J Clin Aesthet Dermatol. 2023;16(10):52–57.
J Clin Aesthet Dermatol. 2023;16(10):52–57.
by Shari R. Lipner, MD, PhD; Julianne M. Falotico, MD; and Adarsh Konda, PharmD
Dr. Lipner is with the Department of Dermatology at Weill Cornell Medicine in New York, New York. Dr. Falotico is with the Renaissance School of Medicine at Stony Brook University in Stony Brook, New York. Dr. Konda is with Ortho Dermatologics, a division of Bausch Health US, LLC, in Bridgewater, New Jersey.
FUNDING: Funding was provided by Ortho Dermatologics, a division of Bausch Health US, LLC.
DISCLOSURES: Dr. Lipner has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, Moberg Pharmaceuticals, and BelleTorus Corporation. Dr. Konda is an employee of Ortho Dermatologics and may hold stock and/or stock options in its parent company.
ABSTRACT: Onychomycosis is a fungal infection of the nail unit affecting approximately five percent of the global population and representing 50 percent of all nail dystrophies seen in clinical practice. Patients with onychomycosis can suffer significant pain in addition to physical and psychological distress, which may seriously impair their quality of life (QoL). It is well established that onychomycosis prevalence is impacted by patient characteristics, including age and systemic comorbidities. However, the impact of patient sex on onychomycosis occurrence and treatment is not well characterized. This narrative review of the literature was conducted to address a dearth of published information on epidemiology, QoL, clinical trial participation, and treatment success specifically in female patients with onychomycosis. Additionally, an analysis of real-world treatment of onychomycosis in female patients is reported, including prescription patterns and the impact of toenail polish on topical treatments for onychomycosis. Understanding sex as a clinically relevant variable may inform onychomycosis treatment strategies and improve treatment outcomes.
Keywords. Fungal infection, toenail, nail disorders, onychomycosis, gender, women, efinaconazole, topical
Onychomycosis is a fungal infection of the toenails or fingernails caused by dermatophytes, nondermatophyte molds, or yeasts.1 Treatment duration of onychomycosis can be long, as achieving complete cure—comprising a normal appearing nail plate, negative nail fungal culture, and negative potassium hydroxide—may take 12 months or more in toenails due to slow nail growth.2 Additionally, the relapse/reinfection rate is at least 20 to 25 percent.3
Patient factors can impact onychomycosis occurrence and are important to consider when evaluating treatment options with antifungals.4 For example, increasing age is a well-accepted risk factor for onychomycosis,5 as are systemic comorbidities such as diabetes.6 Since 2001, NIH-defined Phase III clinical trials must include valid subgroup analysis by sex/gender if a difference in outcome is expected among groups.7 However, published analyses of onychomycosis burden and its treatment in the female sex are sparse.
With this in mind, we present a narrative review of the existing literature on epidemiology, quality of life (QoL), clinical trial participation, and treatment success in female patients with onychomycosis. We also present real-world data of relevance to onychomycosis in female patients, including prescription patterns by sex for antifungals approved in the United States (US) for onychomycosis treatment, and the impact of nail polish on the efficacy of topical antifungals used for onychomycosis therapy.
Prevalence
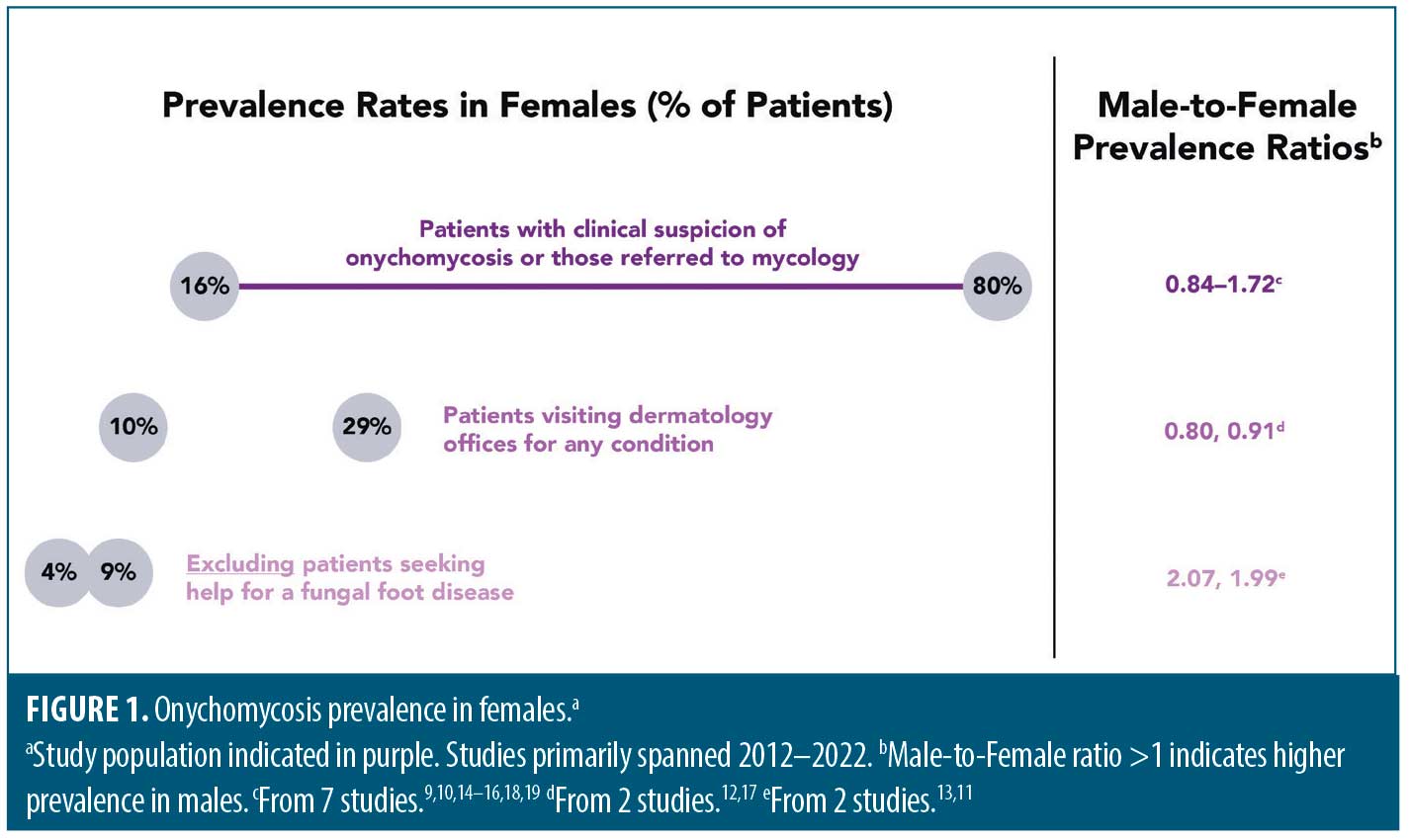
Global prevalence of onychomycosis has been estimated to be approximately 5.5 percent.8 Onychomycosis prevalence in females, however, is difficult to estimate, as reported rates vary greatly. For example, per 11 articles primarily published in the last decade from which female prevalence could be calculated, rates ranged from 4 to 80 percent.9–19 Design differences in these hospital-based studies—including study location, study duration (months to years), patient numbers (hundreds to thousands), and patient populations—could contribute to this variability. As expected, some of the highest female prevalence rates (16–80%) were reported in studies of patients with clinical suspicion of onychomycosis or those referred to mycology, with male-to-female prevalence ratios ranging from 0.84 to 1.72.9,10,14–16,18,19 Intermediate female prevalence rates (10%17 and 29%12) were from patients visiting dermatology offices for any condition, and the male:female prevalence ratio was slightly below 1.0. Finally, the lowest female prevalence rates were in studies that excluded those seeking help for a fungal foot disease (4%11 and 9%13; male:female prevalence ratio of approximately 2) (Figure 1).
The sex differences noted above are unlikely to be due to selection bias, as nine of these 11 studies had a preponderance of females (>50%). The prevalence ratios above are also in line with a systematic review of hospital- and population-based studies from the 1960’s through the early 2000’s, which reported a mean male:female ratio of 1.4 (range: 0.44–3.02).20 Male sex was also associated with onychomycosis in a recent analysis of 121,386 outpatients with continuous insurance coverage.21 The basis for the lower prevalence in females is not clear and warrants further investigation. Some suggest that occupational factors may play a role, with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.22 Differences in hormone levels between males and females also may result in different capacities to inhibit the growth of dermatophytes.22–24 For example, progesterone binds to and inhibits growth of the dermatophytes Trichophyton mentagrophytes and Trichophyton rubrum in vitro.24,25 Human androgenic hormones can also differentially inhibit the growth of dermatophytes.26 How these sex hormones function in vivo to potentially impact fungal infection seen in onychomycosis is yet to be determined. Finally, differences in nail physiology described below may also help explain this sex difference in onychomycosis prevalence.
Physiology
The nail unit is a dynamic structure, with new growth of fingernails and toenails occurring at a rate of 2–3mm and 1mm per month, respectively.27 Reports on nail growth rates varying by sex are conflicting, with the bulk of studies on the topic published in the early- to mid-20th century suggesting no difference in nail growth rate between males and females.28–32 While contemporary literature on nail growth is sparse, one study in which patients self-measured nail growth demonstrated that monthly nail growth rates are slightly faster in healthy, adult American males compared to females (+0.38 [fingernail] and +0.20mm [toenail] per month in males vs. females), although this difference was not statistically significant.33
Nail composition varies between the sexes. Both toenails and fingernails are thicker in males compared to females.34,35 Nail plate and matrix volume is also higher in males than females.36 Male nails have a higher content of disulfide bonds in the α-keratin protein, rendering their fingernail plates stiffer than those of females.37 Such physiological differences in nail biology between males and females may influence treatment success in a sex-specific manner.
Clinical Trials
In general, women and racial/ethnic minorities remain underrepresented in clinical trials in the US despite efforts to diversify research cohorts.38 To determine if this underrepresentation extends to clinical trials for onychomycosis, we analyzed studies of oral only, topical only, or oral and topical antifungal combination therapies for onychomycosis treatment. A literature search on PubMed for onychomycosis randomized controlled/clinical trials was performed on January 9, 2022. Keywords “onychomycosis,” “clinical trial,” and “randomized controlled trial” were used. Studies not reported in English and those using laser/procedural based therapies were excluded. The following data were extracted: author, year of publication, antifungal route of administration (oral, topical, or oral and topical combination), total number of patients, and number of patients by sex. The percentage of patients by sex was calculated using the N values reported.
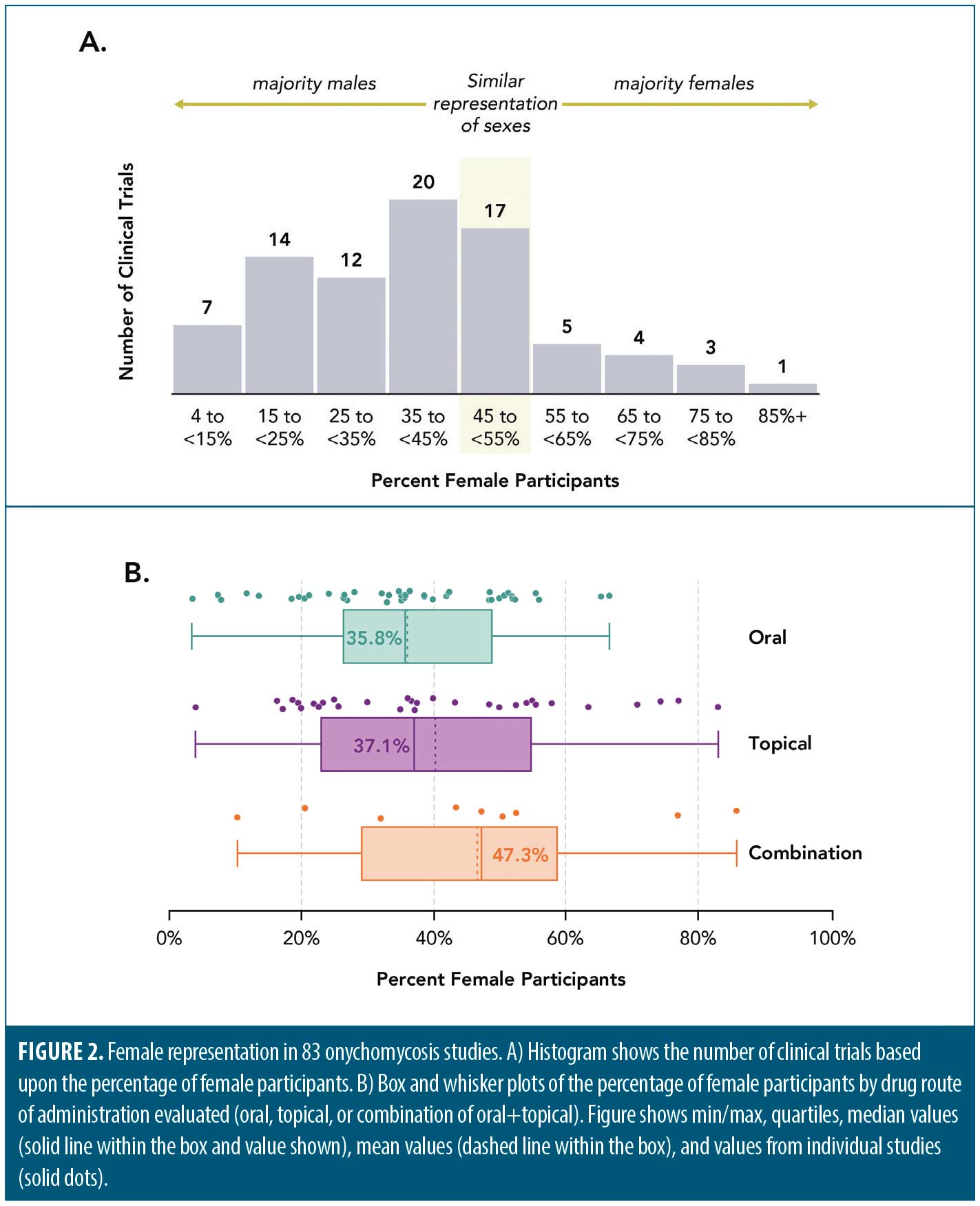
Of 102 studies evaluated (see Supplemental Tables 1 and 2, which can be found on the online version of this article), 83 (81%) reported participants’ sex, and just over a third of all subjects were female (7,121 of 19,994 [36%]). On average, the clinical trials skewed male with respect to sex demographics (Figure 2A–B). In 43 oral-only trials, female participants comprised an average of 36.0 percent (median: 35.8%) of participants across the studies, with an average male:female ratio of 1.77. In 31 topical-only trials, female participants comprised 40.4 percent (median: 37.1%) of the total, with a male:female ratio of 1.47. In nine combination oral-topical trials, female participants comprised 46.7 percent (median: 47.3%) of the total, with a male:female ratio of 1.14. The male:female onychomycosis prevalence ratios from studies over the last 10 years range from 0.8 to 2.07, suggesting the sex demographics in onychomycosis clinical trials appropriately mirrors onychomycosis prevalence reported in the literature.
Supplemental Tables 1–2:
Quality of Life
QoL impairments due to onychomycosis generally impact females more than males.39–48 Early studies, published in the late 1990s, demonstrated that compared with male patients, female patients rated their toenail symptoms as more bothersome.40,41 Females also reported greater pain in their toes and nails, plus greater problems with appearance of their nails and with performing physical activities compared to males.41 Social problems, burden of symptoms, and/or emotional state impairments are more pronounced in females than in males.45,47 Mean scores on the Dermatology Life Quality Index (DLQI) questionnaire were also numerically higher for females compared to males (6.97 vs. 5.73), indicating poorer QoL.43
While antifungal treatments for onychomycosis are associated with QoL improvements,46 there is limited information on potential differences between the sexes. A post-hoc analysis of two Phase III efinaconazole 10% studies found that improvements in QoL were greater in female participants compared to males.22 Scores across all items on the OnyCOE-t questionnaire were lower (worse) in females at baseline (range: males 50.1–72.0, females 32.0–64.9), with greater change from baseline to week 52 (range: males 8.4–26.1, females 12.7–36.0).
Treatment efficacy in females
Onychomycosis treatment efficacy in females is challenging to examine because it is rarely reported in the literature. Of the antifungal drugs approved by the US Food and Drug Administration for the treatment of onychomycosis, analyses by sex have been published for efinaconazole 10% solution (6 publications),22,49–53 tavaborole 5% solution (1),54 oral itraconazole (3),55–57 and oral terbinafine (3).4,56,57 Although the definition of treatment success varied by study, success of onychomycosis treatment was generally greater in women than in men.
Approval of efinaconazole 10% solution was based on two multicenter, double-blind, vehicle-controlled Phase III studies (N=1655), in which 74 to 80 percent of participants were male.58 Though baseline clinical characteristics were similar between male and female participants in the Phase III studies,22 females had higher treatment responses. Following 52 weeks of treatment, complete cure rates were significantly higher in females versus males (27% vs. 16%; P=0.001)50,52 and mean percent affected target toenail was lower (14.4% vs. 20.6%; P value not reported).49 Of 19 participants treated with efinaconazole that were completely cured at Week 24, the majority (12/19; 63%) were female.51 In a Japanese study evaluating efficacy of efinaconazole 10% solution with long-term use for up to 72 weeks (N=219; 66 females), subgroup analysis revealed that treatment success was higher in female compared to male patients (62% vs. 54%; P value not reported).53
Female onychomycosis patients in the tavaborole 5% solution arm in Phase III studies (N=1194, 148 females) saw notable improvements in the appearance of their nail plates over time.54 Nails from tavaborole-treated females had significantly greater increases in the percentage of normal nail (one measure of an improved onychomycosis infection) from baseline to end-of-study at 52 weeks as compared to vehicle-treated nails (31.9% and 7.9%, respectively; P<0.001). In the tavaborole-treated females with more than 50 percent baseline nail plate infection, there were significantly greater increases in normal nail from baseline to end-of-study as compared to patients with 50 percent or less baseline infection (80.9% and 22.4%, respectively; P<0.0001).
For oral antifungals, analyses of treatment success by sex yielded mixed results, with some showing that females were more likely to achieve cure whereas others saw no effect of sex on efficacy outcomes. In a trial comparing continuous terbinafine with intermittent terbinafine in onychomycosis (N=199, 47 females), women were more likely than men to reach clinical cure (defined as a nail without any clinical signs of onychomycosis; odds ratio for males not achieving clinical cure: 2.558 [1.168–5.606]).4 In an analysis of 81 patients (61 females) with onychomycosis due to nondermatophyte molds or Candida in whom treatment with either terbinafine pulse or itraconazole pulse was completed, 51 percent of females versus 15 percent of males were “cured,” with male sex, but not female sex, having significant negative effects on cure rates (P<0.01).56 In a study comparing continuous terbinafine with intermittent itraconazole (N=496, number by sex not reported), univariate prognostic factor analysis showed that no baseline clinical parameter, including sex, had a statistically significant association with mycological cure at 72 weeks.57 Nine months following 4-month treatment with itraconazole in a study of 24 patients (12 females) with onychomycosis, sex of the patient was not correlated with clinical and mycological outcome at the end of the study.55
A caveat to comparing female-specific efficacy results across studies and antifungals is that definitions of success, study populations, disease severity, drug doses, treatment time, and follow up period vary widely. However, available data still suggest that oral and topical antifungals are efficacious in female patients with onychomycosis, and in some instances, efficacy is greater in females compared to males. While the reason for this is unknown, this may be explained, to some degree, by the physiological differences in nail biology between the sexes noted above.
Real-world treatment of onychomycosis in females
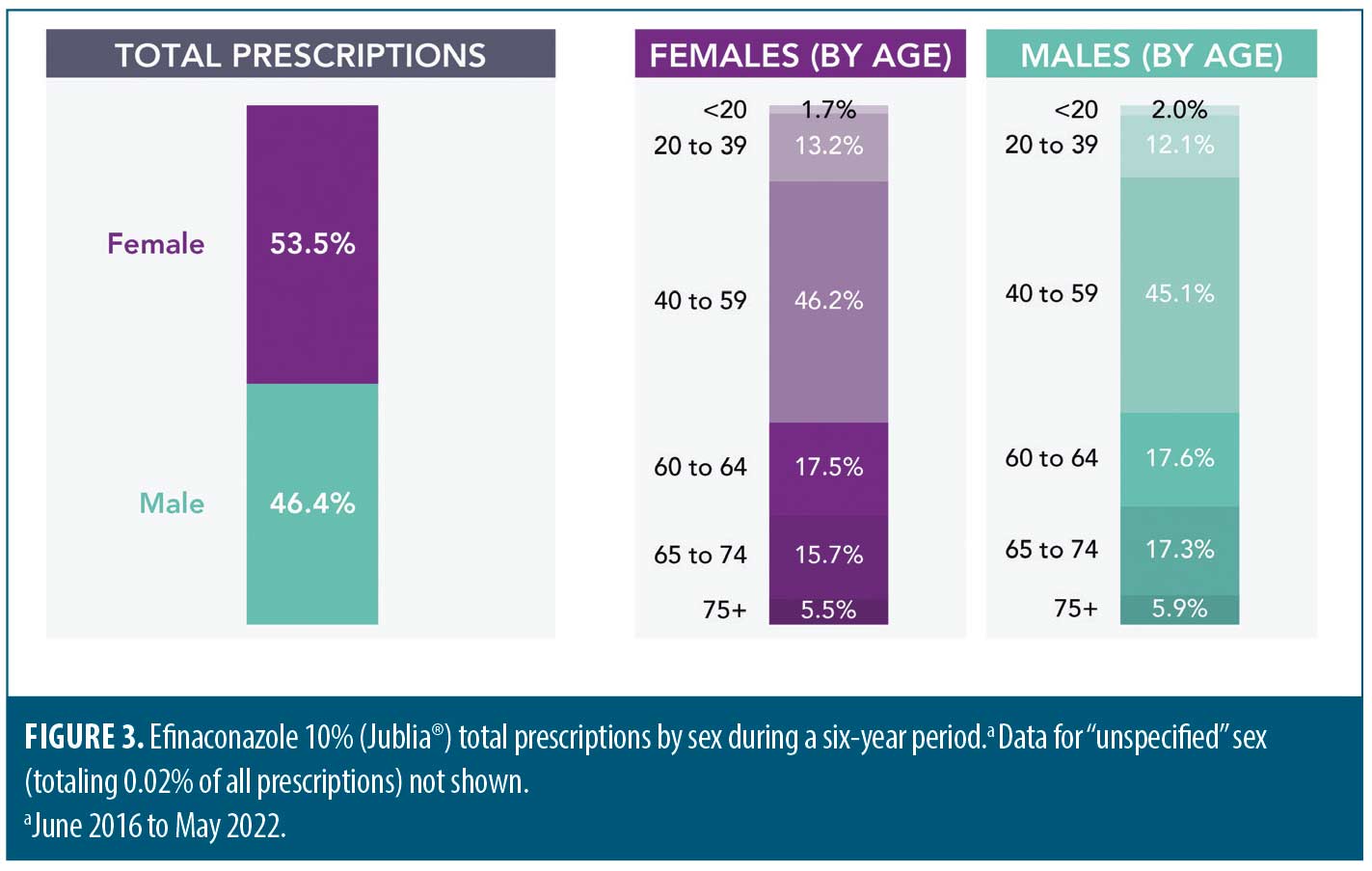
Prescription patterns. Although onychomycosis prevalence generally appears to be greater in men, women represent approximately half or more of patients seeking help or treatment for infected nails. More female patients (57.6% vs. 42.4% male) made visits to physicians’ offices in the US for a possible diagnosis of onychomycosis during an 18-year period from 1993–2010, per the National Ambulatory Medical Care Survey database.59 In the UK between the years 2001 and 2017, a slightly higher proportion of males compared to females (52% vs. 48%) sought treatment for onychomycosis from their general practitioner.60 To explore the treatment of females with onychomycosis in the real world, we analyzed prescription patterns by sex of a commonly used topical antifungal (efinaconazole 10%; Jublia®, Ortho Dermatologics). During the six-year period analyzed, 53.5 percent of prescriptions for topical efinaconazole were to females, indicating that females account for slightly more topical efinaconazole prescriptions than males. (Figure 3).
Topical efficacy in onychomycosis nails treated with polish. Real world treatment of onychomycosis in females is further complicated by the fact that female patients may wish to use nail polish to camouflage nail changes associated with onychomycosis. In clinical trials of topical efinaconazole 10%58 and tavaborole 5%,61 participants were instructed to not apply nail polish;62 such restrictions in the real world may hamper treatment of female patients with onychomycosis.62 Therefore, it is important to understand the compatibility and efficacy of topical antifungals in individuals wearing nail polish, though data on the subject are limited.
Only one study has evaluated the impact of tavaborole 5% solution on nail polish,63 though there are no studies on nail penetrance or clinical efficacy of tavaborole 5% in patients with onychomycosis who concurrently use nail polish. Efinaconazole 10% solution may impact the appearance of nail polish.63,64 However, other data on its compatibility with nail polish are promising. Nail polish did not affect penetrance of efinaconazole 10% through human cadaver nails,65 or clinical efficacy of efinaconazole 10%—measured by onychomycosis severity index, and nail growth and thickness—in patients with onychomycosis.64
Conclusion
There is a paucity of published studies analyzing the impact of onychomycosis on female patients. For reasons that are not well understood, onychomycosis prevalence and the efficacy of current treatments appear to be different in females compared to males. There is a need for more studies assessing real-world factors that may affect treatment of onychomycosis in females, especially the compatibility and efficacy of topical antifungals with concurrent nail polish use. A more thorough understanding of factors that impact onychomycosis development and treatment in females could help mitigate the significant QoL burden experienced by female patients with onychomycosis.
Acknowledgements
Medical writing and editorial support was provided by Kavitha Abiraman, PhD, and Jacqueline Benjamin, PhD, of Prescott Medical Communications Group in Chicago, Illinois, with support from Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC.
References
- Gupta AK, Stec N. Recent advances in therapies for onychomycosis and its management. F1000Res. 2019;8.
- Elewski BE, Gupta AK, Rosen T, et al. Onychomycosis: Does cure equate to treatment success? J Drugs Dermatol. 2016 May;15(5):626–632.
- Tosti A, Elewski BE. Onychomycosis: Practical approaches to minimize relapse and recurrence. Skin Appendage Disord. 2016 Sep;2(1-2):83–87.
- Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010 Jun;24(6):679–684.
- Gupta AK, Venkataraman M, Talukder M. Onychomycosis in older adults: Prevalence, diagnosis, and management. Drugs Aging. 2022 Mar;39(3):191–198.
- Gupta AK, Konnikov N, MacDonald P, et al. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: A multicentre survey. Br J Dermatol. 1998 Oct;139(4):665–671.
- NIH. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2017 [cited 2022 September 01]; Available from: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: An updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14(1):32–45.
- Bitew A, Osman F, Yassin S. Non-dermatophyte mold dominated onychomycosis in patients attending a rank higher specialized dermatology clinic in Addis Ababa, Ethiopia. Clin Cosmet Investig Dermatol. 2022;15:507–518.
- Dhib I, Fathallah A, Yaacoub A, et al. Clinical and mycological features of onychomycosis in central Tunisia: A 22 years retrospective study (1986-2007). Mycoses. 2013 May;56(3):273–280.
- Gupta AK, Gupta G, Jain HC, et al. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30,000 patients visiting physicians’ offices. J Eur Acad Dermatol Venereol. 2016 Sep;30(9):1567–1572.
- Di Chiacchio N, Suarez MV, Madeira CL, et al. An observational and descriptive study of the epidemiology of and therapeutic approach to onychomycosis in dermatology offices in Brazil. An Bras Dermatol. 2013 Feb;88 Suppl 1:3–11.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000 Oct;43(4):641–648.
- Gregoriou S, Mpali N, Vrioni G, et al. Epidemiology of onychomycosis in an academic nail unit in south Greece during a three-year period. Skin Appendage Disord. 2020 Mar;6(2):102–107.
- Iqbal AF, Mahesh TU, Mukesh BS, et al. A clinico-mycological study of onychomycosis at a tertiary care center. Asian Journal of Medical Sciences. 2017 Jan;8(1):48–57.
- Maraki S, Mavromanolaki VE. Epidemiology of onychomycosis in Crete, Greece: A 12-year study. Mycoses. 2016 Dec;59(12):798–802.
- Nkondjo Minkoumou S, Fabrizi V, Papini M. Onychomycosis in Cameroon: A clinical and epidemiological study among dermatological patients. Int J Dermatol. 2012 Dec;51(12):1474–1477.
- Sakkas H, Kittas C, Kapnisi G, et al. Onychomycosis in northwestern Greece over a 7-year period. Pathogens. 2020 Oct;9(10).
- Sylla K, Tine RCK, Sow D, et al. Epidemiological and mycological aspects of onychomycosis in Dakar (Senegal). J Fungi (Basel). 2019 Apr;5(2).
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: A literature study. J Eur Acad Dermatol Venereol. 2014 Nov;28(11):1480–1491.
- Gold JAW, Wu K, Jackson BR, et al. Opportunities to improve guideline adherence for the diagnosis and treatment of onychomycosis: Analysis of commercial insurance claims data, United States. J Am Acad Dermatol. 2023 Mar;88(3):683-686.
- Rosen T. Evaluation of gender as a clinically relevant outcome variable in the treatment of onychomycosis with efinaconazole topical solution 10%. Cutis. 2015 Sep;96(3):197–201.
- Hashemi SJ, Sarasgani MR, Zomorodian K. A comparative survey of serum androgenic hormones levels between male patients with dermatophytosis and normal subjects. Jpn J Infect Dis. 2004 Apr;57(2):60–62.
- Schar G, Stover EP, Clemons KV, et al. Progesterone binding and inhibition of growth in trichophyton mentagrophytes. Infect Immun. 1986 Jun;52(3):763–767.
- Clemons KV, Schär G, Stover EP, et al. Dermatophyte-hormone relationships: Characterization of progesterone-binding specificity and growth inhibition in the genera trichophyton and microsporum. J Clin Microbiol. 1988 Oct;26(10):2110-2115.
- Brasch J, Flader S. Human androgenic steroids affect growth of dermatophytes in vitro. Mycoses.1996 Sep-Oct;39(9-10):387–392.
- Lipner S, Scher R. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Maibach H, Fanian F, Agache P, eds. Measuring the Skin. Springer, Cham; 2015.
- Dawber R. Fingernail growth in normal and psoriatic subjects. Br J Dermatol. 1970 May;82(5):454–457.
- Gilchrist ML. The relation of finger-nail growth to nutritional status. J Anat.1939 Jul;73(Pt 4):575–582.
- Hamilton JB, Terada H, Mestler GE. Studies of growth throughout the lifespan in Japanese: Growth and size of nails and their relationship to age, sex, heredity, and other factors. J Gerontol.1955 Oct;10(4):401–415.
- Hillman RW. Fingernail growth in the human subject; rates and variations in 300 individuals. Hum Biol. 1955 Dec;27(4):274–283.
- Le Gros Clark WE, Buxton LHD. Studies in nail growth. Br J Dermatol. 1938;50(5):221–235.
- Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010 Apr;24(4):420–423.
- Szymoniak-Lipska M, Polanska A, Jenerowicz D, et al. High-frequency ultrasonography and evaporimetry in non-invasive evaluation of the nail unit. Front Med (Lausanne). 2021;8:686470.
- Tosti A, Piraccini B. Chapter 89: Biology of nails and nail disorders. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. McGraw Hill; 2012.
- Wollina U, Berger M, Karte K. Calculation of nail plate and nail matrix parameters by 20 MHz ultrasound in healthy volunteers and patients with skin disease. Skin Res Technol. 2001 Feb;7(1):60–64.
- Brzózka P, Kołodziejski WL. Sex-related chemical differences in keratin from fingernail plates: A solid-state carbon-13 NMR study. RSC Advances. 2017;7:28213–28223.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: A promise yet to be fulfilled. PLoS Med. 2015 Dec;12(12):e1001918.
- Schein JR, Gause D, Stier DM, et al. Onychomycosis. Baseline results of an observational study. J Am Podiatr Med Assoc. 1997 Nov;87(11):512–529.
- Drake LA, Patrick DL, Fleckman P, et al. The impact of onychomycosis on quality of life: Development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol.1999 Aug;41(2 Pt 1):189–196.
- Lubeck DP, Gause D, Schein JR, et al. A health-related quality of life measure for use in patients with onychomycosis: A validation study. Qual Life Res. 1999;8(1-2):121–129.
- Bunyaratavej S, Pattanaprichakul P, Leeyaphan C, et al. Onychomycosis: A study of self-recognition by patients and quality of life. Indian J Dermatol Venereol Leprol. 2015 May-Jun;81(3):270–274.
- Kayarkatte MN, Singal A, Pandhi D. Impact of onychomycosis on the quality of life: Dermatology life quality index-based cross-sectional study. Skin Appendage Disord. 2020 Mar;6(2):115–119.
- Tabassum H, Adil M, Amin SS, et al. The impact of onychopathies on quality of life: A hospital-based, cross-sectional study. Indian Dermatol Online J. 2020 Mar-Apr;11(2):187–194.
- Milobratović D, Janković S, Vukičević J, et al. Quality of life in patients with toenail onychomycosis. Mycoses. 2013 Sep;56(5):543–551.
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: A systematic review. J Am Acad Dermatol. 2021 Nov;85(5):1227–1239.
- Szepietowski JC, Reich A, Pacan P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venereol. 2007 Apr;21(4):491–496.
- Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol.1998 May;38(5 Pt 1):702–704.
- Bhatia N. Managing assessments and expectations: Patient responses following therapy with efinaconazole topical solution, 10%. J Drugs Dermatol. 2015 Jul;14:694–698.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: Impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016 Feb;9(2):42–47.
- Elewski BE, Tosti A, Lin T. Efinaconazole 10% topical solution: Case review of onychomycosis patients who were completely cured at week 24. Skin Appendage Disord. 2018;4(2):67–70.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: A pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014 Jul;13(7):815–820.
- Iozumi K, Abe M, Ito Y, et al. Efficacy of long-term treatment with efinaconazole 10% solution in patients with onychomycosis, including severe cases: A multicenter, single-arm study. J Dermatol. 2019 Aug;46(8):641–651.
- Pariser DM, Wendelken ME, Rycerz AM, Jr., et al. Planimetric post-hoc analysis of women with onychomycosis from tavaborole 5% Phase III studies: Evidence of greater improvements in patients with >50% baseline infection. J Drugs Dermatol. 2018 Feb 1;17(2):168–172.
- Fiallo P, Cardo PP. Age as limiting factor of the efficacy of itraconazole for treatment of onychomycosis. Mycoses. 2001;44(5):191–194.
- Ranawaka RR, de Silva SH. Factors influencing cure rates of non-dermatophyte mold and candida onychomycosis: Analysis of outcomes in 81 patients who completed treatment. Int J Dermatol. 2017 Feb;56(2):202–208.
- Sigurgeirsson B, Paul C, Curran D, Evans EG. Prognostic factors of mycological cure following treatment of onychomycosis with oral antifungal agents. Br J Dermatol. 2002 Dec;147(6):1241–1243.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two Phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013 Apr;68(4):600–608.
- Taheri A, Davis SA, Huang KE, et al. Onychomycosis treatment in the United States. Cutis. 2015 May;95(5):E15–21.
- Sajeed M, Wei L, Murdan S. What can GP data tell us about the treatment of onychomycosis in the UK? Skin Health and Disease. 2022;2(1):e84.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: Results from 2 randomized Phase-III studies. J Am Acad Dermatol. 2015 Jul;73(1):62–69.
- Del Rosso JQ. Application of nail polish during topical management of onychomycosis: Are data available to guide the clinician about what to tell their patients? J Clin Aesthet Dermatol. 2016 Aug;9(8):29–36.
- Vlahovic TC, Coronado D, Chanda S, et al. Evaluation of the appearance of nail polish following daily treatment of ex vivo human fingernails with topical solutions of tavaborole or efinaconazole. J Drugs Dermatol. 2016 Jan;15(1):89–94.
- Canavan TN, Bevans SL, Cantrell WC, et al. Single-center, prospective, blinded study comparing the efficacy and compatibility of efinaconazole 10% solution in treating onychomycosis with and without concurrent nail polish use. Skin Appendage Disord. 2018 Nov;5(1):9–12.
- Zeichner JA, Stein Gold L, Korotzer A. Penetration of ((14)c)-efinaconazole topical solution, 10%, does not appear to be influenced by nail polish. J Clin Aesthet Dermatol. 2014 Sep;7(9):34–36.