J Clin Aesthet Dermatol. 2023;16(10):48–51.
J Clin Aesthet Dermatol. 2023;16(10):48–51.
by Joely Kaufman-Janette, MD; Alex Cazzaniga, BS, MBA; Andrew Jacobson, MD;
Laura L. Eaton Jankov, MSN, APRN, FNP-BC; Karen Copeland, PhD; Atta Behfar, MD, PhD; Saranya Wyles, MD, PhD
Dr. Kaufman-Janette, Mr. Cazzaniga, and Dr. Jacobson are with Skin Research Institute in Coral Gables, Florida. Ms. Jankov is with UltaMed Corporation in Fort Lauderdale, Florida. Dr. Copeland is with Boulder Biostatistics in Steamboat Springs, Colorodo. Drs. Behfar and Wyles are with Mayo Clinic in Rochester, Minnesota.
FUNDING: This study was partially funded by Rion Aesthetics, Inc.
DISCLOSURES: Drs. Jankov, Copeland, and Wyles are consultants for Rion Aesthetics, Inc. Dr. Behfar and the Mayo Clinic have ownership interest in Rion Inc. and Rion Aesthetics, Inc.
ABSTRACT: Objective. The primary objective of this pilot study was to demonstrate the benefits of topical human platelet extract (plated)™ serum for the improvement of persistent facial redness.
Methods. This single-center, open-label pilot study evaluated six subjects using (plated)™ serum containing human platelet extract (HPE) with Renewosome™ technology twice daily for six weeks. The primary efficacy endpoint was a reduction in the Clinical Erythema Assessment (CEA) grade, and a reduction in Patient Subjective Assessment grade at six weeks. Secondary endpoints included an improvement in quality of life related to facial redness, and a reduction in redness by Mexameter™ spectrometry measurement. Safety data included monitoring for adverse events.
Results. Topical HPE serum demonstrated a statistically significant improvement in facial redness at Week 9 when averaging the Mexameter™ spectrometry results across nine regions of the face (p=0.0052). The primary and secondary endpoints were achieved. CEA grade at Week 6 demonstrated that all subjects improved by at least one grade, while one subject improved by two grades. One patient reported dryness. No other adverse effects were observed.
Limitations, Study limitations included a small sample size and lack of darker skin types (Fitzpatrick IV-VI).
Conclusion. This study demonstrates that topical HPE with Renewosome™ technology provides statistically significant reduction in facial redness and is safe and well-tolerated.
Keywords. Human platelet extract, skin rejuvenation, facial redness
Facial appearance plays a considerable role in self-esteem, self-confidence, and in our interactions with others.1 Thus, it is not difficult to understand why patients with persistent facial redness frequently experience fear of social judgment. Historically, facial redness has been stigmatized as a sequalae of alcohol abuse or association with psychiatric or anger management problems.2 Blushing has also been viewed as a sign of betrayal, as the color red is also the color of emotion.2
Facial erythema is one of the most common outpatient complaints in dermatology.3 It can accompany a variety of clinical conditions, including rosacea, actinic photodamage, acne vulgaris, folliculitis, seborrheic dermatitis, atopic dermatitis, episodic flushing, lupus erythematosus, photosensitivity and many others.4 Multiple medical and procedural therapies exist for the treatment of unwanted facial redness, including topical medical therapies (i.e., metronidazole), photodynamic therapy, radiofrequency microneedling, as well as laser and light-based therapies. Pulsed dye laser (PDL) and intense pulsed light (IPL) are the two most common light-based devices used to treat facial redness.3 Vascular lasers reduce facial redness by heating the dermal blood vessels thereby reducing blood vessel diameter.3
The decrease in quality of life of patients with persistent facial redness has been demonstrated in a variety of studies utilizing validated questionnaires such as the Rosacea Quality of Life Index (RosaQoL) and the Dermatology Life Quality Index (DLQI).4,5 If untreated, facial erythema can have serious psychological effects, social consequences, and can lead to physical disfigurement.4
One of the latest developments in aesthetic regenerative medicine is exosome technology in a commercially available topical cosmetic product for the resolution of facial photodamage and aging skin changes. Exosomes are extracellular cargos found in a variety of body fluids.6 They are secreted by most cells to communicate with each other.6 Exosomes have a lipid bilayer structure carrying diverse biomolecules based on their cellular origin.7 The cellular origin of exosomes affects their content and ability to act on recipient cells. Platelets are an ideal source of exosomes due to their wound healing abilities. Exosomes also have extremely low immunogenicity along with high hemocompatibilities.8
Topical HPE (plated)™ serum (Rion Aesthetics, Inc, Rochester, Minnesota) with Renewosome™ technology is sourced from U.S. sourced pooled human apheresed platelets. It has the potential to induce maximal effects on skin regeneration and recovery. HPE serum is produced with consistent exosome batch quality, purity, and potency.9 It is an off-the-shelf cosmetic product for skin rejuvenation that has demonstrated induction to normalized skin health at 4 to 6 weeks, with improved clinical measures of facial photodamage and skin aging.9 This study examines evidence-based outcomes for reduction in facial redness after utilizing HPE in a topical cosmetic application six weeks after twice daily use.
Methods
This prospective, single center, open-label study of the safety and efficacy of (plated)™ HPE (human platelet extract) serum was conducted in accordance with the International Conference on Harmonization, Good Clinical Practice guidelines, Code of Federal Regulations, and the Declaration of Helsinki. The study was approved by the Allendale IRB. All subjects gave informed consent before any study procedures were performed. This study was conducted to assess the safety and effectiveness of topical HPE on facial erythema after six weeks of twice daily use.
Subjects meeting all inclusion and exclusion criteria were enrolled and treated with topical HPE serum. The main criteria for inclusion were male and female adults 18 years or older with persistent (non-transient) facial redness with a baseline erythema score of 3 (moderate) or 4 (severe) on both the validated 5-point Clinician’s Erythema Assessment (CEA) scale10 and the validated 5-point Patient Subjective Assessment (PSA) scale.11 Subjects also needed to be willing to minimize external factors that might trigger flushing flare-ups, such as spicy foods, hot drinks, hot environments, prolonged sun exposure, strong winds, emotional stress, and alcoholic beverages during the study. Major exclusion criteria included subjects with significant history or current evidence of any uncontrolled chronic or serious disease or medical condition, female subjects who were pregnant or nursing, or subjects with inflammatory lesions on the face or forms of rosacea that would interfere with the diagnosis or assessment of study endpoints. Additionally, subjects with transient flushing syndrome, diagnosis of severe rosacea, ocular rosacea, rhinophymatous rosacea, or acne fulminans at baseline were excluded.
Exclusionary concomitant medications and treatments prior to and during the entire study period included the use of cosmetic and/or over-the-counter products for redness reduction and/or skin clearing; topical antibiotics, corticosteroids, antiparasitic agents, medications for rosacea; systemic corticosteroids or antibiotics; intense/excessive ultraviolet radiation, phototherapy, facials, chemical peels, and microdermabrasion.
Cosmetic product application. Subjects were instructed to apply topical HPE serum twice daily (morning and evening) for six weeks.
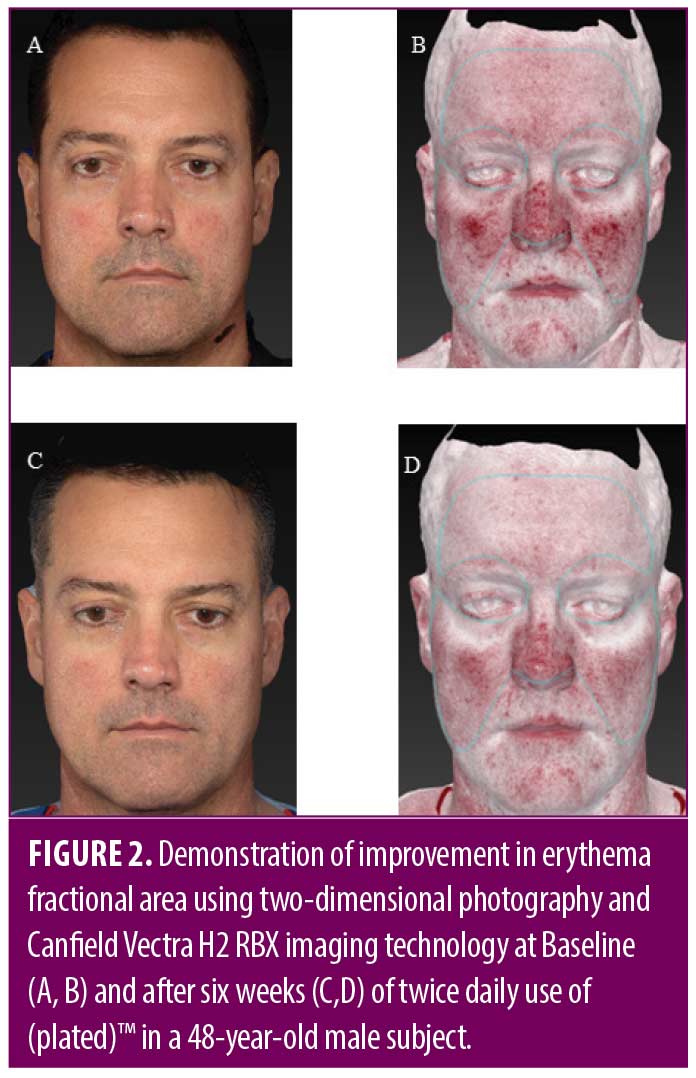
Skin evaluation. Subjects underwent in-office clinical evaluations at baseline (Day 0), Weeks 2, 4, and 6. A follow-up phone call also occurred on Day 7 to assess safety. All office visits included Mexameter™ spectrometry measurements of redness, Canfield Vectra H2 (Canfield Scientific, Parsippany, New Jersey) photography, and evaluation of adverse events. A final follow-up visit was conducted at Week 9 (3 weeks after topical application completion) to assess for lasting efficacy or rebound effect. The end of study visit included photography, Mexameter™ spectrometry, subject opinion survey, and Patient Subjective Assessment (PSA) of redness using the 5-point scale.
Study endpoints and variables. The primary efficacy endpoint was a reduction in the Clinical Erythema Assessment graded by the lead investigator, and a reduction in Patient Subjective Assessment grade at Week 6.
Secondary endpoints included an improvement in Dermatology Life Quality Index (DLQI) related to facial redness, and a reduction in redness by Mexameter™ spectrometry measurement. Safety data included monitoring for adverse events. Subjects also completed a satisfaction survey at Week 9.
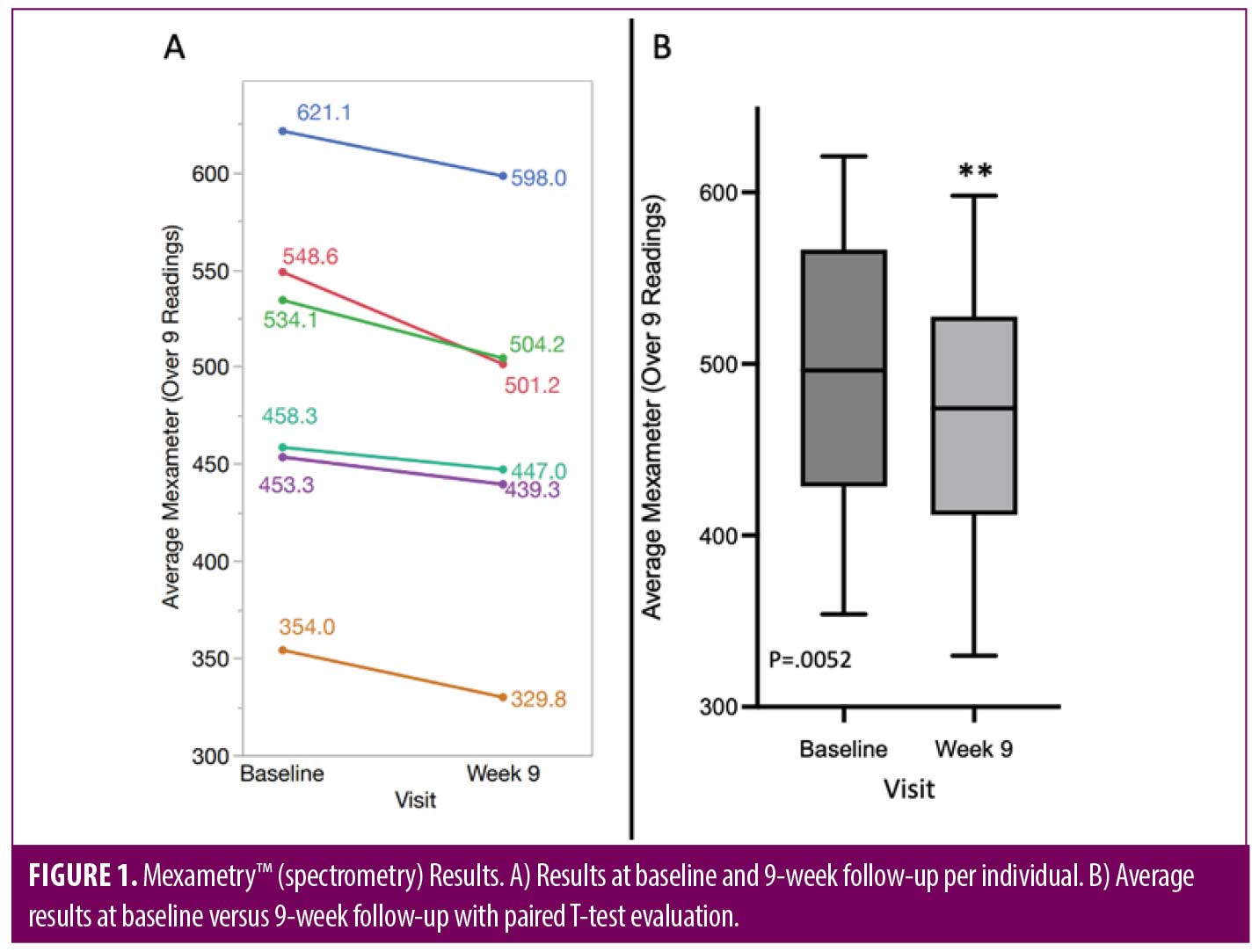
Statistical analysis. Data from surveys and grading is summarized with counts and percentages. The numerical Mexameter™ spectrometry results were averaged across nine regions providing a robust estimate of redness for each subject at each time point. A paired t-test was used to evaluate the improvement from baseline to nine weeks in redness. In addition, a mixed model was used to test for an improvement over time using data across all study visits.
Results
Six participants (4 females and 2 males) with a mean age of 48.8 years (range 42–65 years old) and Fitzpatrick Skin Phototypes II and III were consented and enrolled (Table 1).
The primary endpoint was met. CEA grade by the lead investigator at Week 6 demonstrated that all subjects improved by at least 1 grade, while one subject improved by 2 grades. PSA grade by the subject demonstrated 2 grades of improvement in three (50%) subjects at Week 6, while two subjects had a 1 grade improvement and one subject stated that they did not notice any improvement.
Secondary endpoints were also met. All subjects whose quality of life on the DLQI at baseline showed that they were moderately impacted by their skin problem, improved to small effect by Week 9. Additionally, the majority of subjects (83%; n=5 subjects) reported in an opinion survey that the cosmetic product application positively affected their self-esteem.
Reduction in redness by Mexameter™ spectrometry was statistically significant at Week 9. When averaging the Mexameter™ readings over nine regions per patient, a paired t-test shows a statistically significant average difference of 25 units from baseline to nine weeks (p=0.0052). The range of differences observed was 11 to 47 units. A mixed model, using the data from all five visits, showed a significant effect of time confirming the finding of the paired t-test (Figure 1). For example, a representative 48-year-old male subject is shown using 2D photography and Canfield H2 RBX imaging technology to demonstrate progression of improvement in erythema fractional area from baseline to Week 6 (Figure 2).
Subject opinion survey results at Week 9 (end of study visit) indicated that all subjects experienced improvement in redness, skin irritation, skin tone, texture, and smoothness. All subjects were either satisfied or very satisfied, agreed that their skin appeared healthier, and would recommend the cosmetic serum to a friend. All but one subject experienced minimal to moderate improvement in fine lines and wrinkles as well as improvement in self-esteem. One patient reported dryness as a side effect. No other adverse events were reported.
Discussion
Proper evaluation of facial redness is needed to establish the cause and diagnosis. In this pilot study, topical HPE facilitated a reduction in facial redness with no evidence of complications or safety concerns. The majority of subjects reported an improvement in their self-esteem, which is an important component of psychological well-being in subjects suffering from persistent facial redness.
Indeed, the molecular and cellular biology of sensitive skin that leads to persistent facial redness is complex with limited published reports on improving facial redness with topical formulations.12 We sought to improve stratum corneum moisturization and antioxidant properties with topical HPE, a regenerative nanotechnology. This study showed that daily use of topical HPE offers patients a long-term, non-invasive cosmetic serum that is less invasive than laser procedures. The experiences of participants in this study are consistent with those in previous reports, as topical HPE has also shown benefit in reducing signs of skin aging13 and pigmentation.
Furthermore, facial redness can be caused by abnormalities in clearance of free radial damage caused by UV photodamage and other sources of oxidative stress.14,15 Topical HPE has anti-inflammatory and anti-oxidant properties, which involve hampering the production of free radicals and scavenger-like activity as reported in other platelet sources such as platelet rich plasma.16 Platelet derivatives including growth factors, cytokines, and exosomes harbor anti-inflammatory effects through chemokines such as PF4 (CXCL4) that shape the functional phenotype of macrophages toward the alternative activation (M2) pathways with anti-inflammatory and healing functions.17 Other anti-inflammatory mechanisms from platelet regenerative biocargo have been suggested such as the interference with NF-κB pathway.18
Limitations. A limitation of the facial redness study was that enrollment was based on investigator diagnosis of persistent facial erythema without the diagnosis of rosacea. Patients with persistent centrofacial erythema may also experience sensitive skin and diverse sensory symptoms (e.g., burning, itching, or stinging), which may occur with or without clinical signs (e.g. redness, dryness, or papules19). Therefore, it is plausible that subjects with variety of skin types were enrolled in the facial redness study. Another limitation is the lack of a vehicle-control or comparative product and only changes from baseline levels were assessed. It is noted, however, many studies evaluating improvement in facial redness used a similar approach.20–22 Other study limitations included a small sample size, conducted at a single-center and lack of darker skin types (Fitzpatrick IV-VI). Our results warrant future studies with an expanded population.
Conclusion
This study demonstrates that a novel human platelet-derived extracellular vesicle-based serum with a unique proprietary regenerative technology provides statistically significant improvement in facial redness. Most subjects noticed a positive improvement in their quality of life and self-esteem. All subjects noted improvement in skin tone, skin texture, and skin smoothness with less irritation and redness. Topical HPE is safe and well-tolerated under normal conditions of use. Topical HPE did not show irritant, allergenic, photo-irritation, or photo-sensitization potential. It is an effective first-line cosmetic formulation for persistent facial redness, including rosacea-prone skin.
References
- Heisig M. and A. Reich. Psychosocial aspects of rosacea with a focus on anxiety and depression. Clinical, Cosmetic and Investigational Dermatology. 2018. ume 11:103–107.
- Cribier B. [The red face: art, history and medical representations]. Ann Dermatol Venereol. 2011. 138 Suppl 2: p. S116–123.
- Yepuri V, et al. Light-Based Devices for the Treatment of Facial Erythema and Telangiectasia. Dermatol Ther (Heidelb). 2021. 11(6): p.1879–1887.
- Loyal J, et al. Updates and Best Practices in the Management of Facial Erythema. Clinical, Cosmetic & Investigational Dermatology. 2021. 14:601–614.
- van der Linden MM, et al. Health-related quality of life in patients with cutaneous rosacea: a systematic review. Acta Derm Venereol. 2015. 95(4):395–400.
- Kalluri R and VS LeBleu. The biology, function, and biomedical applications of exosomes. Science. 2020. 367(6478).
- Xiong M, et al. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacological Research. 2021.166.
- Yang GH, et al. Overcome the barriers of the skin: exosome therapy. Biomater Res. 2021. 25(1):22.
- Proffer SL, et al. Efficacy and Tolerability of Topical Platelet Exosomes for Skin Rejuvenation: Six-Week Results. Aesthetic Surgery Journal. 2022. 42(10):1185–1193.
- Tan J, et al. Reliability of Clinician Erythema Assessment grading scale. J Am Acad Dermatol. 2014. 71(4):760–763.
- Tan J and Leoni M. Erythema of Rosacea: Validation of Patient’s Self-Assessment Grading Scale. J Drugs Dermatol. 2015. 14(8): 841–844.
- Nisbet SJ, et al. Clinical and in vitro evaluation of new anti-redness cosmetic products in subjects with winter xerosis and sensitive skin. Int J Cosmet Sci. 2019. 41(6): p. 534–547.
- Proffer SL, et al. Efficacy and Tolerability of Topical Platelet Exosomes for Skin Rejuvenation: Six-Week Results. Aesthet Surg J. 2022 Sep 14;42(10):1185–1193.
- Chen J, et al. Oxidative stress in the skin: Impact and related protection. Int J Cosmet Sci. 2021. 43(5):495–509.
- Tsuchida K and Kobayashi M. Oxidative stress in human facial skin observed by ultraweak photon emission imaging and its correlation with biophysical properties of skin. Scientific Reports. 2020. 10(1):9626.
- Andia I and Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med. 2013. 8(5):645–658.
- Gleissner CA, et al. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010. 184(9):4810–4818.
- Bendinelli P, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010. 225(3):757–766.
- Marriott M, et al. The complex problem of sensitive skin. Contact Dermatitis. 2005.53(2):93–99.
- Grivet-Seyve M, Santoro F, and Lachmann N. Evaluation of a novel very high sun-protection-factor moisturizer in adults with rosacea-prone sensitive skin. Clin Cosmet Investig Dermatol. 2017. 10:211-219.
- Jovanovic Z, et al. Efficacy and Tolerability of a Cosmetic Skin Care Product With Trans-4-t-butylcyclohexanol and Licochalcone A in Subjects With Sensitive Skin Prone to Redness and Rosacea. J Drugs Dermatol. 2017. 16(6):605-610.
- Weber TM, et al. Skin tolerance, efficacy, and quality of life of patients with red facial skin using a skin care regimen containing Licochalcone A. J Cosmet Dermatol. 2006. 5(3): 227–232.