 J Clin Aesthet Dermatol. 2021;14(1):55–58
J Clin Aesthet Dermatol. 2021;14(1):55–58
by Michael Roberts, DO; Nam H. Vo, DO; David Crasto, DO; and David Roy, DO, FAOCD, FAAD
Dr. Roberts is with Merit Health Wesley in Hattiesburg, Mississippi. Mr. Vo and Mr. Crasto are with the William Carey University College of Osteopathic Medicine in Hattiesburg, Mississippi. Dr. Roy is with Pine Belt Dermatology and Skin Cancer Center in Petal, Mississippi.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Necrotizing fasciitis (NF) is a progressive inflammatory infection of the fascia that is often aggressive and advances insidiously. NF can be preceded by traumatic injury or surgical intervention or may occur spontaneously. Here, we describe a patient who presented with monomicrobial subacute necrotizing fasciitis due to Serratia marcescens. Dermatology was consulted at the local hospital for suspected cellulitis with no resolution using appropriate antibiotics. Our patient failed to show improvement with antibiotic treatment and experienced an above-the-knee amputation. We have also included a review of the current literature on subacute necrotizing fasciitis and S. marcescens. A case review of 25 patients resulted in rates of 52.1% for overall mortality, 72% for mortality among patients treated with only antibiotics, and 40% for mortality among patients treated with surgical debridement. There needs to be a high suspicion for necrotizing fasciitis even in patients presenting with cellulitis without systemic symptoms. If S. marcescens is isolated, more aggressive treatment may be warranted. More cases of subacute necrotizing fasciitis and S. marcescens need to be reported in the literature to better understand early diagnosis and best management practices.
KEYWORDS: Subacute, necrotizing, fasciitis, Serratia marcescens
Necrotizing fasciitis (NF) is a progressive inflammatory infection of the fascia that is often aggressive and advances insidiously. NF can be preceded by traumatic injury or surgical intervention may occur spontaneously.1 The infection initially causes necrosis of the fascia and subcutaneous tissue, while sparing the muscle and dermal tissue. The bacteria express toxins and progress along the fascial plane, causing devascularization of muscle and cutaneous tissue.2 Devascularization occurs as a result of thrombosis of the cutaneous perforating vessels, causing rapid tissue necrosis.3 The current recommended guidelines for NF treatment are broad empiric antibiotics, clindamycin for group A Streptococcus infection, and prompt surgical consultation.4
NF most commonly affects the extremities but can involve any part of the body. The staging system proposed by Wang et al5 describes the common presentation of NF. Stage 1 (early) is frequently associated with erythema, swelling, and pain, with palpation beyond the presenting site. Stage 2 (intermediate) is evidenced by the formation of bullae or serous filled blisters. Stage 3 (late) manifests as skin anesthesia, crepitus, hemorrhagic bullae, and dusky discoloration.1,6,7
Necrotizing soft tissue infections are classified on the basis of anatomic location, depth, and microbial cause.8 The depth of infection can reach adipocytes, fascia, and muscle. There are three types of microbial classifications. Type I contains mixed microbes, including aerobic and anaerobic bacteria. Type II is monomicrobial and most commonly involves group A Streptococcus or Staphylococcus aureus. Type III involves Vibrio vulnificus, although this classification is not universally agreed upon.9 Multimicrobial infections are more common than monomicrobial infections. Monomicrobial infections tend to affect younger, healthier individuals with a history of minor trauma.10
Serratia marcescens is a rare cause of NF. S. marcescens is a motile, Gram-negative, facultative anaerobe and a member of the Enterobacteriaceae family, which is maintained in the normal colonic flora.11 In recent years, reports of S. marcescens respiratory-tract infections, urinary-tract infections, septicemia, meningitis, and wound infections have increased.11 The factors predisposing individuals to infections include but are not limited to trauma, alcoholism, peripheral vascular disease, immunosuppression, diabetes mellitus, antibiotic use, steroid use, intravenous lines, lacerations, and abscesses.12 One study reported a 52-percent mortality rate for S. marcescens NF.12
Limited reports have described a rare, subacute presentation of NF that lacks the aggressive clinical features typically seen with NF and instead demonstrates an indolent course followed by sudden, rapid deterioration.13–17 Vascular insufficiency, cellulitis, or other soft-tissue infections can easily mimic this condition due to the lack of apparent severity at onset. The definitive treatment includes surgical intervention via fasciotomy and debridement to prevent limb loss and potentially death. Establishing accepted criteria is critical to support early disease recognition.14
Here, we present a case of subacute NF that was caused by S. marcescens and resulted in partial amputation. We also discuss the current literature and diagnostic parameters.
Case Report
A 73-year-old African-American female patient with a medical history of hypertension and hypercholesterolemia presented to the emergency department with a two-week history of weakness and painful swelling in her bilateral lower extremities. Seven days prior, she had sought care at a local outpatient clinic, where she received furosemide for a suspected chronic heart failure exacerbation. At that time, examination of her legs revealed erythema, tenderness to palpation, and edema, which was worse on the right leg. There was no mention of induration or bullae.
The patient later presented to the emergency department. At that time, her right lower leg and foot displayed erythema, induration, and small, tense bullae of the right lower extremity. After admission, laboratory analysis indicated a white blood cell count of 7.4×109/L, a blood glucose level of 65mg/dL, and a lactic acid level of 3.6mmol/L. She was treated empirically for cellulitis with suspected sepsis. Blood and wound cultures revealed S. marcescens. Her condition continued to deteriorate despite the addition of aztreonam and ceftriaxone. Over the course of six days, tense, painful hemorrhagic bullae with significant edema of the dorsum of the foot developed, extending into the distal phalanges (Figures 1 and 2). Induration of the tissue extended beyond clinically apparent erythema. At no point during her course did she develop an elevated white blood cell count, pyrexia, or elevated glucose levels.
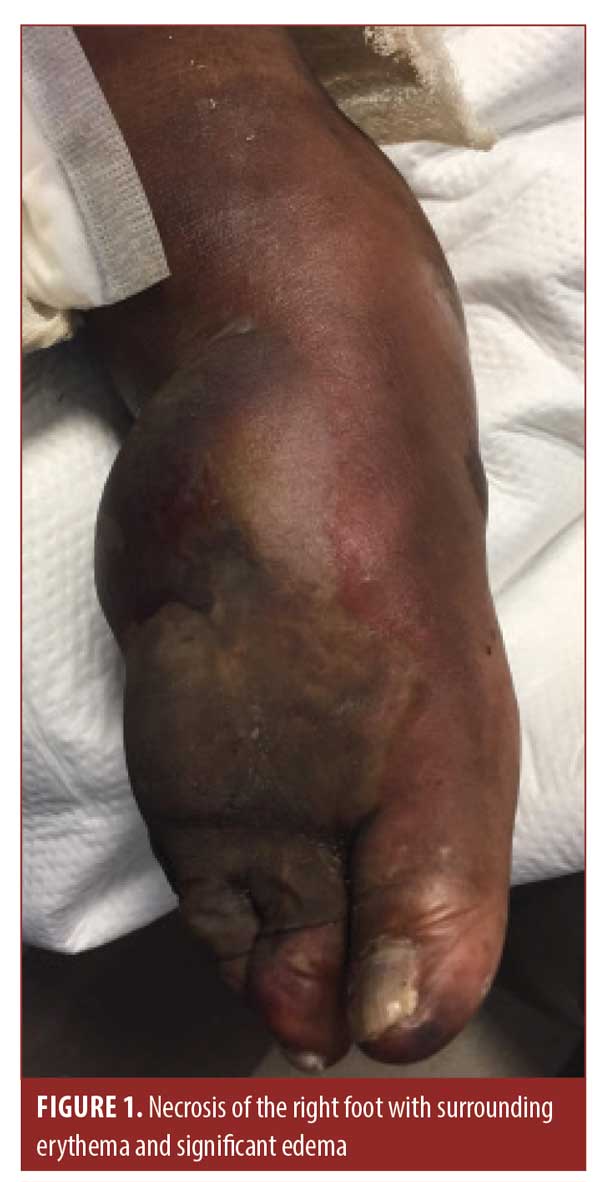
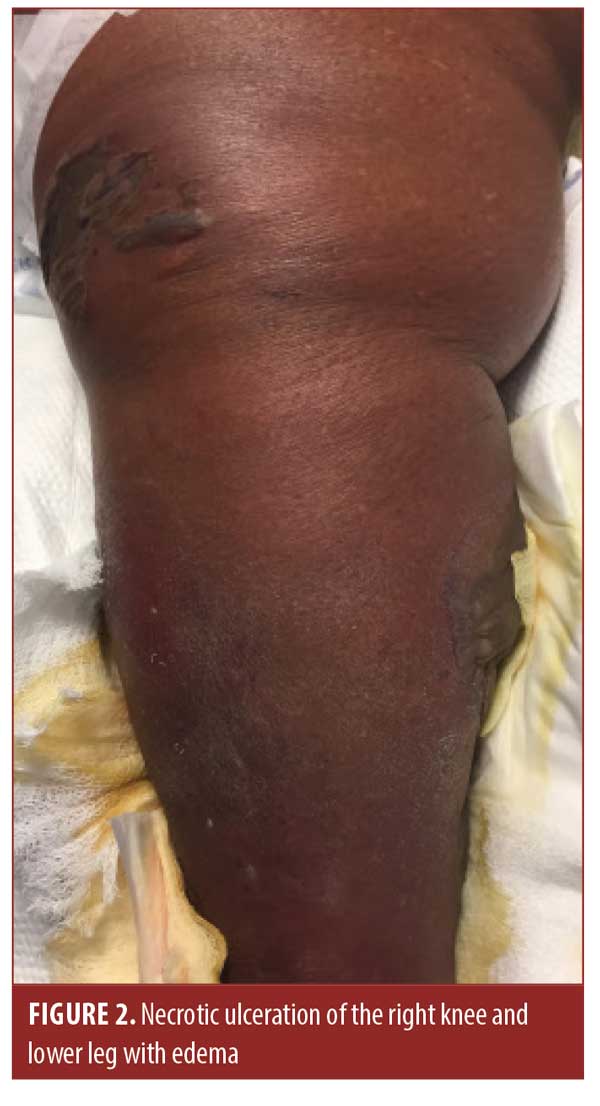
The dermatology department was consulted and a diagnosis of NF was made after examination. The surgery department was immediately consulted and the patient underwent an above-the-knee amputation of the right lower extremity. She was later transferred to a transitional care facility and was ultimately able to return home.
Discussion
NF is often misdiagnosed at the time of admission to the hospital, and consequently, can lead to high morbidity and mortality rates.15 The mortality rates reported in studies are approximately 24 to 34 percent and are lower with early diagnosis and treatment.18,19 This patient’s subacute presentation was a slowly worsening leg pain of two weeks’ duration, with no additional symptoms aside from weakness. At presentation to the emergency department, she was tachycardic, without meeting Systemic Inflammatory Response Syndrome criteria. Her laboratory results also did not meet the criteria for sepsis or suggest NF and she was treated with the proper antibiotics. She had a delayed diagnosis and an acute deterioration, which resulted in an above-the-knee amputation.
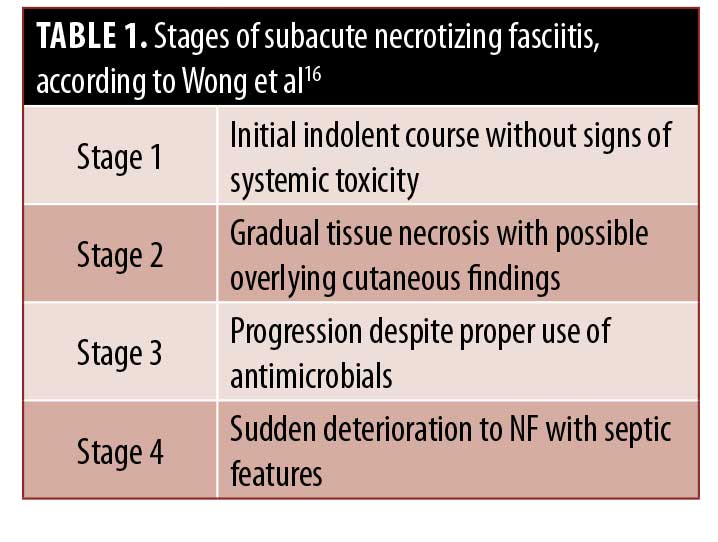
The true incidence of subacute NF is likely misrepresented in the literature.14 Because of the subtle features and lack of symptomatic severity, the diagnosis of subacute NF can be exceedingly difficult.14,15 Furthermore, the point at which the clinical decline will occur is difficult to predict accurately. According to a review by Wong and Tan16 in 2006, NF features can present as early as six days or greater than one month after the onset of symptoms.16 Failure to consider NF as a diagnosis during this indolent period can be detrimental to patients because early surgical debridement is key to reducing morbidity and mortality rates.14–17 Wong and Tan16 proposed a four-stage grading system, which our patient also followed (Table 1).
 Our patient presented with type II monomicrobial S. marcescens NF of the right lower leg. S. marcescens has increasingly been identified as a causative pathogen in NF. A case review by Lakhani et al12 described 15 cases from 1987 to 2015, and 10 new cases have since been added to the literature (Table 2), two of which were multimicrobial.12,19,36 There is currently insufficient statistical power to understand the effects of S. marcescens on NF. The average age of infection is 49.2 (± 21.9 years) and a 52.1 percent rate of mortality has been reported, not counting two patients who were lost to follow-up after leaving the hospital against medical advice. Patients treated with only antibiotics show a 72-percent mortality rate, while those treated with surgical debridement present a 40-percent mortality rate.
Our patient presented with type II monomicrobial S. marcescens NF of the right lower leg. S. marcescens has increasingly been identified as a causative pathogen in NF. A case review by Lakhani et al12 described 15 cases from 1987 to 2015, and 10 new cases have since been added to the literature (Table 2), two of which were multimicrobial.12,19,36 There is currently insufficient statistical power to understand the effects of S. marcescens on NF. The average age of infection is 49.2 (± 21.9 years) and a 52.1 percent rate of mortality has been reported, not counting two patients who were lost to follow-up after leaving the hospital against medical advice. Patients treated with only antibiotics show a 72-percent mortality rate, while those treated with surgical debridement present a 40-percent mortality rate.

Our case has two interesting features—subacute presentation of NF and S. marcescens as a pathogen—that must be reported more often. When S. marcescens is diagnosed, there appears to be a greater mortality rate than that in other cases of NF. S. marcescens is associated with a 52.1-percent mortality rate as compared with that of 34-percent for general NF cases.18 Factors leading to increased mortality may include an immunocompromised state, delayed treatment, or subacute presentation. This patient presented without systemic or laboratory signs, thus delaying biopsy and debridement. More of these cases must be added to the literature to expand our knowledge of how to diagnose subacute NF to decrease morbidity and mortality rates.
References
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis BMJ. 2005;330(7495):830–833.
- Goldstein EJC, Anaya DA, Dellinger EP. Necrotizing Soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705–710.
- Kiat HJ, En Natalie YH, Fatimah L. Necrotizing fasciitis: how reliable are the cutaneous signs?. J Emerg Trauma Shock. 2017;10(4):205–210.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147–159.
- Wang YS, Wong CH, Tay YK. Staging of necrotizing fasciitis based on the evolving cutaneous features. Int J Dermatol. 2007;46(10):1036–1041.
- Stoneback J, Hak D. Diagnosis and management of necrotizing fasciitis. Orthopedics. 2011;34(3):196.
- Arif N, Yousfi S, Vinnard C. Deaths from necrotizing fasciitis in the United States, 2003–2013. Epidemiol Infect. 2016;144(6):1338–1344.
- Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208(2):279–288.
- Ahmad H, Haider J, Siddiqui SS, et al. Subacute peristomal necrotizing fasciitis detected during adjuvant chemotherapy for adenocarcinoma rectum: case report on a unique presentation and description of a simple surgical strategy for treatment. Cureus. 2018;10(1):e2075.
- Puvanendran R, Huey JC, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55(10):981–987
- Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903–912.
- Lakhani NA, Narsinghani U, Kumar R. Necrotizing fasciitis of the abdominal wall caused by Serratia marcescens. Infect Dis Rep. 2015;7(2):5774.
- Jarrett P, Rademaker M, Duffill M. The clinical spectrum of necrotising fasciitis. A review of 15 cases. Aust N Z J Med. 1997;27(1):29–34.
- Wong CH, Tan SH. Subacute necrotising fasciitis. Lancet. 2004;364(9442):1376.
- Saliba WR, Goldstein LH, Raz R, et al. Subacute necrotizing fasciitis caused by gas-producing Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2003;22(10):612–614.
- Wong CH, Wang YS. What is subacute necrotizing fasciitis?: a proposed clinical diagnostic criteria. J Infect. 2006;52(6):415–419.
- Ahmad H, Haider J, Siddiqui SS, et al. Subacute peristomal necrotizing fasciitis detected during adjuvant chemotherapy for adenocarcinoma rectum: case report on a unique presentation and description of a simple surgical strategy for treatment. Cureus. 2018;10(1):e2075.
- Pasternack MS, Swartz MN. Cellulitis, necrotizing fasciitis, and subcutaneous tissue infections. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia (PA): Churchill Livingstone Elsevier; 2015: 1195–1216.
- Tyler K, Patterson A, Hebra A. Necrotizing fasciitis in a healthy pediatric patient caused by Enterobacter cloacae and Serratia marcescens: a discussion of diagnosis and management. Am Surg. 2016;82(9):e292–e293.
- Rimailho A, Riou B, Richard C, Auzepy P. Fulminant necrotizing fasciitis and nonsteroidal anti-inflammatory drugs. J Infect Dis. 1987;155(1):143–146.
- Bornstein PF, Ditto AM, Noskin GA. Serratia marcescens cellulitis in a patient on hemodialysis. Am J Nephrol. 1992;12(5):374–376.
- Zipper RP, Bustamante MA, Khatib R. Serratia marcescens: a single pathogen in necrotizing fasciitis. Clin Infect Dis. 1996;23(3):648–649.
- Huang JW, Fang CT, Hung KY, et al. Necrotizing fasciitis caused by Serratia marcescens in two patients receiving corticosteroid therapy. J Formos Med Assoc. 1999;98:851–854.
- Liangpunsakul S, Pursell KJ. Community-acquired necrotizing fasciitis caused by Serratia marcescens: case report and review. Eur J Clin Microbiol Infect Dis. 2001;20(7):509–510.
- Newton CL, Delemos D, Abramo TJ, et al. Cervical necrotizing fasciitis caused by Serratia marcescens in a 2 year old. Pediatr Emerg Care. 2002;18(6):433–435.
- Bachmeyer C, Sanguina M, Turc Y, et al. Necrotizing fasciitis due to Serratia marcescens. Clin Exp Dermatol. 2004;29(6):673–674.
- Curtis CE, Chock S, Henderson T, Holman MJ. A fatal case of necrotizing fasciitis caused by Serratia marcescens. Am Surg. 2005;71:228–230.
- Statham MM, Vohra A, Mehta DK, et al. Serratia marcescens causing cervical necrotizing oropharyngitis. Int J Pediatr Otorhinolaryngol. 2009;73(3):467–473.
- Motsitsi NS. Fatal necrotizing fasciitis following human bite of the forearm. J Hand Surg Eur Vol. 2011;36(7):605–605.
- Vano-Galvan S, Álvarez-Twose I, Moreno-Martín P, Jaén P. Fulminant necrotizing fasciitis caused by Serratia marcescens in an immunosuppressed host. Int J Dermatol. 2012;53(1).
- Prelog T, Jereb M, Cucek I, Jazbec J. Necrotizing fasciitis caused by Serratia marcescens after venous access port implantation in a child with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2012;34(6).
- Wen YK. Necrotizing fasciitis caused by Serratia marcescens: a fatal complication of nephrotic syndrome. Ren Fail. 2012;34(5):649–652.
- Rehman T, Moore TA, Seoane L. Serratia marcescens necrotizing fasciitis presenting as bilateral breast necrosis. J Clin Microbiol. 2012;50(10):3406–3408.
- Cope TE, Cope W, Beaumont DM. A case of necrotising fasciitis caused by Serratia marsescens: extreme age as functional immunosuppression? Age Ageing. 2013;42(2):266–268.
- Majumdar R, Crum-Cianflone NF. Necrotizing fasciitis due to Serratia marcescens: case report and review of the literature. Infection. 2015;44(3):371–377.
- Hagiya H, Ojima M, Yoshida T, et al. Necrotizing soft tissue infection caused by Serratia marcescens: a case report and literature review. J Infect Chemother. 2016;22(5):335–338.
- Lin WL, Yeh TC, Lu CH, Huang HK, Chiu WY. A catastrophic cervical necrotizing fasciitis after tooth extraction. Intern Emerg Med. 2015;11(8):1135–1136.
- Heigh EG, Maletta-Bailey A, Haight J, Landis GS. Necrotizing fasciitis of the lower extremity caused by Serratia marcescens. J Am Podiatr Med Assoc. 2016;106(2):144–146.
- Marin L, Rowan R, Mantilla A, et al. Lower-extremity infections caused by Serratia marcescens. J Am Podiatr Med Assoc. 2017;107(3):231–239.

