 J Clin Aesthet Dermatol. 2022;15(4):31–35.
J Clin Aesthet Dermatol. 2022;15(4):31–35.
by Marwan Dawood, MD; Christina Sizopoulou, MD; Shoshe Greenberger, MD; Aviv Barzilai, MD; and Felix Pavlotsky, MD
Dr. Dawood is with the Rambam Health Care Campus in Haifa, Israel. Drs. Sizopoulou, Greenberger, Barzilai, and Pavlotsky are with the Department of Dermatology at Sheba Medical Center in Tel Hashomer, Israel. Drs. Greenberger, Barzilai, and Pavlotsky are also with the Sackler School of Medicine, Tel Aviv University in Tel Aviv, Israel.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Narrowband UVB (NB UVB) has been suggested as an option for lichen planus control. However, the literature is lacking in reports of long-term results.
Objective. We sought to evaluate the long-term results of NB UVB therapy in lichen planus.
Methods. This was a prospective, retrospective analysis of patients with lichen planus treated with NB UVB at our institution since 2004. The clinical response and relapse rate were recorded according to age, sex, mouth involvement, skin phototype, number of treatments, and total radiation dose.
Results. One hundred thirty-seven of 192 (71%) eligible patients had a major response (MR) and 102 (74%) patients had no recurrence after an average follow-up period of 58.7 months. MR was achieved in 66 percent and 75 percent of men and women, respectively (p=0.021) and 76 percent and 68 percent of patients with Fitzpatrick Skin Types I and II and Skin Types IV and V, respectively (p=0.017). Age at the onset of the disease, number of treatments, and total UVB dose had no effect on the MR rate. The disease-free periods were 131 and 101 months for male and female patients, respectively (p=0.06), and 128 and 103 months for patients 40 years or younger and older than 40 years of age, respectively (p=0.07). Conclusion. Based on our results, female patients and patients with lighter skin phototypes appeared to have higher MR rates. However, female and older patients appear to be at increased risk of recurrence.
Keywords: Lichen planus, narrowband UVB, phototherapy
Lichen planus (LP) is a T-cell–mediated disease involving the skin and/or the mucous membranes, with a worldwide occurrence affecting 0.2 to 1 percent of the population.1
When activated T-cells (primarily CD8+ cells) are recruited to the dermal-epidermal junction, they induce apoptosis to the basal keratinocytes.2 It is known that the interaction between T lymphocytes and basal keratinocytes is enhanced by increased expression of intercellular adhesion molecule-1 (ICAM-1) by basal keratinocytes.3 Many chemokines, such as CXCL10, are expressed in basal cells that are attacked by the cytotoxic T cells.4
Treatment for LP is disappointing and controversial. Reported treatment options include topical and systemic steroids, retinoids, azathioprine, dapsone, cyclosporine A, griseofulvin, methotrexate, cyclophosphamide, hydroxychloroquine, tacrolimus, and interferon-gamma.5–7
Several reports support the role of phototherapy in the treatment of LP. In earlier reports, various types of photochemotherapy (PUVA) were used.8–10 Recently, narrowband (NB) ultraviolet (UV) B became a replacement for PUVA due to the reduced risk of side effects and greater convenience. Several groups, including ours,9–13 have reported on the high short-term efficacy of this treatment. However, there are no sufficient data on the long-term results of LP therapy in general and of phototherapy in particular.
Here, we present our long-term experience of the relapse rate in a large group of patients following their first NB UVB course, focusing on the possible predictive parameters for a major and/or prolonged response.
Methods
This was a prospective, retrospective historical cohort study of 192 patients with typical cutaneous LP treated by NB UVB at Sheba Medical Center since 2004. In 71 percent of patients, histological confirmation was available.
Patient records were reviewed and the following data recorded and stratified: sex, age at disease onset, accompanying medical conditions and medications, clinical characteristics, disease duration at the beginning of treatment, Fitzpatrick phototype, the number of treatments and the total dose, concurrent topical or systemic treatment, and response rate and duration.
Clinical response and duration were recorded from medical files by the unit dermatologists. We assessed the clinical response using simplified lesion and pruritus scores previously described by Ramesh et al,14 where a major response (MR) was defined as at least 90-percent subsidence of lesions, absence of pruritus, and no new lesions; a partial response was defined as 50- to 90-percent subsidence of lesions, mild pruritus, and no new lesions; and no response was defined as less than 50-percent subsidence of lesions, moderate-to-severe pruritus, and/or new lesions.
Follow-up data were recorded at 1 to 9 years after the cessation of treatment by a telephone questionnaire in a small number of cases lost to follow-up. Follow-up data recorded by clinical examination and/or chart review and/or telephone questionnaire included current clinical status, pruritus severity, and remission duration.
NB UVB treatment was given in Waldmann 1,000 L booths (Waldman AG, Schwenningen, Germany). The irradiance was checked routinely with a UVB detector (Waldman AG) with an average of 1.6 mW/cm2. The starting and incremental doses per treatment were 0.05 to 0.1 J/cm2 according to the patient’s phototype. All patients were initially treated thrice weekly on nonconsecutive days with genital areas shielded. The NB UVB dose was increased with each treatment according to skin type by 0.05 to 0.1 J/cm2 (for a maximum of 3 J/cm2).
Institutional review board approval was obtained and the need for patient consent was waived due to the noninterventional nature of this study.
Statistical analysis. The clinical response according to age, number of treatments, and total radiation dose was analyzed by one-way analysis of variance, while Pearson’s chi-squared test was used to check the clinical response according to sex, mouth involvement, and skin phototype. The relapse rate was also compared using the chi-squared test. Because of the difference in the treatment groups regarding the follow-up period, Kaplan-Meier disease-free analysis was performed using a log-rank test.
Results
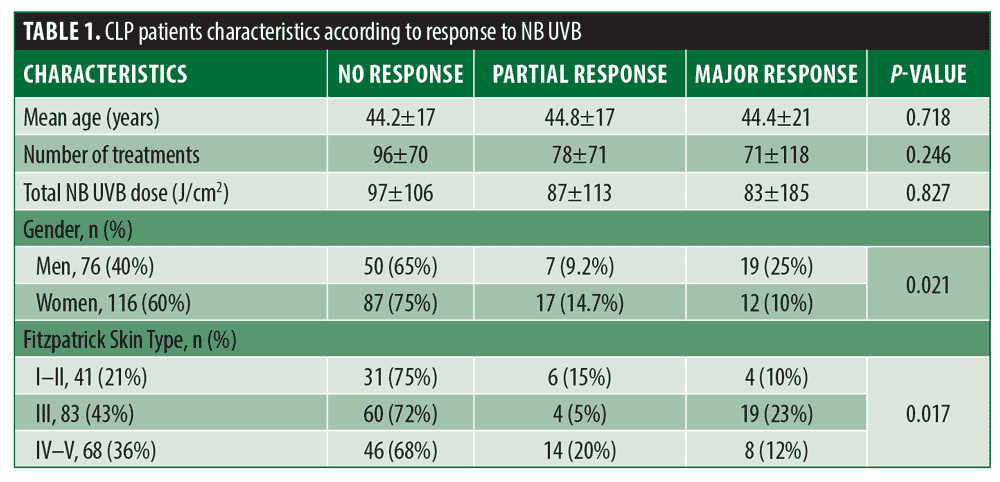
During the study period, 192 patients with LP treated by NB UVB were included in our study. Table 1 summarizes the demographic and clinical characteristics of the study population.
Of interest, only two patients had hepatitis C (1%), and no one had hepatitis B. Fifty-five patients (27%) presented with at least one component of metabolic syndrome. More specifically, 10 patients (5%) had diabetes, 21 patients (10%) had hypertension, and 24 patients (12%) had hyperlipidemia.
Out of 192 patients treated by NB UVB, 41% (n=80) were also taking an additive therapy; of these 80 patients, 50(62%) were on moderate to potent topical steroids, 19 were receiving systemic therapy with acitretin (24%), four (5%) were on prednisone, and four (5%) were on methotrexate. Three (4%) patients were on both topical and systemic therapy (acitretin+clobetasol).
The mean total dose of the first NB UVB phototherapy cycle was 45.4 J/cm2.
Major, partial, and no responses, respectively, were experienced by 137 (71%), 24 (13%), and 31 (16%) patients. Also, 74 percent of the patients with MR had no recurrence after an average follow-up of 58.7 months. Age at the onset of the disease, number of treatments, and the total UVB dose had no effect on the MR rate (p=0.718, p=0.246, and p=0.827, respectively). MR was achieved in 66 percent and 75 percent of men and women, respectively (p=0.021), and in 76 percent and 68 percent of patients with Fitzpatrick Skin Types I and II and Skin Types IV and V, respectively (p=0.017).
Five patients (2%) reported burning and four patients developed herpetic (simplex or zoster) infection. One patient experienced dizziness and weakness and another had hypertension.
Long-term efficacy was assessed in all of the 137 patients with MR. The median time of maintaining the response after complete treatment cessation was 55 months after a median follow-up period of 1 to 163 months.
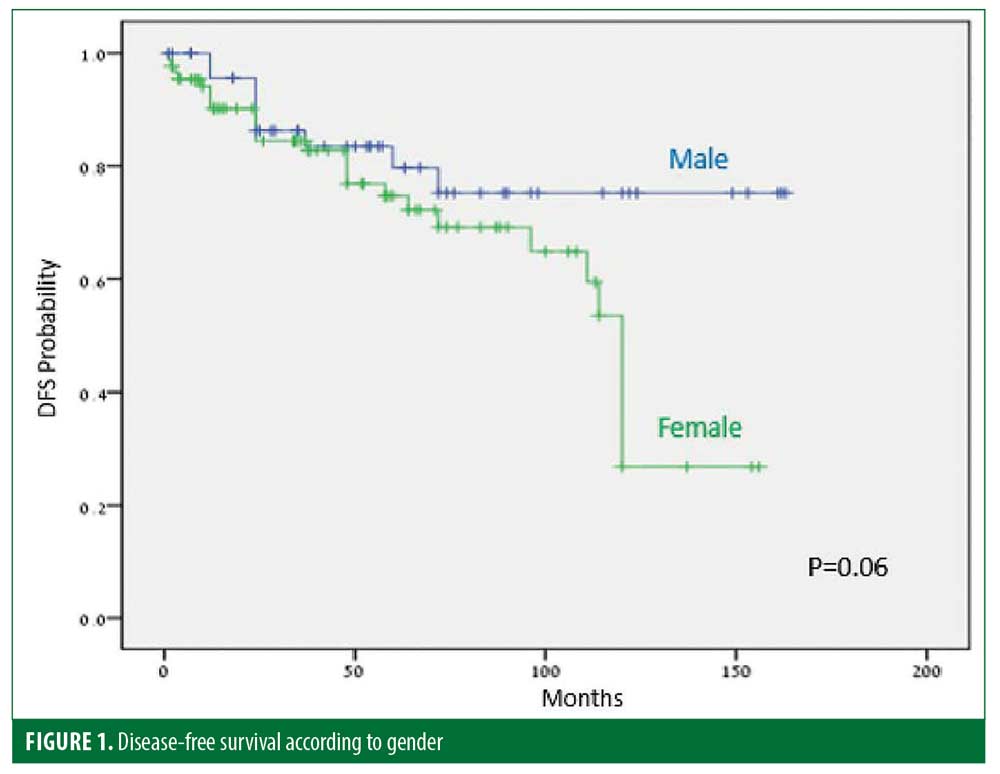
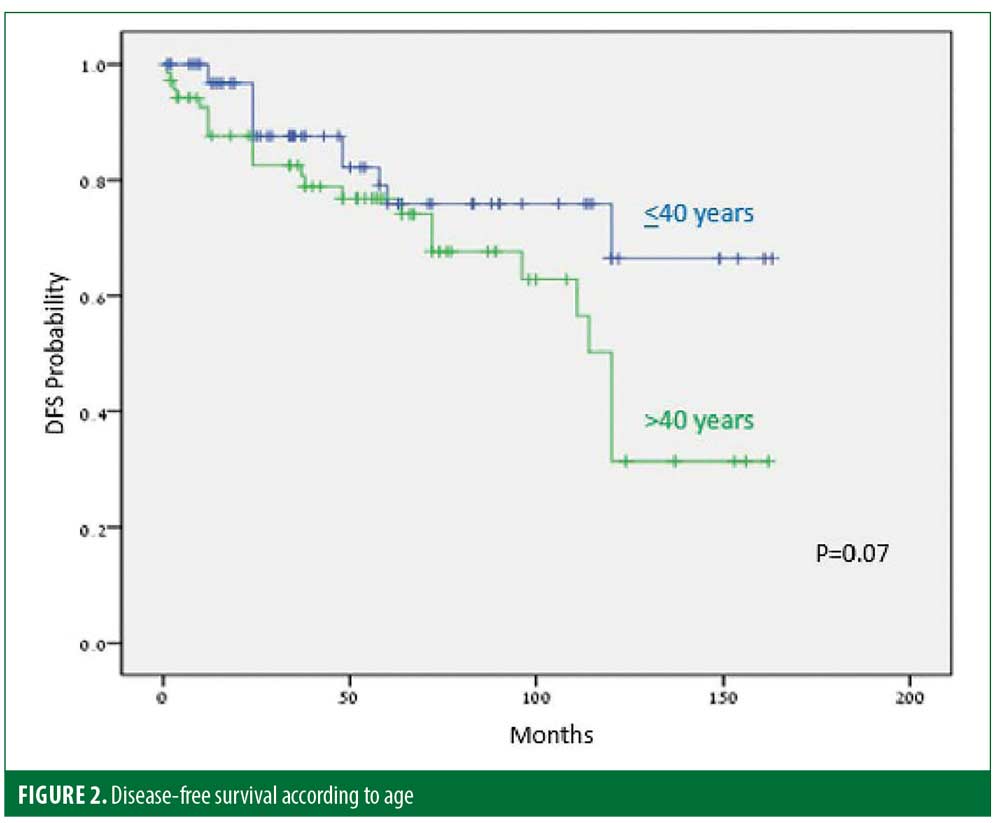
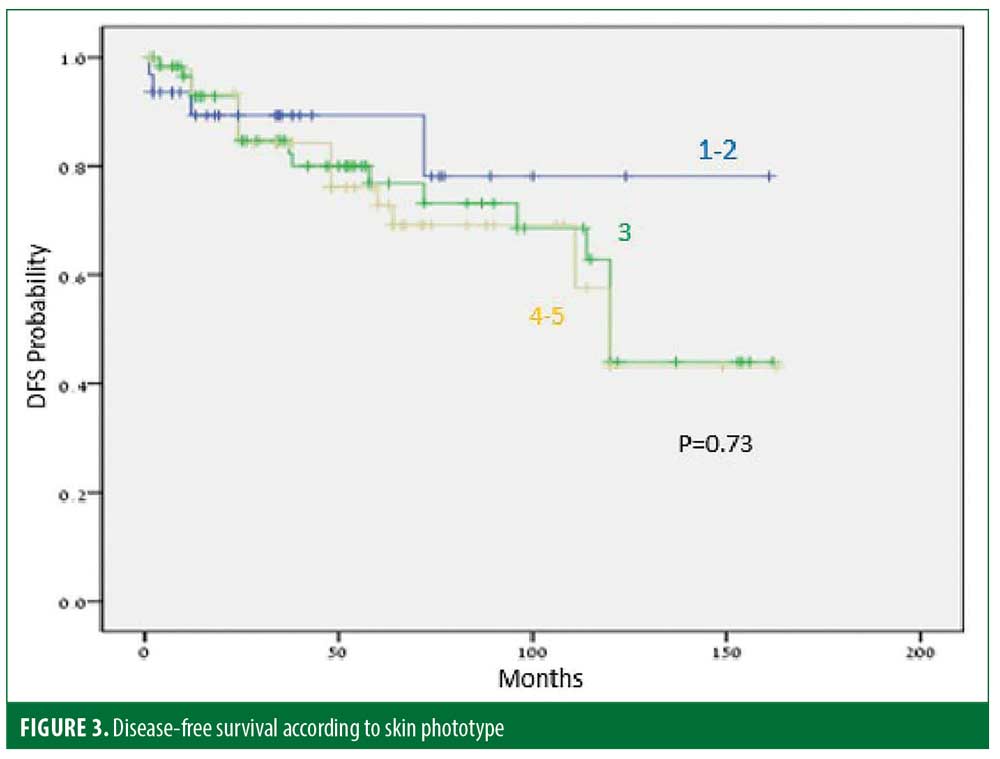
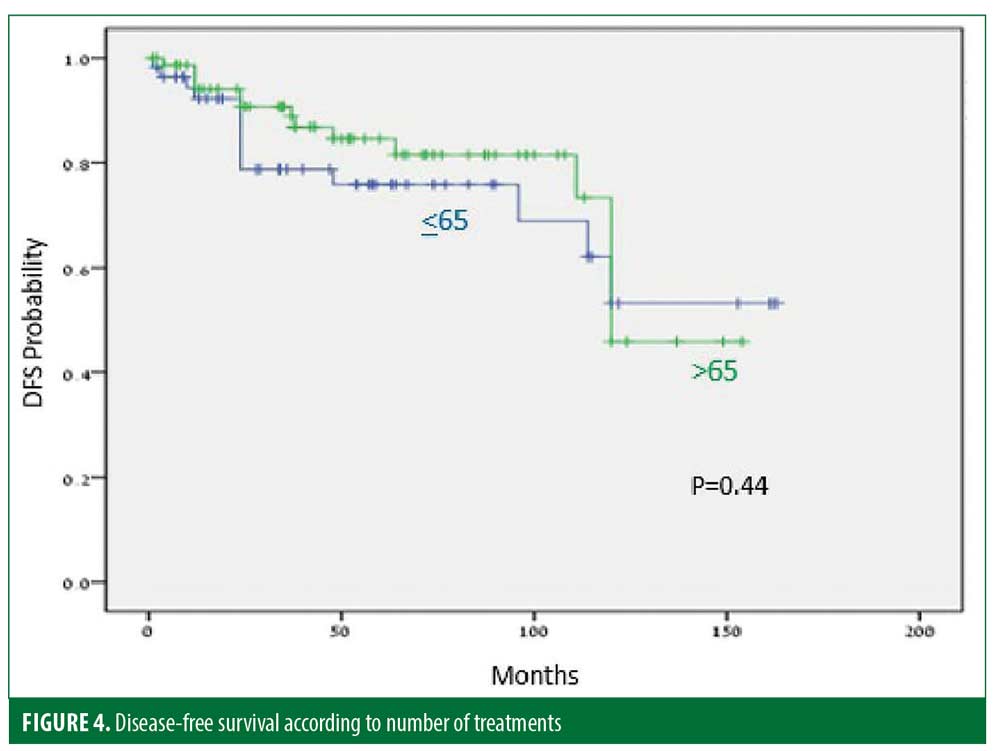
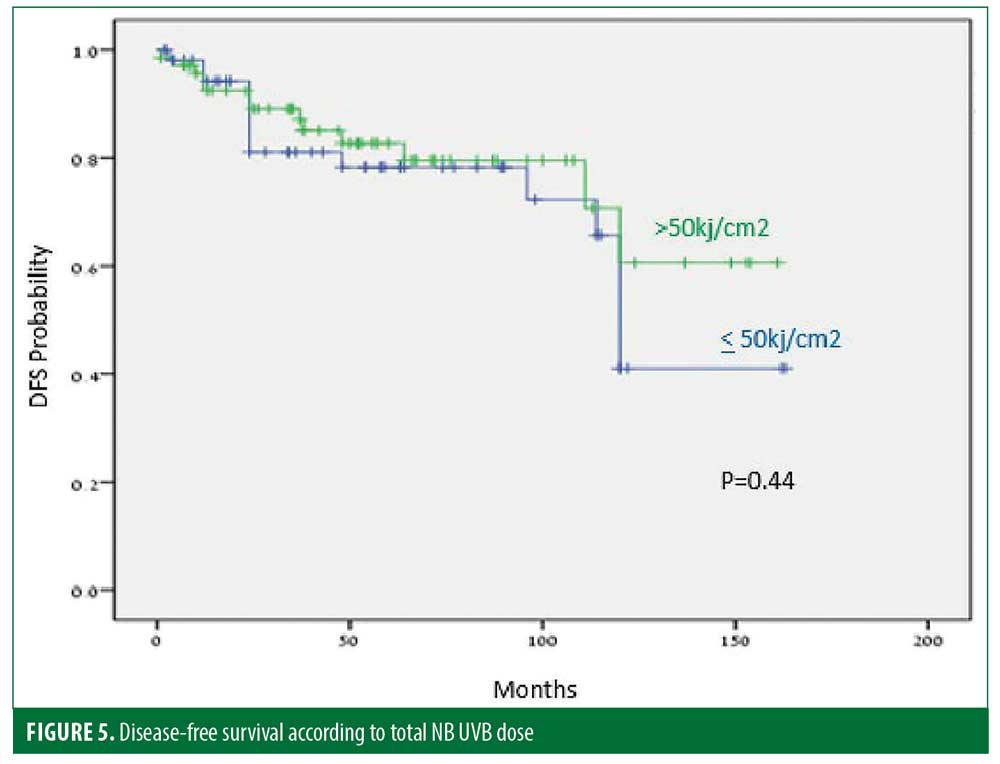
Thirty-five (25%) patients experienced recurrence after a median of 45.9 months. The disease-free period (DFP) was 131 and 101 months for male and female patients, respectively (p=0.06) (Figure 1), and 128 and 103 months for patients 40 years or younger and older than 40 years of age, respectively (p=0.07) (Figure 2), while skin type (Figure 3) and the number of treatments (Figure 4) and total treatment dose (Figure 5) had no effect on the DFP (p=0.73, p=0.44, and p=0.44, respectively).
Discussion
None of the reported systemic therapies for LP (steroids, acitretin, dapsone, azathioprine, methotrexate, cyclophosphamide, cyclosporine, and interferon-gamma) have been shown to be highly effective and/or to offer prolonged remission.15–17
Meanwhile, regarding phototherapy, PUVA was beneficial in two small studies.18,19 Sixty-five percent of 75 patients treated with bath and ointment trioxsalen PUVA had a complete response (CR) with a relapse rate of 25 percent after two to five years.20
A 76-percent versus a 50-percent response rate and a 55-percent versus a 23-percent cure rate at 10 to 15 months of follow-up were recorded with bath versus oral PUVA, respectively.21 Another study reporting on 1 to 4 years of follow-up of 145 PUVA-treated patients described a 26.1-percent recurrence rate.22
UVB is more convenient and has fewer side effects than PUVA. Broadband UVB use in LP is cited in old textbooks, but hardly mentioned in modern articles.23 Today, NB UVB is the preferred treatment for LP. Taneja and Taylor24 described five patients treated with NB UVB with sustained remission achieved after five months in three of them but only in one after 20 months. Saricaolu et al10 reported CR in 8 of 10 patients, with five were still in remission after one year. Yashar et al25 reported moderate or significant improvement in three of their four patients. In a comparative study, PUVA led to a better CR rate of 67 percent versus 31 percent with UVB. On the other hand, the relapse rate was 47 percent versus 30 percent with PUVA and UVB, respectively.26 Solak et al27 reported on 24 patients treated by NB UVB, where two-thirds (66%) demonstrated CR, with recurrence happening in six out of 16 after 12 months. Similarly, Gamil et al28 reported a 68.75 percent CR rate in 16 patients, while our group has shown the same results in a large group of 50 patients.11 However, no follow-up was reported in both of the studies.
In a recent meta-analysis by Atzmony5 comparable efficacy was reported for oral and bath PUVA and NB UVB. Again, no conclusions regarding long-term efficacy were detailed.
Previous research suggested spontaneous remission of LP can be observed in up to two-thirds of patients after one year.29 However, our clinical experience is that LP is a much more chronic disease. Moreover and as mentioned above, data on the DFP following MR are limited and, when available, follow-up periods are relatively short. Furthermore, in many existing reports, patients were still on maintenance therapy, thus not allowing conclusions to be drawn on the disease-modifying effect of the therapy as well as the possible cure.
The purpose of the present study was to examine the long-term benefits of NB UVB treatment for LP. The response rates in 192 patients were comparable to those of previous NB UVB and PUVA cohorts,8,11,13,19,20–22,25,27 with 71 percent, 13 percent, and 16 percent major, partial, and no response rates, respectively.
Age at the onset of the disease, number of treatments, total UVB dose, and mouth involvement had no effect on the MR rate, while MR was achieved in 66 percent and 75 percent of men and women, respectively (p=0.021), and 76 percent and 68 percent of Fitzpatrick Skin Types I and II and Skin Types IV and V, respectively (p=0.017). Side effects were rare and mild.
The long-term efficacy in 137 patients with MR was assessed. The median DFP after complete treatment cessation was 55 months. Thirty-five of 137 (25%) patients relapsed within a median period of 45.9 months. This rate is similar to that described in previous PUVA studies.19,20,22 DFPs were 131 and 101 months for male and female patients, respectively (p=0.06), and 128 and 103 months for patients 40 years and younger and older than 40 years of age, respectively (p=0.07).
In summary, more than half of the 192 studied patients were disease-free for at least 4.8 years after a single course of NB UVB, therefore suggesting a curative potential of this therapy. Younger or male patients might have a greater chance to achieve this outcome.
Limitations. The limitations of our study are its retrospective nature, some of the participants were on additive therapies, and the study site was a single center. Thus, further and possibly prospective randomized studies are needed.
References
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London, England: Mosby Elsevier; 2012: 183–196.
- Pittelkow MR, Daoud MS. Lichen planus. In: KGL Wolff, SI Katz, BA Gilchrest, et al., eds. Fitzpatrick’s Dermatology in General Medicine. Vol. 1, 7th ed. New York, New York: McGraw-Hill; 2008: 244–255.
- Norris DA. Cytokine modulation of adhesion molecules in the regulation of immunologic cytotoxicity of epidermal targets. J Invest Dermatol. 1990;95 (6 Suppl.):111S–120S.
- Wenzel J, Tuting T. An IFN-associated cytotoxic cellular immune response against viral, self, or tumor antigens is a common pathogenetic feature in “interface dermatitis”. J Invest Dermatol. 2008;128(1):2392–2402.
- Atzmony L, Reiter O, Hodak E, et al. Treatments for cutaneous lichen planus: a systematic review and meta-analysis. Am J Clin Dermatol. 2016;17(1):11–22.
- Antiga E, Caproni M, Parodi A, et al. Treatment of cutaneous lichen planus: an evidence based analysis of efficacy by the Italian Group for Cutaneous Immunopathology. G Ital Dermatol Venereol. 2014;149(6):719–726.
- Shiohara T, Mizukawa Y. Fixed drug eruption: the dark side of activation of intraepidermal CD8+ T cells uniquely specialized to mediate protective immunity. Chem Immunol Allergy. 2012;97:106–121.
- 8. Iraji F, Faghihi G, Asilian A, et al. Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: a randomized clinical trial. J Res Med Sci. 2011;16(12):1578–1582.
- Helander I, Jansén CT, Meurman L. Long-term efficacy of PUVA treatment in lichen planus: comparison of oral and external methoxsalen regimens. Photodermatol. 1987;4(5):265–268.
- Sharma L, Mishra MK. A comparative study of PUVASOL therapy in lichen planus. Indian J Dermatol Venereol Leprol. 2003;69(3): 212–213.
- Pavlotsky F, Nathansohn N, Kriger G, et al. Ultraviolet-B treatment for cutaneous lichen planus: our experience with 50 patients. Photodermatol Photoimmunol Photomed. 2008;24(2):83–86.
- Habib F, Stoebner PE, Picot E, et al. [Narrow band UVB phototherapy in the treatment of widespread lichen planus]. Ann Dermatol Venereol. 2005;132(1):17–20.
- Saricaolu H, Karadogan SK, Bakan EB, et al. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19(5):265–267.
- Ramesh M, Balachandran C, Shenoi S D, Rai VM. Efficacy of steroid oral mini-pulse therapy in lichen planus: an open trial in 35 patients. Indian J Dermatol Venereol Leprol. 2006;72(2):156–157.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. An evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134(12):1521–1530.
- Capella GL, Finzi AF. Psoriasis, lichen planus, and disorders of keratinization: unapproved treatments or indications. Clin Dermatol. 2000;18(2):159–169.
- Sharma R, Maheshwari V. Childhood lichen planus: a report of fifty cases. Pediatr Dermatol. 1999;16(5):345–348.
- Ortonne JP, Thivolet J, Sannwald C. Oral photochemotherapy in the treatment of lichen planus (LP). Clinical results, histological and ultrastructural observations. Br J Dermatol. 1978;99(1):77–88.
- Gonzalez E, Momtaz-T K, Freedman S. Bilateral comparison of generalized lichen planus treated with psoralens and ultraviolet A. J Am Acad Dermatol. 1984;10(6):958–961.
- Karvonen J, Hannuksela M. Long term results of topical trioxsalen PUVA in lichen planus and nodular prurigo. Acta Derm Venereol Suppl (Stockh). 1985;120:53–55.
- Helander I, Jansen CT, Meurman L. Long-term efficacy of PUVA treatment in lichen planus: comparison of oral and external methoxsalen regimens. Photodermatol. 1987;4(5):265–268.
- Narwutsch M, Narwutsch M. Recurrence and morphologic resistance phenomena of PUVA-treated lichen ruber planus cutaneous. Dermatol Monatsschr. 1989;175(3):148–154.
- Nanda A, Al-Ajmi HS, Al-Sabah H, et al. Childhood lichen planus: a report of 23 cases. Pediatr Dermatol. 2001;18(1):1–4.
- Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41(5):282–283.
- Yashar S, Gielczyk R, Scherschun L, et al. Narrow-band ultraviolet B treatment for vitiligo, pruritus, and inflammatory dermatoses. Photodermatol Photoimmunol Photomed. 2003;19(4):164–168.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23(1):15–19.
- Solak B, Sevimli Dikicier B, Erdem T. Narrow band ultraviolet B for the treatment of generalized lichen planus. Cutan Ocul Toxicol. 2016;35(3):190–193.
- Gamil H, Nassar A, Saadawi A, et al. Narrow-band ultraviolet B phototherapy in lichen planus. J Eur Acad Dermatol Venereol. 2009;23(5):589–590.
- Mignogna MD, Muzio LL, Russo LL, et al. Oral lichen planus: different clinical features in HCV-positive and HCV-negative patients. Int J Dermatol. 2000;39(2):134–139.

