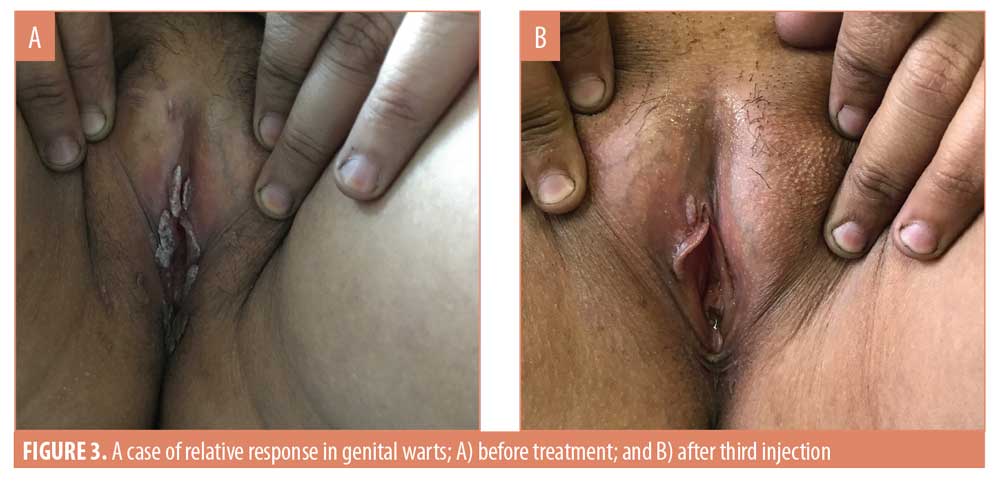
After three weeks of administering the first MMR vaccine, an average 42.4-percent improvement was seen in the 33 patients. After the second vaccine, the response in the patients was 75.8 percent. After the last vaccine, the average response was near 98 percent in all the patients. In the patients who saw complete clearance of their genital warts, a 48.7-percent response to the first vaccine and 74.6-percent to the second vaccine was observed (Figures 1 and 2). However, in the patients who showed a relative response, a 31.3-percent response to the first vaccine, a 77.9-percent to the second vaccine, and a 93.2-percent response to the third MMR vaccine was observed (Table 4) (Figures 3 and 4).
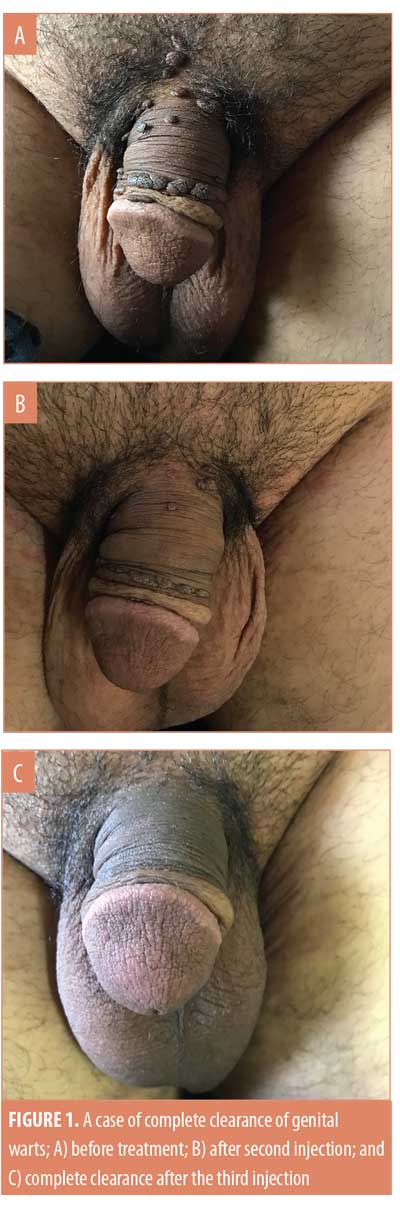
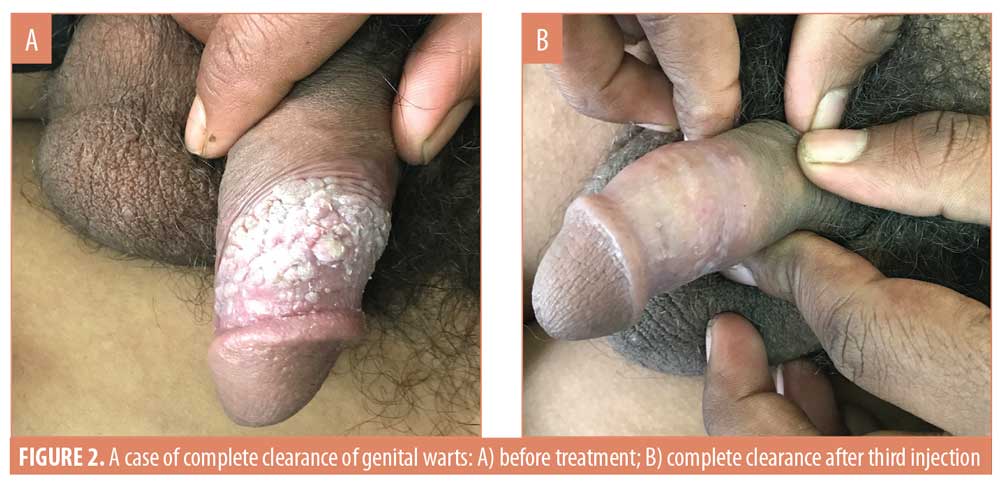
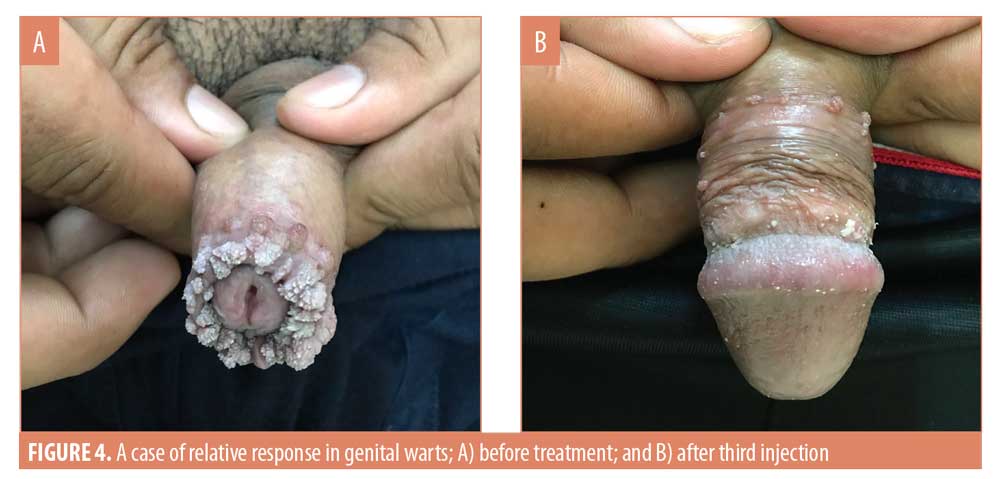
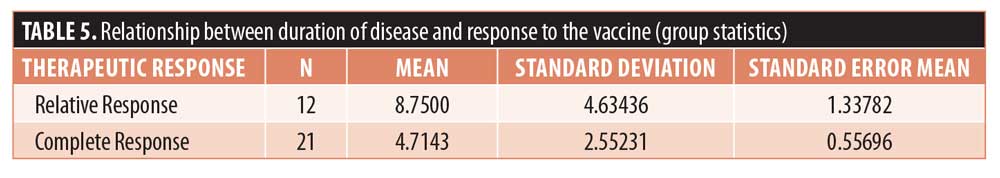
The average duration of disease in the patients who showed complete response was 4.7±2.6 weeks and that in the patients who showed relative response to the MMR vaccine was 8.8±4.6 weeks. Thus, the duration of disease in the patients who saw complete clearance of their genital warts was comparatively smaller (Table 5). P-value after Levene’s tests was 0.045, less than 0.05, and the p-value for the t-test was 0.014, less than 0.05; thus, there was a significant difference in the duration of disease in the patients from two different response groups (Table 6).
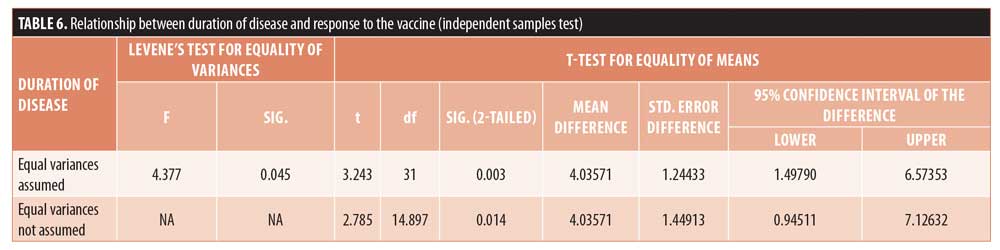
Adverse effects were evaluated at every visit, and the only adverse event reported was swelling following injection, which was seen in 18.2 percent of patients (Table 7).
Discussion
Intralesional immunotherapy has recently gained popularity in the treatment of warts. It utilizes the ability of the immune system to mount a delayed type hypersensitivity response to various antigens or wart tissue leading to production of Th1 cytokines which stimulate cytotoxic T cells and natural killer cells to eradicate HPV infection. This stimulated immune response has a potential to resolve the distant warts as well and not the wart alone that has been primarily injected.4
Intralesional immunotherapeutic agents used for the treatment of genital and extragenital include Mw vaccine, BCG vaccine, the MMR vaccine, Candidial extract, and Trichophyton antigen.5 Immunotherapy using the MMR vaccine has been widely used for the treatment of cutaneous warts due to favorable results with reduced adverse effects and lower recurrence rates.6 We could not find any study in the literature where the MMR vaccine had been used specifically for the treatment of genital warts; however, studies reporting on the use of intralesional mumps antigen for genital warts are available.7
In our study, a 42.4-percent response was seen in the 33 patients, on average, after three weeks of administering the first MMR vaccine. After the second vaccine, the response in the patients was 75.8 percent. After the last vaccine, the average response was near 98 percent in all patients. In the patients who saw complete clearance of their warts, a 48.7-percent response to the first vaccine and 74.6-percent to the second vaccine was observed. However, in the patients who showed relative response, a 31.3-percent response to the first vaccine, 77.9-percent response to the second vaccine, and 93.2-percent response to the third MMR vaccine was observed.
In our study, we observed the average duration of disease in the patients who showed complete response to be 4.7±2.6 weeks. Patients who showed relative response to the MMR vaccine had an average disease duration of 8.8±4.6 weeks; thus, the disease duration of the patients who saw complete clearance of their condition was comparatively smaller. The p-value according to Levene’s tests was 0.045 and t-test was 0.014 at 95% confidence level. Thus, there was a significant difference in the duration of disease in the patients from two different response groups. The mean duration of disease is smaller with low variability in the patients who showed complete response to the vaccine. Thus, patients with a shorter duration of disease showed a relatively better response to the MMR vaccine, and such patients have a higher chance of seeing complete clearance of their warts.
Many studies have demonstrated the effectiveness of the MMR vaccine in the treatment of extragenital warts. A study by Mohamad et al8 evaluated the effects of the MMR vaccine in the treatment of plantar warts in 100 patients. The investigator observed a significantly higher rate of complete clearance compared to the control group (82% vs. 0%, respectively). The rate of partial response was six percent versus 30 percent, and the rate of no response was 12 percent versus 70 percent, respectively.
A study by Nofal et al9 also studied the effects of the MMR vaccine in 135 patients with single or multiple recalcitrant or nonrecalcitrant common warts. The investigators observed complete response in 57 patients (81.4%), partial response in seven patients (10%), and no response in six patients (8.6%) of the MMR group.
Previous studies have evaluated the MMR vaccine for the treatment of cutaneous warts, including Zamanian et al,10 who observed a 75-percent response to MMR injections; Gamil et al,11 who reported an 87-percent complete response; Dhope et al,12 who observed a complete response in 65 percent of patients; Raju et al,13 who observed complete remission in 70.4 percent of patients; and Naseem et al,14 who observed a complete response in 81 percent of patients. However, none of these studies evaluated the efficacy of the MMR vaccine for AGWs specifically.
Our study demonstrates the therapeutic potential of the MMR vaccine in treatment of genital warts. Similar to our study, many studies have demonstrated the efficacy of other intralesional immunotherapeutic agents on AGWs. A study by King et al7 retrospectively studied the efficacy of immunotherapy with mumps, Candida, and/or Trichophyton skin test antigens in patients with AGWs and reported complete resolution in 5 of 10 patients who completed the therapy. In a pilot study of intralesional injection of Mw vaccine, 8 of 10 patients with AGWs showed complete resolution.15 Eassa et al16 reported complete clearance in nearly half of the pregnant women with AGWs who received intralesional purified protein derivative of Mycobacterium tuberculosis.
In our study, the only adverse event reported was swelling following injection, which was seen in 18.2 percent of patients, while in other studies where MMR vaccine has been used for the treatment of extragenital warts, pain during injection and flu-like symptoms were commonly reported side effects.10,17,18
Limitations. Limitations to this study include small sample size and the short duration of follow-up period. Further randomized, double-blinded, and vehicle-controlled studies with larger sample sizes, are required to support our findings.
Conclusion
Our results suggest that intralesional immunotherapy with the MMR vaccine can serve as an effective therapy for the treatment of anogenital warts. To our knowledge, this is the first study where the MMR vaccine is used for the treatment of anogenital warts, as previous studies have been limited to the evaluation of the MMR vaccine for the treatment of extragenital warts. Larger, prospective, randomized, controlled trials are needed to confirm our results.
References
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350.
- Cox JT, Petry KU, Rylander E, RoyM. Using imiquimod for genital warts in female patients. J Womens Health (Larchmt). 2004;13(3):265–271.
- Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4:273–293.
- Chandrashekar L. Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol. 2011;77:261–263.
- Sinha S, Relhan V, Garg VK. Immunomodulators in warts: Unexplored or ineffective? Indian J Dermatol. 2015;60:118–129.
- Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida and trichophyton skin test antigens: a single-blinded, randomized and controlled trial. Arch Dermatol. 2005;141(5):589–594.
- King M, Johnson SM, Horn TD. Intralesional immunotherapy for genital warts. Arch Dermatol. 2005;141(12):1606–1607.
- Mohamad NS, Badran F, Yakout E. Evaluation of the efficacy of a combination measles, mumps, and rubella vaccine in the treatment of plantar warts. Our Dermatol Online. 2013;4:463–467.
- Nofal A, Nofal E. Intralesional immunotherapy of common warts: successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol. Venereol. 2010;24(10): 1166–1170.
- Zamanian A, Mobasher P, Jazi GA. Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart. Adv Biomedic Res. 2014;3:107.
- Gamil H, Elgharib I, Nofal A, Abd-Elaziz T. Intralesional immunotherapy of plantar warts: Report of a new antigen combination. J Am Acad Dermatol. 2010;63:40–43.
- Dhope A, Madke B, Singh AL. Effect of measles mumps rubella vaccine in treatment of common warts. Indian J Drugs Dermatol. 2017;3:14–19
- J Raju, AV Swamy, BL Nanjunda Swamy, et al. Intralesional Measles, Mumps and Rubella (MMR) Vaccine-An Effective Therapeutic Tool in The Treatment of Wart. Journal of Evidence-based Medicine and Healthcare. 2015; 2(50):8548–8551.
- R Naseem, S Aamir. The Efficacy of Intralesional Measles, Mumps, Rubella (MMR) Antigen in Treatment of Common Warts. Pakistan Journal of Medical and Health Sciences. 2013;7(4):1130–1133.
- Gupta S, Malhotra AK, Verma KK, et al. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of anogenital warts: an open label pilot study. J Eur Acad Dermatol Venereol. 2008;22(9):1089–1093.
- Eassa BI, Abou-Bakr AA, El-Khalawany MA. Intradermal injection of PPD as a novel approach of immunotherapy in anogenital warts in pregnant women. Dermatol Ther. 2011;24(1):137–143.
- Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol. 2001;137(4): 451–455.
- Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens: a safe and effective therapy. J Drugs Dermatol. 2004;3(3):263–265.

