J Clin Aesthet Dermatol. 2023;16(10):44–46.
J Clin Aesthet Dermatol. 2023;16(10):44–46.
by Mikel Muse, DO; Blair Harris, DO; Jesse Dewey, DO; Sherlyn Saju, DO; Alexandra Taylor, DO; Luke Maxfield, DO; Christopher Cook, DO; and Rene Bermudez, DO
Drs. Muse, Harris, and Dewey are with Campbell Dermatology at Sampson Regional Medical Center in Lillington, North Carolina. Ms. Saju and Ms. Taylor are with the Campbell University School of Osteopathic Medicine in Lillington, North Carolina. Drs. Maxfield, Cook, and Bermudez are with Campbell University at Sampson Regional Medical Center Dermatology Residency Program in Lillington, North Carolina.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. We sought to record the incidence and risk factors associated with upstaging squamous cell carcinoma in situ (SCCIS) to squamous cell carcinoma (SCC) during Mohs surgery with the largest sample size to date.
Methods. Patient records of preoperative biopsy-proven SCCIS being treated with Mohs between January 2019 to March 2022 were identified and reviewed. Postoperative diagnoses of invasive SCC proven by dermal infiltration on pathology were identified as upstaged SCCIS.
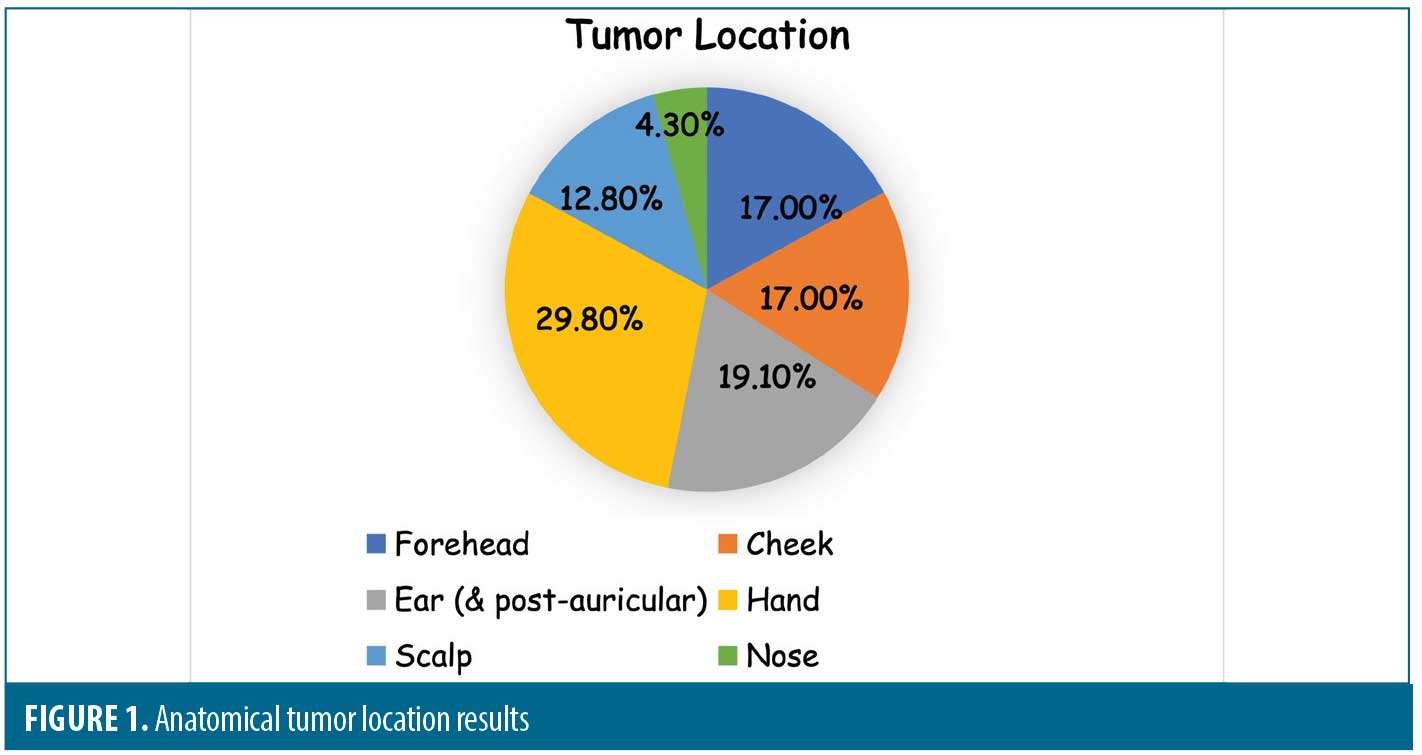
Results. From 2,043 cases of preoperative diagnosed SCCIS, 47 (2.3%) were upstaged to SCC during Mohs surgery. Of the 47 invasive tumors, a large proportion on the hands (29.8%) and lesions with larger preoperative sizes had a higher risk of being upstaged to invasive SCC in this study.
Limitations. All of the patients included were from rural and suburban areas of North Carolina. The only sections obtained were those reviewed for margin analysis, which may significantly underestimate the actual number of invasive SCC, as only the deepest and furthest portions were examined.
Conclusion. This retrospective study concluded that 2.3 percent of preoperatively diagnosed SCCIS were upstaged to SCC during treatment with Mohs surgery. Large lesions (>2cm) and lesions on the hand were more likely to be upstaged (29.8%). Treatment must be individualized considering the size of the lesion, the anatomic location, and the possibility that in some cases the initial biopsy may not have been able to accurately distinguish SCCIS from SCC. Although there is a myriad of treatment options for SCC, select patients with increased risk factors for upstaged SCC must be considered for margin assessed treatment modalities.
Keywords. Squamous cell carcinoma in situ, squamous cell carcinoma, Mohs surgery, basal cell carcinoma
Over one million cases of invasive squamous cell carcinoma (SCC) are diagnosed annually in the United States.1 With its increasing incidence, invasive SCC represents a compelling public health concern.2 According to a study analyzing Medicare databases from 2006 to 2012, there has been a 35 percent increase in non-melanoma skin cancers (NMSC) with the ratio of SCCs to basal cell carcinomas (BCC) approaching a 1:1 ratio versus the previously believed ratio of 1:4.3 Several strategies targeting education, surveillance and monitoring have been underlined in the US Surgeon General’s Call to Action to Prevent Skin Cancer in 2014, in an effort to address the alarming increase in this trend.4
Squamous cell carcinoma in situ (SCCIS) clinically presents as an erythematous scaly patch or plaque and is recognized to be the preceding lesion of SCC.5,6 Numerous therapeutic surgical or non-surgical interventional options can be used in the treatment of SCCIS, giving providers the option to risk-stratify lesions and their treatments using the National Comprehensive Cancer Network guidelines released in 2018.7 Treatment options for SCCIS include topical chemotherapy agents, such as fluorouracil, and topical imiquimod, radiation therapy and surgical procedures, such as electrodessication and curettage, excision, and Mohs surgery. The choice of treatment often depends on the size, anatomical location of the tumor, and the patient’s comorbidities.
Mohs surgery is a tissue sparing surgical treatment for SCC utilized in cosmetically or functionally sensitive areas or for types of skin cancer with aggressive pathology. During Mohs surgery, the physician is both the surgeon and the pathologist, and 100 percent of the tissue margin is reviewed to ensure tumor removal. In some cases, upon treatment with Mohs surgery, the surgeon may see a more aggressive subtype of cancer than originally diagnosed with the initial skin biopsy. This may be due to various dermatopathologists’ approach to reading skin biopsies and/or the biopsy technique, specifically comparing shallow versus deep shave biopsies. For conventional excisions, it is widely understood that only 1 to 2 percent of the actual margins are checked histologically using the traditional dermatopathology “bread loaf” technique.8 Transection of the tumor base may lead to underestimation of the depth and true tumor diagnosis, resulting in inaccurate treatment choice and therefore an upstaging of the final diagnosis.9 This phenomenon is largely provider dependent in terms of biopsy technique and tendency to report on the transected tumor base.9
According to Morton et al,10 the risk of untreated SCCIS progressing to invasive SCC ranges from 3 to 10 percent. Published studies analyzing the incidence of SCCIS being upstaged to SCC during Mohs surgery to date have been limited in number and sample size. A five-year single institution study conducted from 2007 to 2012 by Eimpunth et al9 at the University of California San Diego had a sample size of 556 and an invasion rate of 16.3 percent. More recently, a six-year retrospective study published in 2019 by Newsom et al11 showed an invasion rate of 3.5 percent in a sample size of 428. This same study prospectively analyzed consecutive tumor blocks from Mohs surgery, where instead of only sectioning the margins, the central tumor block was vertically frozen sectioned. It was a six month prospective study with a sample size of 69, revealing an invasion rate of 10.1 percent.11 The authors aim to record the incidence and risk factors associated with upstaging SCCIS to SCC during Mohs surgery with the largest sample size to date compared to these previous studies.
Taking into consideration the pressure to minimize cost on the healthcare system, the most efficacious yet cost-effective treatment choice for patients is desirable. According to a retrospective comparative cohort study conducted by van Lee et al, cutaneous SCC treated with Mohs had a three times lower risk of recurrence than standard excision after being adjusted for tumor size and deep tumor invasion.12 The Mohs Appropriate Use criteria has been developed to help triage treatment decisions for non-melanoma skin cancer. Not all patients are ideal candidates for Mohs surgery, so all treatment options should be considered and tailored to the individual patient based on their individual tumor, its location, size, and patient risk factors, such as immunosuppression.
Methods
A retrospective cohort study was performed by reviewing patient records at a multi-site, multi-provider, private practice group from January 1, 2019, through March 1, 2022. Inclusion criteria encompassed having the initial histologic diagnosis of SCCIS, which was then treated with Mohs surgery. Postoperative diagnosis of invasive SCC with dermal infiltration seen on fresh frozen section was identified as upstaged SCCIS. The study was approved by The Campbell University Institutional Review Board.
Medical records of cases who met inclusion criteria had the following data extracted during review: age, sex, location, size of lesion at initial encounter, biopsy technique, preoperative histopathology, date of Mohs surgery, postoperative histopathology, and final defect size.
Results
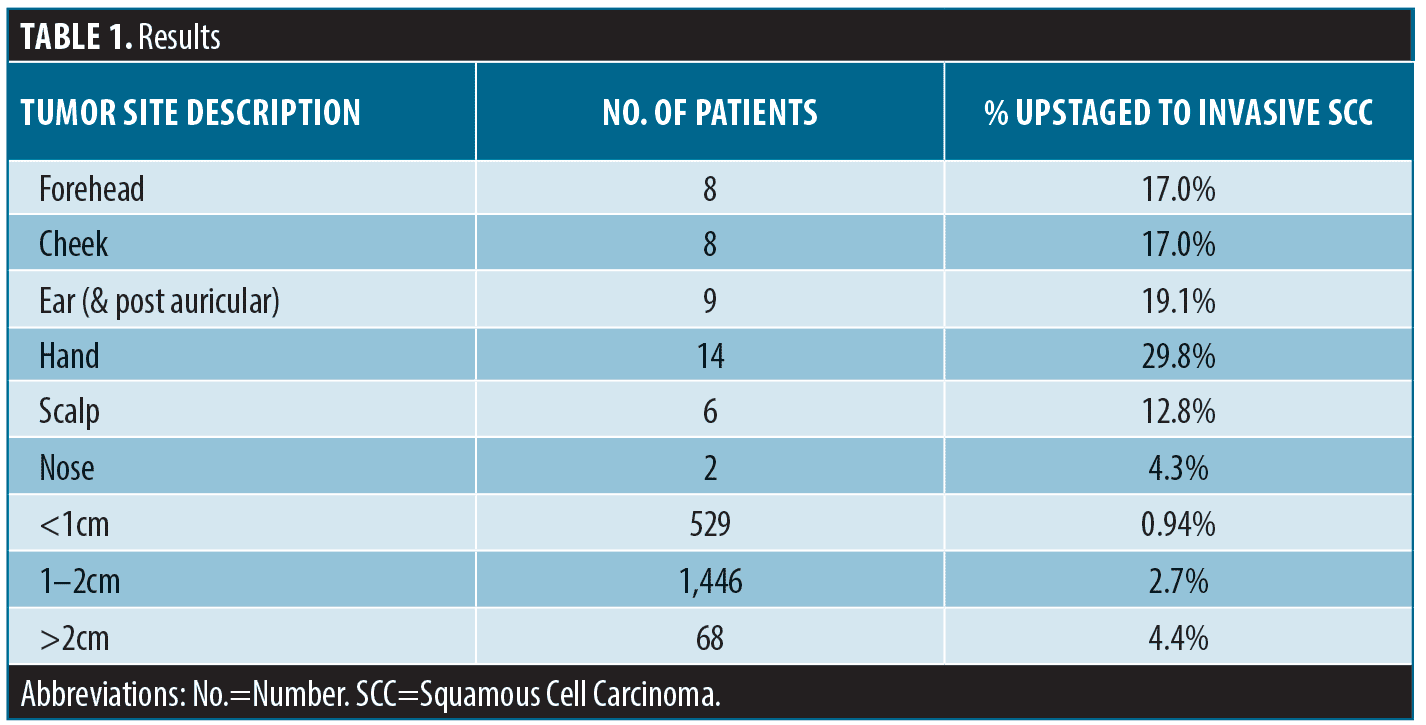
Operative notes from four providers during the three-year period yielded a sample size of 2,043. Of the 2,043 lesions that had the original diagnosis of SCCIS, 47 (2.3%) were upstaged to invasive SCC based on post-operative histology. These specimens were further stratified based on location and size. The authors included the postauricular areas as part of the location of the ear. Of the 47 specimens, 14 (29.8%) came from the hand, nine (19.1%) from the ear, eight (17.0%) from the forehead, eight (17.0%) from the cheek, six (12.8%) from the scalp and two (4.3%) from the nose (Table 1 and Figure 1.) The hands were the anatomic location associated with highest risk of upstaged SCC in this study.
Larger lesions were noted to be associated with an increased likelihood of invasion. Of the 2,043 specimens, 68 (3.3%) specimens had a diameter greater than 2cm and three (4.4%) of these lesions were upstaged to invasive SCC (Table 1). 1,446 specimens had a diameter between 1cm to 2cm and only 39 (2.7%) were upstaged to invasive SCC. From the data extracted over the three-year retrospective study, preoperative diameter size was associated with a higher risk of upstaged SCC.
Conclusion
The incidence of non-melanoma skin cancer is rising, with the overall ratio of SCC to BCC also on the rise. With this increase, Mohs Micrographic Surgery is increasingly becoming utilized. Mohs Appropriate Use Criteria lists SCCIS as appropriate for Mohs surgery in high risk areas including the face, ears, and hands. Our study, with the largest sample size to date evaluating SCCIS upstaged to SCC postoperatively, showed an increased incidence of invasion in these high risk areas, such as the hand, and speaks to the appropriate use of Mohs for SCCIS in these areas. Previous studies were limited in number and furthermore had smaller sample sizes, but showed similar rates of invasion, ranging from 3.3 to 31 percent. Increased sample size increases the power of a study, and the probability of rejecting a false null hypothesis.13 Although this study showed a small number of upstaged lesions, the authors were able to establish that anatomic location and preoperative diameter size were clinical predictors of upstaged SCC and confirmed the appropriate use of Mohs surgery in these areas.
Not every tumor needs to be treated with Mohs surgery and thankfully as dermatologists we have many possible treatment options for SCCIS depending on the anatomical location and individual patient. When deciding if it may be appropriate to de-escalate treatment to a less aggressive, cost effective method, the authors recommend clinically evaluating the lesion for induration or signs of a more aggressive tumor, especially if it is on the hands. In addition, this highlights the importance for Mohs surgeons to thoroughly document if their patients had a more aggressive invasive cancer upon treatment with Mohs surgery as this is supportive documentation that the patient even more strongly warranted treatment with Mohs surgery.
Limitations. Limitations to this study include the aspect that all of the patients included were from rural and suburban areas of North Carolina. The only sections obtained were those reviewed for margin analysis, which may significantly underestimate the actual number of invasive SCC, as only the deepest and furthest portions were examined. In addition, the diagnosis of invasive SCC post-treatment was confirmed by each individual Mohs surgeon but not confirmed with a second physician or dermatopathologist. A small prospective study looking deeper into the tumor blocks found significantly higher rates of invasion (10-30%), and this may be a more meaningful way to study rates of invasion for our cutaneous tumors treated with Mohs surgery.
References
- Guzman AK, Schmults CD, Ruiz ES. Squamous Cell Carcinoma. Dermatologic Clinics. 2023;41(1):1–11.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatology. 2015;151(10):1081–1086.
- U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. Washington, DC: U.S. Dept of Health and Human Services, Office of the Surgeon General; 2014.
- Combalia A, Carrera C. Squamous Cell Carcinoma: An Update on Diagnosis and Treatment. Dermatology Practical & Conceptual. 2020;10(3).
- Shimizu I, Cruz A, Chang KH, et al. Treatment of squamous cell carcinoma in situ: a review. Dermatologic Surgery: Official Publication for American Society for Dermatologic Surgery [et Al]. 2011;37(10):1394–1411.
- Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78(3):560–578.
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019 May;180(5):1240–1241.
- Eimpunth S, Goldenberg A, Hamman MS, et al. Squamous Cell Carcinoma In Situ Upstaged to Invasive Squamous Cell Carcinoma: A 5-Year, Single Institution Retrospective Review. Dermatologic Surgery. 2017;43(5):698–703.
- Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen’s disease) 2014. Br J Dermatol. 2014;170(2):245–260.
- Newsom E, Connolly K, Phillips W, et al. Squamous Cell Carcinoma In Situ With Occult Invasion: A Tertiary Care Institutional Experience. Dermatologic Surgery. 2019;45(11):1345–1352.
- van Lee CB, Roorda BM, Wakkee M, et al. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br J Dermatol. 2019; 181: 338–343.
- Serdar CC, Cihan M, Yücel D, et al. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021;31(1):010502.