 J Clin Aesthet Dermatol. 2020;13(9):45–48
J Clin Aesthet Dermatol. 2020;13(9):45–48
by Angela Yen Moore, MD and Stephen Moore
Dr. Moore is with the Arlington Research Center in Arlington, Texas, Baylor University Medical Center in Dallas, Texas, and University of Texas Medical Branch at Galveston in Galveston, Texas. Mr. Moore is with the Arlington Research Center in Arlington, Texas.
FUNDING: The authors received partial funding from Biofrontera, Inc., for retrospective chart review, data analysis, and manuscript preparation.
DISCLOSURES: AY Moore has received funds as consultant and clinical study investigator for Biofrontera. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
ABSTRACT: Background: Photodynamic therapy (PDT) is a well-known treatment modality for actinic keratosis (AK). The largest surface area approved by the FDA is 20cm2 with 10% 5-aminolevulinic acid hydrochloride gel (10% ALA gel).
Objective: This retrospective study assessed the tolerability of PDT with 10% ALA gel in areas ranging from 75cm2 to 300cm2.
Method: The medical records of 203 patients with AKs treated with 376 PDT sessions using 10% ALA gel were reviewed. Face and ears were incubated with 10% ALA gel for 60 minutes without occlusion while all other areas were incubated for 90 minutes with plastic wrap occlusion followed by 10J/cm2 blue light. Patients were given specific post-PDT care directions. Patient outcomes data was collected.
Results:Skin irritation was reported in 27 (7%) PDT sessions in 25 patients (12%). These occurred primarily on the face (n=17), hands (n=4,) and scalp (n=3). Of the 349 PDT treatments (93%) without irritation, these subjects reported adherence to a specific post-PDT regimen using zinc oxide and healing creams for 48 hours.
Limitations: This was a retrospective study observing safety and tolerability. Clearance data was not collected.
Conclusions: Based on this retrospective observational case series, PDT with ALA gel appears to be safe for treating patients with AKs covering surface areas 75 to 300cm2. Irritation might be mitigated by post-PDT care regimens.
Keywords: Actinic keratosis, photodynamic therapy, retrospective study, 5-aminolevulinic acid gel, safety, tolerability
Actinic keratoses (AK) are precancerous lesions that generally result from excessive exposure to ultraviolet light, especially sun-exposed areas of fair-skinned individuals.1 Early treatment of AKs might prevent progression to invasive squamous cell carcinomas.2
A meta-analysis of 25 studies reviewed the efficacy of current therapies for AKs in 5,562 patients.3 Treatments included photodynamic therapy (PDT), cryotherapy, imiquimod, diclofenac, fluorouracil, and ingenol mebutate. Typical adverse events included application-site erythema, burning, pain, scaling, and crusting.
The mechanism of PDT involves the application of a photosensitizing agent followed by irradiation with light of an appropriate wavelength. The two most frequently used photosensitizers in dermatology are the pro-drugs methyl-aminolevulinate (MAL) and 5-aminulevulinic acid (ALA gel), which are preferentially absorbed into AK lesions metabolized into the active ingredient protoporphyrin which is activated by a light source.4 The subsequent generation of reactive oxygen species results in targeted cell death.5 Protoporphyrin has maximum absorption efficiency for blue light while red light provides maximum tissue penetration.4
Interest in field cancerization has increased because subclinical AK lesions can be found by histology in areas surrounding visible AKs.6 PDT with ALA gel is approved by the United States (US) Food and Drug Administration (FDA) for actinic keratosis in field-directed treatment areas measuring 20cm2 or smaller. Pivotal trials treated fields of cancerization with ALA gel over areas approximately 20cm2.7 Following one or two treatment sessions with a 635nm red light, 91 percent of those subjects achieved complete lesion clearance after three months. Complete clearance was maintained in 63 percent of subjects at 12 months following field application.7
Dermatologists in the US often face the challenge of patients with AKs on surface areas larger than 20cm2 that need treatment, but little patient-outcome data exists related to tolerability in larger treatment areas or with blue light illumination. This retrospective study assessed the safety of PDT when 10% ALA gel was applied to AKs in areas of field cancerization between 75cm2 and 300cm2.
Methods
This study was conducted in accordance with the principles of the Declaration of Helsinki and the principles of Good Clinical Practice and satisfied all applicable regulatory requirements, per International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines.8 The protocol for this study was approved by Schulman IRB, Columbia, MD (#201707236).
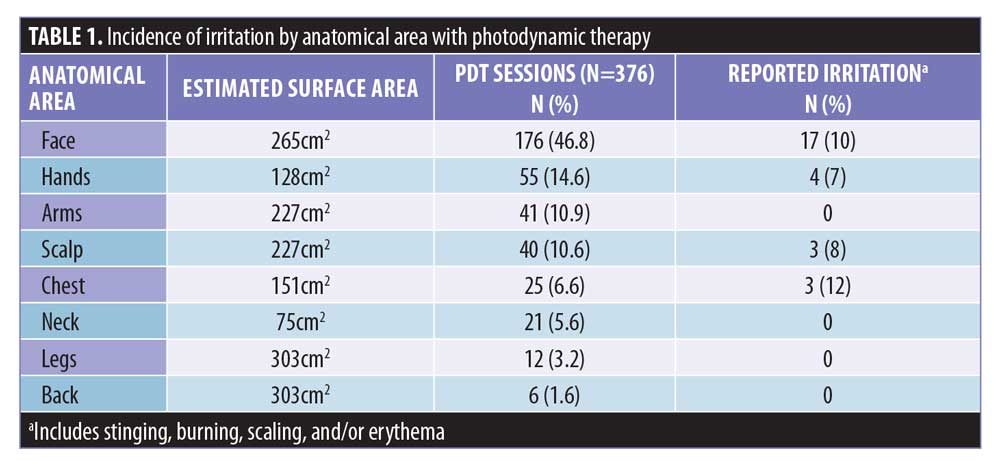
The medical records of 203 patients with AKs treated with 376 PDT sessions using 10% ALA gel (Biofrontera, Inc., Woburn, Massachusetts) in a single dermatology clinic in North Texas between April 2017 and May 2018 were reviewed. Planned treatment areas were degreased robustly with acetone-soaked 4×4 gauze in all patients and then debridement was performed on hyperkeratotic lesions. A full tube (200g) of 10% ALA gel was then applied to estimated surface areas of 265cm2 on full face and ears (Face), 130cm2 on bilateral dorsal hands (Hands), 230cm2 on bilateral dorsal wrists to elbows (Arms), 150cm2 on scalps (Scalp), 150cm2 on decolletés (Chest), 75cm2 on anterior or posterior necks (Neck), 300cm2 on bilateral shins or calves (Legs), and 300cm2 on upper backs (Back) (Table 1).9 The face and ears were incubated with 10% ALA gel for 60 minutes without occlusion, while other anatomical areas were incubated for 90 minutes with plastic wrap occlusion. Patients were instructed to stay within the building but could walk around inside the covered atrium or office building during their incubation period.
Illumination was performed with 10J/cm2 blue light (417nm blue light for a duration of 16 minutes and 40 seconds) (DUSA Pharmaceuticals, Inc., Wilmington, Massachusetts). Immediately after illumination, a specific post-PDT regimen to expedite healing and decrease irritation was recommended. This regimen included a physical sunblock with >10% zinc oxide simultaneously with healing creams containing zinc and/or hyaluronic acid, which were applied every two hours during waking hours by the patient for 8 to 96 hours following PDT treatment. Patients were advised to avoid prolonged sunlight and tanning beds for 48 hours after PDT and to adhere to the post-PDT regimen even if indoors, because sources of blue light other than the sun also include digital screens (e.g., TVs, computers, laptops, smart phones, and tablets), electronic devices, and fluorescent and LED lighting.10 Patients were instructed to keep a record of any irritation, including burning, stinging, scaling, or redness, and to call if these symptoms occurred despite usage of the post-PDT cream regimen. The severity of the symptoms was patient-reported based on the patient complaint, according to each of the parameters. During the first week following PDT, patient-reported symptoms of discomfort and clinician-confirmed signs of skin irritation were collected on stinging or burning, dryness, scaling, erythema or redness, crusting, and persistence of symptoms for >24 to 48 hours. Irritation data was also collected on treatment area, adherence to post-PDT care, and the month when treatment occurred. Tolerability was defined as the lack of any reported irritation symptoms for the week following PDT.
Follow up was performed at the routine follow up visit within two weeks after PDT or phone calls between visits as indicated. All of the irritation subsided within two weeks. For those who had incomplete follow-up data within two weeks of PDT for any reason, an inquiry was made to the patient regarding irritation at the subsequent appointment within four weeks.
Results
Of the 203 patients, 59.6 percent (121/203) were males and 40.4 percent (82/203) were females. Ages ranged from 54 to 95 years. Fitzpatrick Skin Types included Type 2 (n=98; 48.3%), Type 3 (n=96; 47.3%), and Type 4 (n=9; 4.4%). Fifty (13.3%) of the 376 PDT sessions involved debridement of at least one hyperkeratotic lesion, while 326 (86.7%) did not. Of the 376 sessions, PDT treatments included the face (n=176; 46.8%), hands (n=55; 14.6%), arms (n=41; 10.9%), scalp (n=40; 10.6%), chest (n=25; 6.6%), neck (n=21; 5.6%), legs (n=12; 3.2%), and back (n=6; 1.6%) (Table 1).
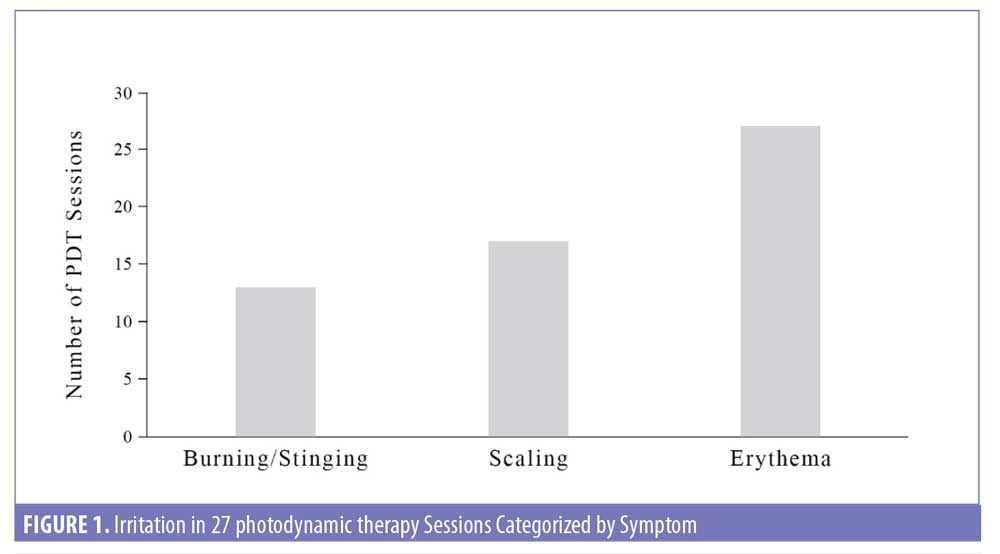
Of the 376 PDT sessions, irritation was not observed in 93 percent (349/376) but observed in 7 percent (27/376) of sessions. Irritation after PDT occurred in 12 percent (25/203) of the patients. Reports of irritation after PDT occurred primarily on the Face (n=17, 63%), followed by Hands (n=4, 15%), Scalp (n=3, 11%), and Chest (n=3, 11%), with none on Arms, Neck, Legs, and Back (Table 1). Characteristics of irritation included persistent burning or stinging after 13 of the PDT sessions (3.5%), scaling after 17 PDT sessions (4.5%), and redness or erythema after 27 of the PDT sessions (7.2%) (Figure 1). Symptoms of burning or stinging were described as severe by the patient in eight cases and mild in five cases. Symptoms of scaling were described as more severe in eight, mild in five, and minimal in four. Of those with redness, symptoms persisted for up to one week in 17 cases but resolved within 24 to 48 hours in 10 cases.
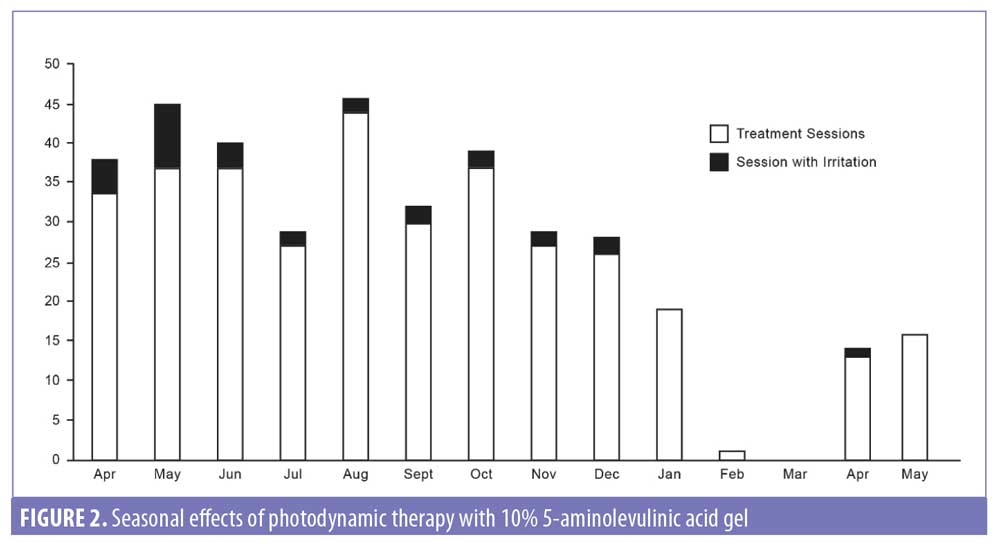
Overall, irritation persisted for more than 24 hours in 17 of the 27 (4.5%) PDT sessions in which irritation was reported. Among the 25 patients reporting skin irritation, most (n=17; 68%) had irritation with the first PDT session associated with poor adherence to the recommended post-PDT regimen. However, after strict adherence to the PDT post-care regimen, these same patients experienced no irritation in subsequent PDT sessions. When the frequency of PDT-related irritation events was analyzed by month, no significant seasonal changes were found (Figure 2).
Discussion
In contrast to long-term topical therapies, PDT is an office-based treatment and reimbursed well by Medicare. As such, PDT provides an ideal treatment for patients with numerous AK lesions over large solar-damaged surface areas, and notably in areas of cosmetic concern.11 Topical treatments for AKs, such as imiquimod, fluorouracil, diclofenac, and ingenol mebutate require patient adherence, have the potential to cause irritation, and have been reported to be poorly reimbursed by Medicare.3 One study reported nonadherence rates of over 50 percent for AK topical therapies requiring four weeks of treatment and over 70 percent for topical therapies requiring 6 to 12 weeks of treatment.2
Favorable tolerability and safety data for PDT with 10% ALA gel for surface areas larger than 20cm2 would allow for larger surface areas to be treated, thus treating both visible AKs and subtle actinic damage and decreasing frequency of patient visits to treat smaller areas. Nonadherence to post-PDT care leads to more irritation, but its effect on efficacy is unknown. Our results suggest that a longer incubation time leads to higher irritation. During the clinical development of 10% ALA gel, its safety and efficacy for treating AKs were assessed in three Phase III, double-blind, placebo-controlled trials.7,12,13 When 10% ALA gel was used for lesion-directed treatment, the overall incidence of irritation associated with locally applied 10% ALA gel followed by red light illumination was 96.4 versus 72.4 percent for placebo-treated subjects.11 In these pivotal Phase III studies, 10% ALA gel was incubated on the face and scalp for 180 minutes under an occlusive dressing followed by 635nm red light illumination.7,12,13 This incubation period with 10% ALA gel was considerably longer than the 60 to 90 minutes of incubation used in this study. The incidence of one or more clinician-confirmed signs of skin irritation, defined as application site erythema, edema, and exfoliation, in Phase III clinical trials was 77.8 percent, and the incidence of one or more patient-reported symptoms of discomfort-related irritation (e.g., burning, pain, pruritis, etc.) was 87.1 percent. When the treatment area was increased to 20cm2, irritation occurred in 100 percent of subjects, versus 69 percent of placebo-treated subjects.7 The most common events (>10%) were mild-to-moderate erythema, pain, burning, irritation, edema, pruritus, exfoliation, scab, induration, and vesicles.7
Our results suggest that treating a larger surface area in a single PDT session does not increase irritation. In the present study, blue light illumination following application of 10% ALA gel to 265cm2 for 60 minutes on the face and ears without occlusion and 90 minutes with occlusion to surface areas ranging from 75cm2 to 300cm2 on other treatment areas resulted in irritation among only seven percent of PDT sessions and 12 percent of treated subjects. Nestor et al14 reported a similar decrease in irritation with shorter incubation periods. In that study, 10% 5-ALA gel was applied to AKs on the face or scalp over a 25cm2 area for one hour incubation followed by 1,000 seconds of blue light illumination.14 PDT was repeated after 28 days. Although our study does not evaluate efficacy, Nestor et al observed complete clearance in 86.8 percent of AK treatment areas, and 97.1 percent clearance of AK lesions at post-treatment eight weeks after two PDT sessions with similar incubation periods as described in the current study.14
Patient-reported symptoms and clinician-confirmed signs of irritation of 7.2 percent erythema, 4.5 percent scaling, 3.5 percent burning/stinging in this study are lower than the irritation reported in the Nestor study.14 In the Nestor study, treatment-reported application site reactions included erythema (15.4%), scaling/dryness (10.3%), and crusting (5.1%) within three days after the first PDT. Three days after the second PDT, local skin reactions included erythema (18.4%), scaling/dryness (2.6%), and crusting (2.6%).
Other factors that might account for the decreased irritation in the current study compared to the Phase III pivotal trials and the Nestor study include the lack of an occlusive dressing on the face and ears and the post-PDT regimen implemented with strict admonition on avoidance of sunlight, tanning beds, and blue light for 48 hours after PDT and directions on application of physical sunblock with zinc oxide >10% and healing creams every two hours during waking hours for 48 hours after PDT. Notably, no irritation occurred in 93 percent of PDT sessions in patients who were adherent to the recommended post-PDT regimen. In the 27 PDT sessions (7%) in which irritation occurred, nonadherence by the patients with the recommended post-PDT regimen was noted, while strict adherence to the recommended PDT post-care regimen eliminated irritation in 63 percent of subsequent PDT sessions in these patients.
The results of the current study also demonstrate that liberal acetone for skin degreasing prior to application of 10% ALA gel did not increase irritation compared to isopropyl alcohol, which was applied in the Phase III clinical trials of 10% ALA gel.7,12,13 Anecdotally, dermatologists have expressed concerns about increased irritation during the summer months due to greater exposure to sunlight, especially in geographic areas with intense summer heat. In the current study, the PDT sessions were performed in Texas, and no significant seasonal variability emerges in the frequency of PDT-related irritation.
Conclusion
Patient tolerability data in this retrospective analysis of PDT with 10% ALA gel and blue light illumination on surface areas ranging from 75cm2 to 300cm2 suggests that PDT treatment of surface areas larger than 20cm2 is safe. Irritation might be further mitigated with 48-hour post-PDT regimens that include sunblock with zinc oxide >10% and healing creams applied simultaneously every two hours during waking hours. Overall, irritation was reported in seven percent of PDT sessions (12% of subjects), with the remaining 93 percent of PDT sessions showing no irritation when patients were adherent to the post-PDT regimen. Among the 25 patients reporting irritation, 68 percent had no irritation in a subsequent PDT session after strict adherence to the PDT post-care regimen despite irritation after the first PDT session and poor adherence to the recommended post-PDT regimen after the first PDT session. The reduced frequency of irritation might also be due to the 60-minute incubation period on the face and neck and 90-minute incubation on other anatomical areas. A randomized, prospective study might stratify the importance of ALA gel incubation duration, larger treatment areas, tolerability, seasonality, and post-PDT care regimens.
Acknowledgements
Authors received partial funding from Biofrontera, Inc., Woburn, Massachusetts, for an investigator-initiated study including a retrospective chart analysis and manuscript preparation. The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., for citations in the introduction and discussion sections of this manuscript.
References
- Sheth VM, Pandya AG. Melasma: A comprehensive update: Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:4–7.
- Fernandez Figueras MT. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol. 2017;31:5–7.
- Gupta a K, Paquet M. Network meta-analysis of the outcome “participant complete clearance” in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169: 250–259.
- Tampa M, Sarbu MI, Matei C, et al. Photodynamic therapy: a hot topic in dermato-oncology. Oncol Lett. 2019;17:4085–4093.
- Benov L. Photodynamic therapy: current status and future directions. Med Princ Pract. 2015;24.
- Szeimies RM, Torezan L, Niwa A, et al. Clinical, histopathological and immunohistochemical assessment of human skin field cancerization before and after photodynamic therapy. Br J Dermatol. 2012;167:150–159.
- Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz®) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED® lamp. Br J Dermatol. 2016;175: 696–705.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6(R1), 1996. Available: https://www.ich.org. Accessed May 2019.
- Agarwal P, Sahu S. Determination of hand and palm area as a ratio of body surface area in Indian population. Indian J Plast Surg. 2010;43(1):49–53.
- Blue Light Exposed site. Blue Light Exposed. http://www.bluelightexposed.com. Accessed 30 Sept 2019.
- Dirschka T, Radny P, Dominicus R, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind Phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol. 2012;166:137–146.
- Szeimies RM, Radny P, Sebastian M, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br J Dermatol. 2010;163:386–394.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825–836.
- Nestor MS, Berman B, Patel J, Lawson A. Safety and efficacy of aminolevulinic acid 10% topical gel versus aminolevulinic acid 20% topical solution followed by blue-light photodynamic therapy for the treatment of actinic keratosis on the face and scalp: a randomized, double-blind study. J Clin Aesthet Dermatol. 2019;12:32–38.

