 J Clin Aesthet Dermatol. 2020;13(9):41–44
J Clin Aesthet Dermatol. 2020;13(9):41–44
by Marina Agozzino, MD; Gaetano Licata, MD; Caterina Maria Rosaria Giorgio, MD; Graziella Babino, MD; Amalia Lupoli, MD; Isabella Sperduti, MD; Giuseppe Argenziano, MD; and Marco Ardigò, MD
Drs. Agozzino, Licata, Giorgio, Babino, Lupoli, and Argenziano are with Dermatolgy Unit at the University of Campania Luigi Vanvitelli in Naples, Italy. Dr. Ardigò is with the Department of Dermatological Clinic at the San Gallicano Dermatological Institute-IRCCS in Rome, Italy. Dr. Sperduti is with the Biostatistical Unit, IRCCS at the Regina Elena National Cancer Institute in Rome, Italy.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
BACKGROUND: The management of melasma remains a challenge in dermatology, despite the availability of a variety of therapies, such as sunscreens, hypopigmented topical treatments, and chemical peels. The ideal treatment for melasma should be efficient, conclusive, and free from side effects.
Objective: The aim of this study is to assess the efficacy and tolerability of a depigmenting agent complex, based on the synergy of oligopeptide-68, phytic acid, glycolic acid, lactic acid, and octinoxate measured by clinical and reflectance confocal microscopy outcomes in melasma patients.
Methods: Twenty female patients exhibiting clinical evidence of melasma, aged between 29 and 61 years, were randomly enrolled in the study. Reflectance confocal microscopy was performed at baseline and after three months of treatment.
Results: Clinically, all patients showed significant improvement in melasma after treatment without adverse reactions. A relevant improvement in the treated skin areas was also assessed using reflectance confocal microscopy analysis.
Conclusion: This study suggests that a combination of topical agents containing oligopeptide-68, phytic acid, glycolic acid, lactic acid, and octinoxate produces significant rapid improvement in melasma at clinical and cellular levels.
Keywords: Melasma, reflectance confocal microscopy, depigmenting agents
Melasma is a common pigmentary disorder caused by abnormal melanin deposits in the skin occurring most frequently in women with Fitzpatrick Skin Phototypes 3 through 5. It can be caused or aggravated by prolonged sun exposure and high levels of sex hormones as a result of pregnancy or extensive use of birth control pills. Other risk factors include use of certain drugs, such as tetracycline or antimalarial drugs. The excessive pigmentation has been attributed to both the increased number of melanocytes and the production of melanin.1
The treatment of melasma is challenging and an accurate clinical evaluation of anatomical location of the affected area, skin phototype, age, duration of disease, pattern of onset, and identification of possible aggravating factors is important.2 There are a variety of treatment options, including sunscreens, hypopigmented topical treatments, and chemical peels. No single agent has proved to be effective for all patients, a combination of two or three agents is often tried in order to achieve optimum results. Physical therapies are frequently used with good clinical success rates, but they are often associated with side effects, recurrence rates, and post-inflammatory hyperpigmentation.3 The ideal treatment for melasma should be efficient, conclusive, and free from side effects; unfortunately, it remains a challenge, and a mild improvement is all that is obtained in a majority of patients. To assess the efficacy of a topical product in melasma by means of biopsy is difficult and is not feasible because of its invasiveness. However, a skin biopsy is not the best way to dynamically monitor skin changes due to the impossibility of evaluating the same area over time and to the inflammatory effects in the surrounding tissues occurring during wound-healing. The choice of the best therapeutic approach depends on the identification of the location and extension of pigment deposition. It is possible to classify melasma into three main subtypes on the basis of Wood light examination: epidermal, dermal, and mixed type.4 However, numerous studies have demonstrated that the Wood light might not be accurate enough to define the location of the pigment deposition. In this regard, reflectance confocal microscopy (RCM) can be used as a noninvasive tool to locate precisely abnormal deposits of pigment, providing additional cytological details for diagnosis and treatment monitoring.5–6 Based on RCM, melasma is classified into two major types, epidermal and mixed type, showing complete coherence with histology. Few reports apply RCM to the evaluation of melasma.7-8 Moreover, RCM can also be used to visualize dynamic skin processes, allowing the in-vivo follow-up of skin lesions after invasive and noninvasive treatments.9,10,11 The aim of our study is to evaluate the efficacy and tolerability of depigmenting topic agents based on the synergy of oligopeptide-68, phytic acid, glycolic acid, lactic acid, and octinoxate, the Obagi® Nu-Derm System’s (Obagi Cosmeceuticals LLC, Long Beach, California) key ingredients, by means of RCM.
Methods
Twenty female patients, aged between 29 and 61 years, exhibiting clinical evidence of melasma were randomly enrolled in the study. Informed consent was obtained from each patient. After cleaning the skin, patients applied the depigmenting agents once daily for 12 weeks. Chemical sunscreens (SPF 50) were applied daily for the entire treatment duration. Each patient underwent a clinical dermatologic examination before (T0) and three months (T1) after the beginning of the treatment. Clinical examinations, photographic documentation, and confocal microscopy evaluations were performed with all patients. In patients with melasma, we selected a target hyperpigmented skin area located on the cheek or forehead for RCM examination. The anatomical site investigated with RCM during the follow-up was identified using photographic reference.
Reflectance confocal microscopy. RCM images were acquired using a reflectance confocal laser scanning microscope (Vivascope® 1500, Caliber I.D., Rochester, New York) that scans the skin horizontally from the epidermis up to the papillary dermis (diode laser, 830-nm laser beam with a maximum power of 35mW). Instruments and acquisition methods were described previously.12 A 4×4 mm VivaCube® composed of four mosaics with a 25-?m step was acquired, starting from the stratum corneum. The presence of pigment located at all levels of the skin (epidermis, dermo-epidermal junction [DEJ], upper dermis) of patients with melasma was first considered. Then the presence of pigment was scored using a qualitative scale of pigmentation, including the following categories: absent (0), low (+), medium (++) or high (+++) (Table 1). At the level of the DEJ the presence of bright polycyclic papillary contours and bright hair follicles/rings, as signs of increased brightness, were also considered. Moreover, in the epidermis and upper dermis, presence of bright cells due to inflammatory cells/melanophages or other structures (e.g., melanin particles) were taken into account.
Results
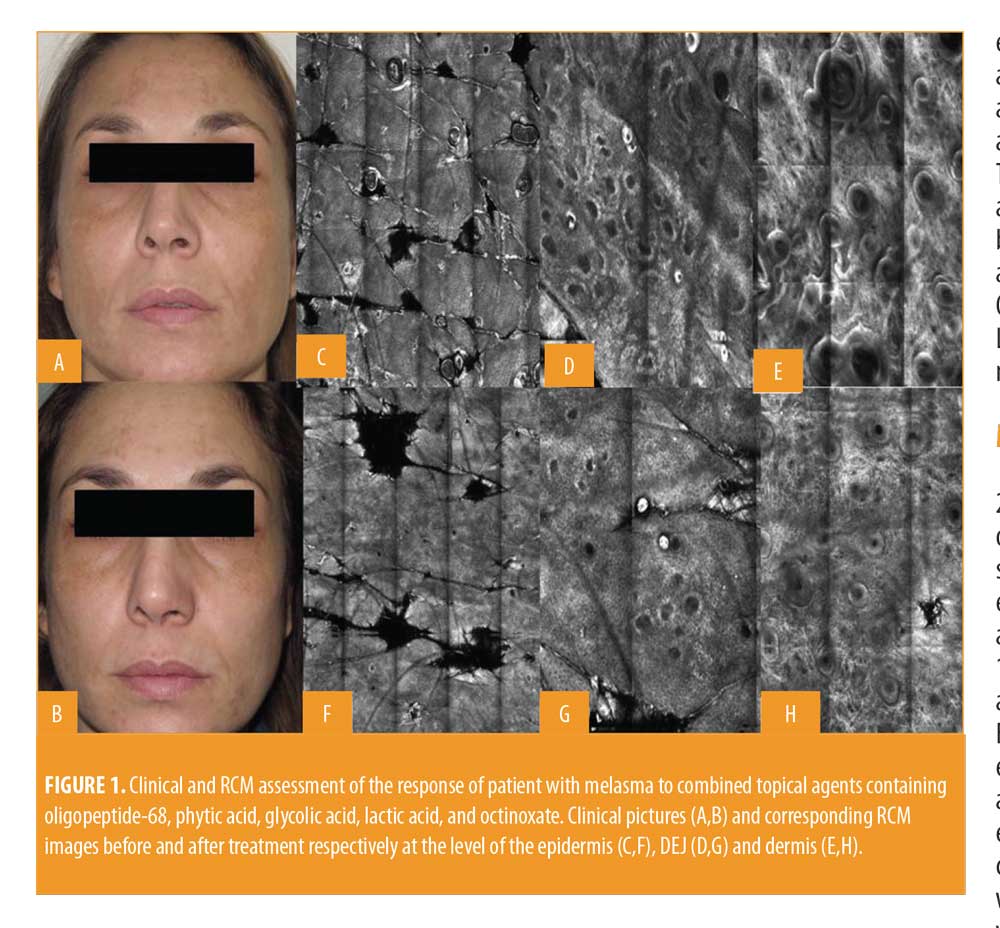
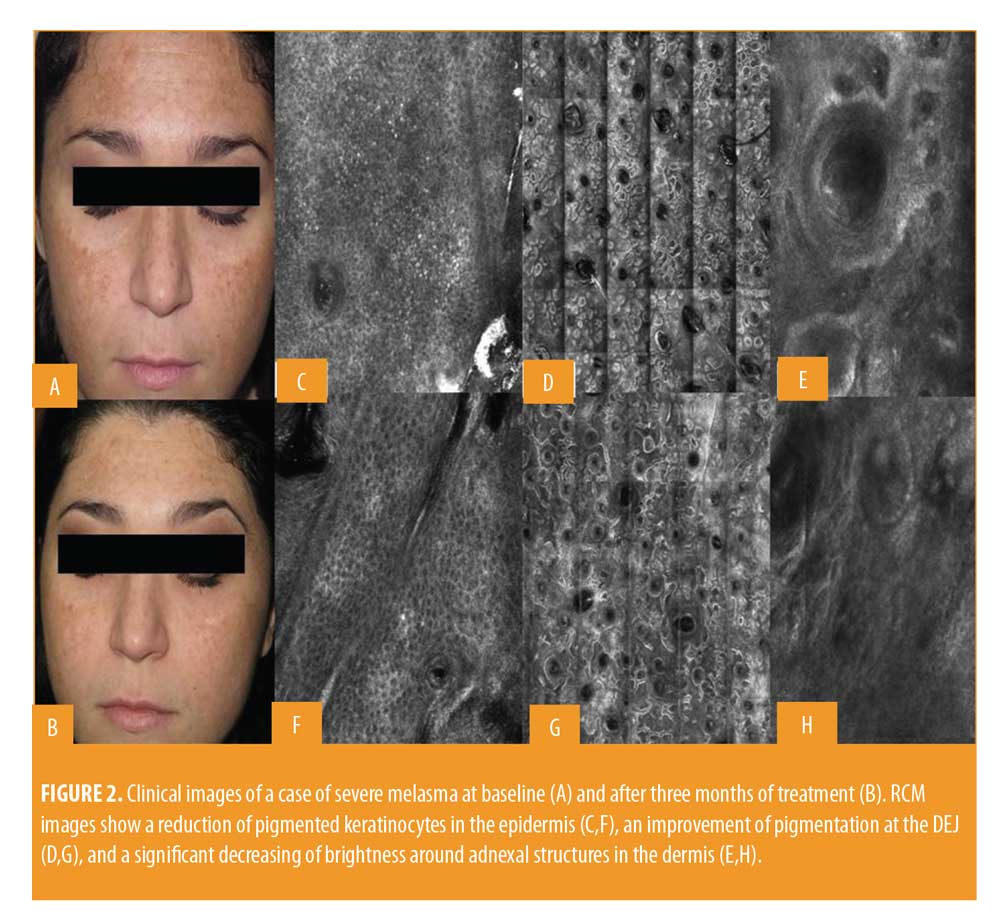
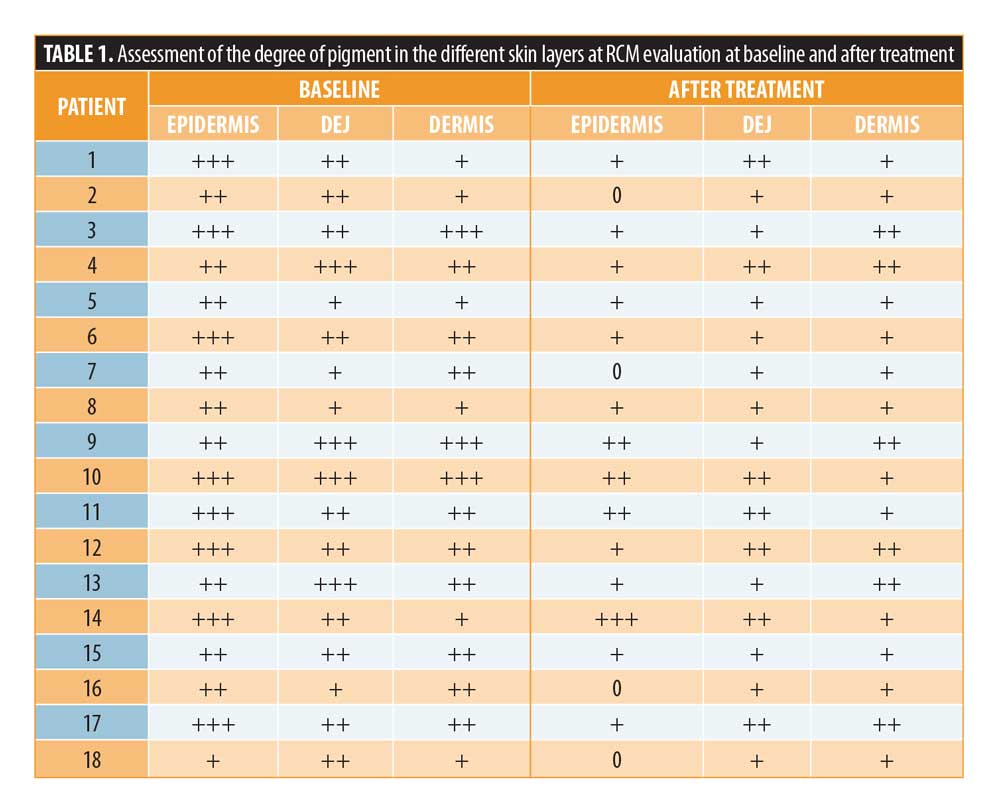
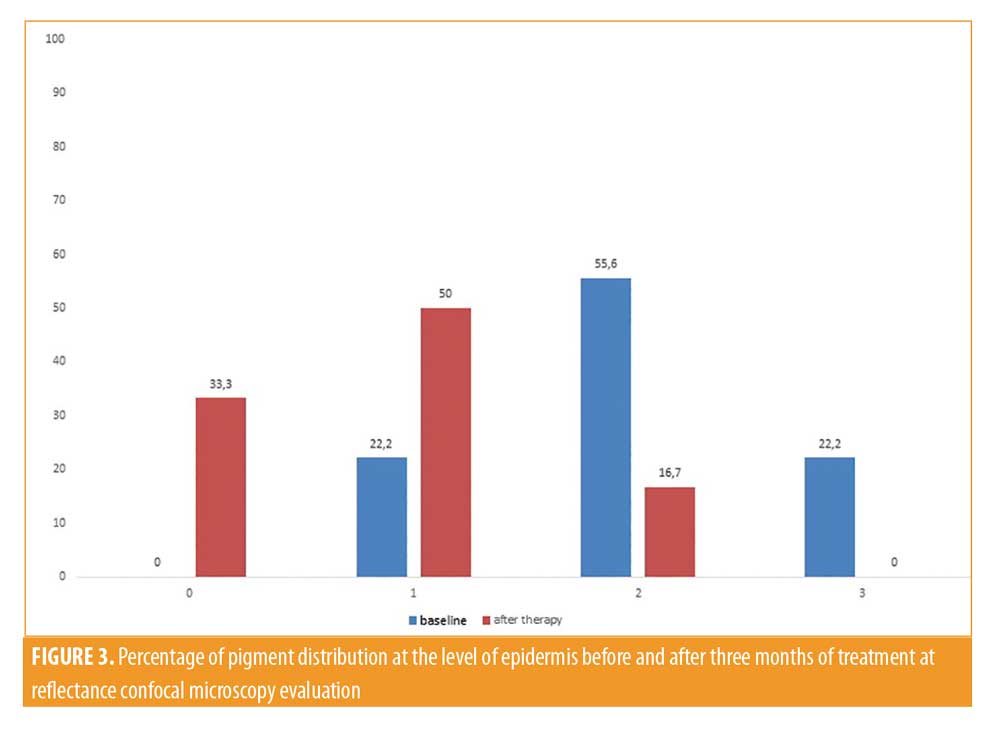
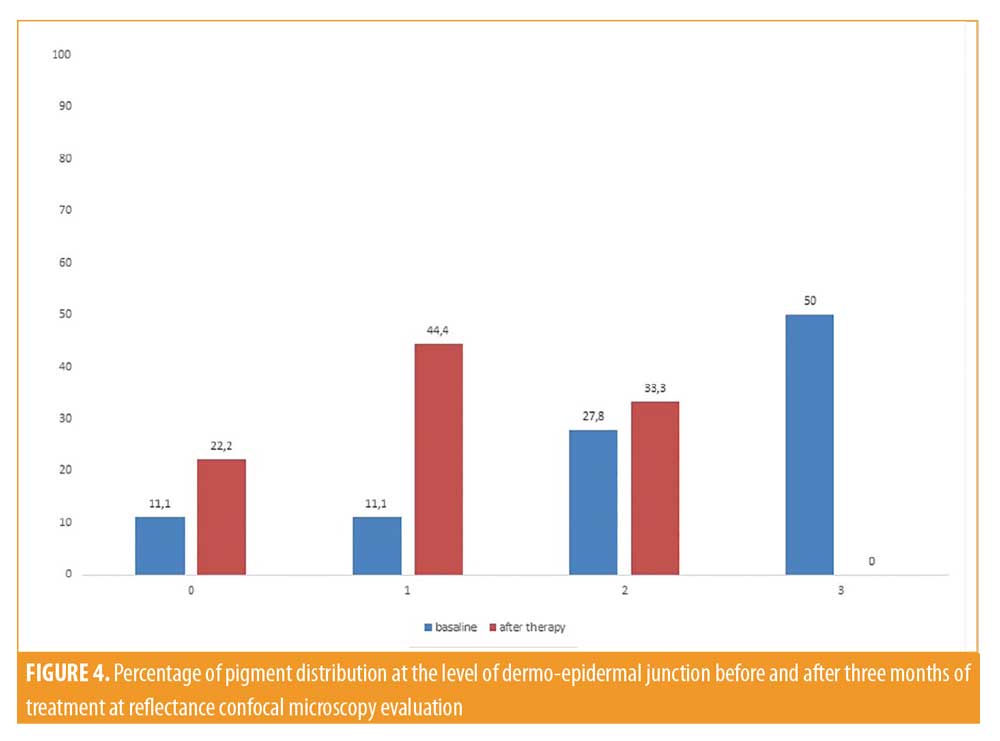
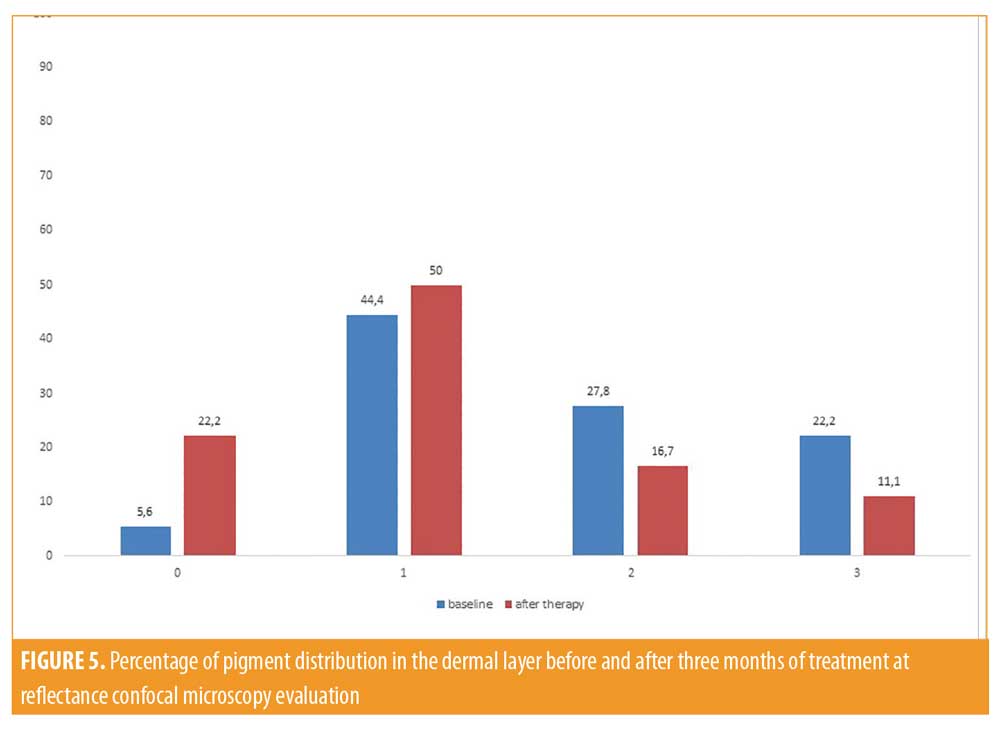
All patients included in the study finished the treatment, but two patients did not continue participating in the study during follow-up. Clinically, all patients showed an improvement in melasma after treatment without adverse reactions like erythema, itching, scarring, or post-inflammatory hyperpigmentation (Figures 1 and 2). A relevant improvement of skin areas treated with the depigmenting agent was also assessed using RCM analysis (Table 1). In particular on the RCM and at baseline at the epidermis level, an elevated degree of pigmentation was observed due to highly refractile keratinocytes with very prominent nuclei. In detail, the pigmentation was high (+++) in 4/18 cases (22.2%), medium (++) in 10/18 cases (55.6 %), and low (+) in 4/18 cases (22.2%) (Figure 3). At the DEJ, presence of visible papillary rings around the dermal papillae, a sign of increased pigmentation, was strong (+++) in 9/18 (50%) cases, mild (++) in 5/18 (27.8%) cases, low (+)in 2/18 cases (11.1%), and absent in 2/18 cases (11.1%) (Figure 4). Finally, dermal pigmentation was high in 4/18 cases (22.2%), medium (++) in 5/18 cases (27.8%), low in 8/18 cases (44.4%), and absent in 1/18 patients (5.6%) (Figure 5).
Twelve weeks from baseline, a microscopic reduction in pigmented keratinocytes at the level of the epidermis and around dermal papillae associated with a slight decrease in periadnexal brightness, a sign of progressive clinical improvement, were disclosed by the RCM (Figures 1 and 2). In particular, we observed a complete disappearance of bright large keratinocytes in the epidermal layer in 33.3 percent of cases (6/18 patients). At the DEJ, 22.2 percent of patients (4/18 cases) exhibited an absence of pigmentation. Moreover, in the upper dermis, only 5.6 percent of patients showed a complete disappearance of residual pigmentation. Notably after treatment, at the levels of the epidermis and DEJ, no patient showed a high pigmentation compared to baseline (Figures 3 and 4).
Discussion
Traditional treatment approaches using topical agents and chemical peels are the first line therapy for melasma. The main purpose of these treatments is to induce a disruption of the enzymatic process that leads to pigment production within the melanocytes. These types of treatments include hydroquinone, tretinoin, and azelaic, kojic, and ascorbic acid that are inhibitors of melanogenesis with an important antioxidant effect. Another decisive approach is improving skin turnover using agents such as glycolic acid, lactic acid, retinoid acid, and linoleic acid. These topical agents should improve the elimination of transepidermal melanin without side effects, such as inflammation or irritation, and avoiding post-inflammatory hyperpigmentation. We evaluated the effectiveness and tolerability of combined topical agents containing oligopeptide-68, phytic acid, glycolic acid, lactic acid, and octinoxate, with effects of inhibition of melanogenesis, antioxidant, and skin turnover improvement. To demonstrate the absence of side effects, such as inflammation and post inflammatory hyperpigmentation, we followed the patients using RCM. After 12 weeks of treatment, a significant reduction of pigmentation at the epidermis level and brightness of dermal papillae at the DEJ were detected using RCM. Confocal microscopy assessment of the response to treatment showed a statistically significant decrease in brightness, mainly at the epidermis and DEJ levels. The Wilcoxon matched pair test returned P-value of P<0.001 (epidermis) and P<0.003 (DEJ) and P<0.005 (upper dermis), revealing a more significant effect in the superficial layers of the skin (epidermis and DEJ) compared to the dermal layer (Figures 3,4, 5).
In addition, RCM analysis showed no signs of inflammation and no worsening of pigmentation after therapy, confirming the depigmenting effect and the safety of the tested depigmenting agents in vivo.
Conclusion
This study suggests that a combination of topical agents containing oligopeptide-68, phytic acid, glycolic acid, lactic acid, and octinoxate produces significant improvement in melasma at a clinical and cellular level and demonstrates the potential of RCM to monitor and predict efficacy of depigmenting agents in the treatment of melasma. However, further studies are necessary to confirm this data in a larger number of patients and for a longer follow-up period.
References
- Sheth VM, Pandya AG. Melasma: a comprehensive update: part I. J Am Acad Dermatol. 2011;65(4):689–697.
- Kang HY, Ortonne JP. What should be considered in treatment of melasma. Ann Dermatol. 2010;22(4):373–378.
- Kang HY, Bahadoran P, Suzuki I et al. In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp Dermatol. 2010;19:e228–e233.
- Sanchez NP, Pathak MA, Sato S, et al. Melasma: a clinical, light microscopy, ultrastructural and immunofluorescence study. J Am Acad Dermatol. 1981;4:698–710.
- Kang HY, Bahadoran P, Ortonne JP. Refectance confocal microscopy for pigmentary disorders. Exp Dermatol. 2010;19(3):233–239.
- Ardigò M, Cameli N, Berardesca E, Gonzales S. Characterization and evaluation of pigment distribution and response to therapy in melasma using in vivo refectance confocal microscopy: a preliminary study. J Eur Acad Dermatol Venereol. 2010;24:1296–1303
- Liu H, Lin Y, Nie X et al.Histological classification of melasma with reflectance confocal mi-croscopy: a pilot study in Chinese patients. Skin Res Technol. 2011;17(4):398–403.
- Funasaka Y, Mayumi N, Asayama S, et al. In vivo reflectance confocal microscopy for skin imaging in melasma. J Nippon Med Sch. 2013;80(3):172–173
- Jo DJ, Kang IH, Baek JH, et al.Using reflectance confocal microscopy to observe in vivo melanolysis after treatment with the picoseconds alexandrite laser and Q-switched Nd:YAG laser in melasma. Lasers Surg Med. 2018;51(5):423–429.
- Goberdhan LT, Mehta RC, Aguilar C et al. Assessment of a superficial chemical peel com-bined with a multimodal,hydroquinone-free skin brightener using in vivo reflectance confocal microscopy. J Drugs Dermatol. 2013;12(3):S38–S41.
- Tsilika K, Levy JL, Kang HY et al. A pilot study using reflectance confocal microscopy (RCM) in the assessment of a novel formulation for the treatment of melasma. J Drugs Dermatol. 2011;10(11):1260–1264.
- Cameli N, Abril E, Agozzino M, et al.Clinical and instrumental evaluation of the efficacy of a new depigmenting agent containing a combination of a retinoid, a phenolic agent and a antiox-idant for the treatment of solar lentigines. Dermatology. 2015;230:360–366.
- Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers Surg Med. 2017;49:7–19.

