 J Clin Aesthet Dermatol. 2021;14(1):45–54.
J Clin Aesthet Dermatol. 2021;14(1):45–54.
by Asha Gowda, MD; Brayden Healey, DO; Harib Ezaldein, MD; and Miesha Merati, DO
Drs. Gowda and Ezaldein are with the Department of Dermatology, University Hospitals Cleveland Medical Center in Cleveland, Ohio. Dr. Healey is with the Department of Medicine, St. Joseph Mercy Hospital in Ann Arbor, Michigan. Dr. Merati is with The Parker Skin and Aesthetic Clinic in Beachwood, Ohio.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Microneedling is a relatively safe therapeutic procedure used to treat many dermatological conditions, including acne vulgaris, alopecia, melasma and other pigmentary disorders, as well as to promote skin rejuvenation, rhytide reduction, and scar remodeling. Given its popularity among patients and increasing use in the clinic and at home, we aim to explain the adverse effects associated with microneedling procedures.
Objective. We reviewed the current literature describing microneedling and the complications that may accompany this therapeutic procedure. PubMed was searched to identify studies that involved microneedling procedures using the standard roller microneedling, stamp microneedling, pen-type microneedling, and/or fractional radiofrequency microneedling devices. The resulting publications included clinical trials, retrospective studies, and case reports, which were then thoroughly reviewed for description of potential or observed complications that arose secondary to the microneedling procedure.
Results. In this systematic review, a total of 51 articles were reviewed, which included 1,029 patients who received microneedling procedures for a variety of different skin conditions. Overall, this review found that microneedling, regardless of the specific device used, is a relatively safe procedure with minimal adverse effects, including, but not limited to, expected erythema, pain, edema, and temporary skin irritation.
Conclusions. Microneedling has become an attractive treatment option for many patients with dermatological conditions. We advise that clinicians and patients be informed about the adverse side effects associated with microneedling so that the risk of preventable complications can be reduced or avoided.
KEYWORDS: Microneedling, adverse effects, systematic review
The utility of needles in treating scars was initially described more than two decades ago.1 Microneedling (MN) or percutaneous collagen induction therapy (PCI) has recently gained popularity given its effectiveness in skin rejuvenation, rhytide reduction, acne vulgaris, alopecia, scar remodeling, melasma, and other pigmentary disorders. The minimally invasive procedure uses instruments containing up to 540 needles that puncture the epidermis and/or dermis, creating microscopic channels.2 These needles can range in size from 0.1 to 0.25mm in diameter and 0.5 to 3mm in length.2 The small size of the needles allows for penetration into the dermis, where this controlled skin injury triggers a release of growth factors such as transforming growth factors alpha and beta (TGFalpha, TGFbeta), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF).2 Ultimately, the microtrauma that occurs promotes the neogenesis of collagen, elastin, capillaries, and other dermal substances. Additionally, MN is also frequently used with various topical agents or other technologies to increase the therapeutic efficacy of topical agents or the procedure itself.
MN is thought to be relatively safe; however, unexpected side effects and reactions do occur. Recent literature has focused mainly upon the efficacy and potential therapeutic benefits. Due to the increased use in the clinic and widening availability of MN in the market, we aim to comprehensively discuss the associated adverse effects (AEs) in this review of the current literature.
Methods
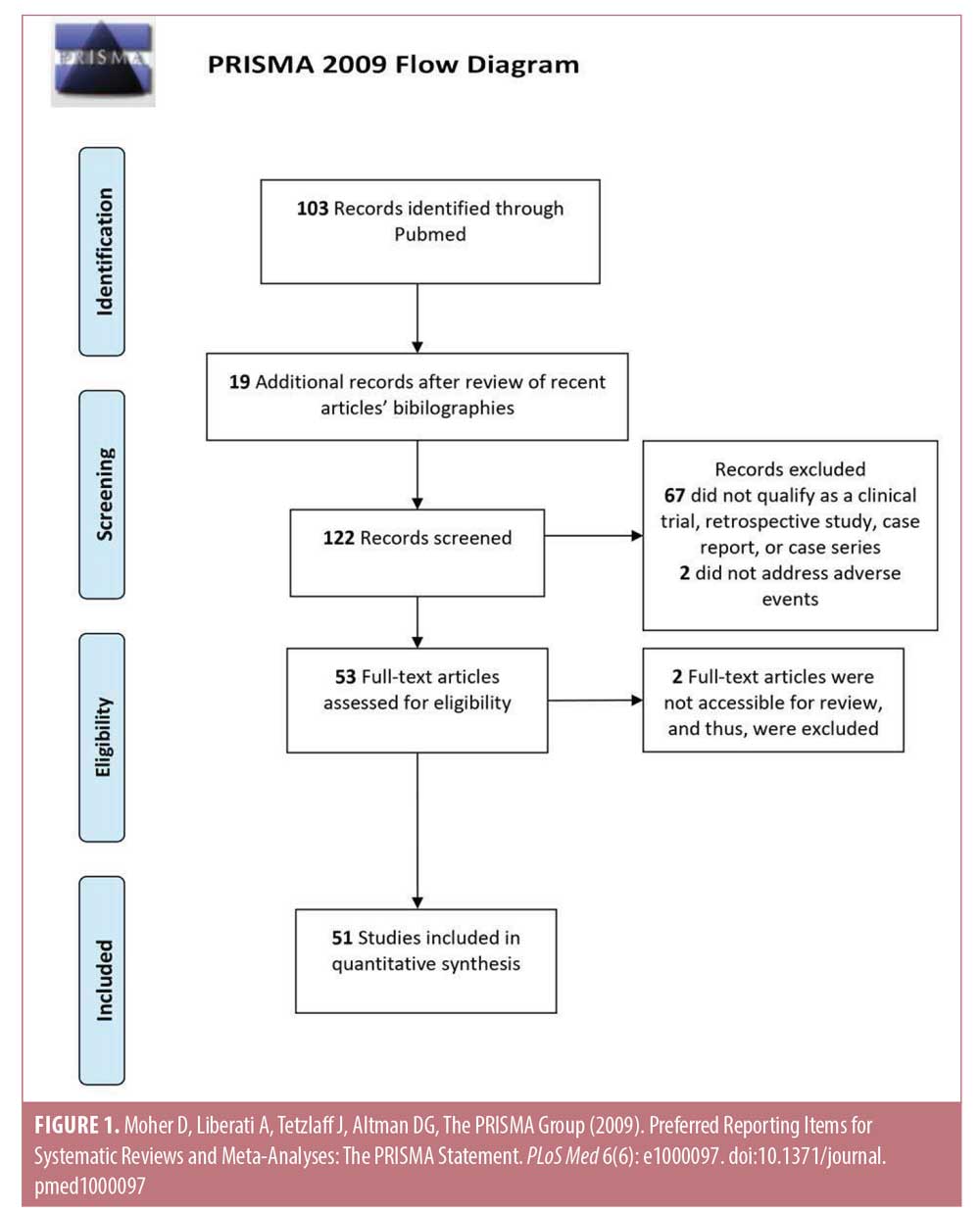
The literature assessed for this study was selected by conducting a PubMed database search, concordant with other published topic reviews on MN. The search terms used included a combination of the following words: “microneedling,” “percutaneous collagen induction,” “collagen induction,” “dermaroller,” “dermal needling,” “dermal rolling,” “micro needle,” and “skin needling” together with “side effect,” ” “side effects,” “adverse effect,” “adverse effects,” “adverse,” “adverse reaction,” “adverse reactions,” “reaction,” or “reactions.” In total, 103 articles resulted from this search and a review of these papers’ bibliographies yielded an additional 19 reports. These 122 reports were reviewed and, based on the exclusion criteria, a total of 51 full-text articles were deemed appropriate for inclusion in this review. Exclusion criteria included articles that could not be classified as either a clinical trial, retrospective study, case series, or case study; did not address AEs; or were not accessible for this review (Figure 1). AEs were categorized based on the MN modality studied as follows: roller MN (RMN) device, dermastamp, pen-type MN (PNM) device, and fractional radiofrequency MN (RFMN) device. Any AEs that developed after combination treatment with MN were grouped into the appropriate aforementioned categories. Statistical analysis was conducted by determining all quantified side effects and calculating the rate of occurrence of each side effect within each device category possible. The comparison of one device to another was conducted using a one-tailed, two-proportions Z-test with an alpha value of 0.05.
Results
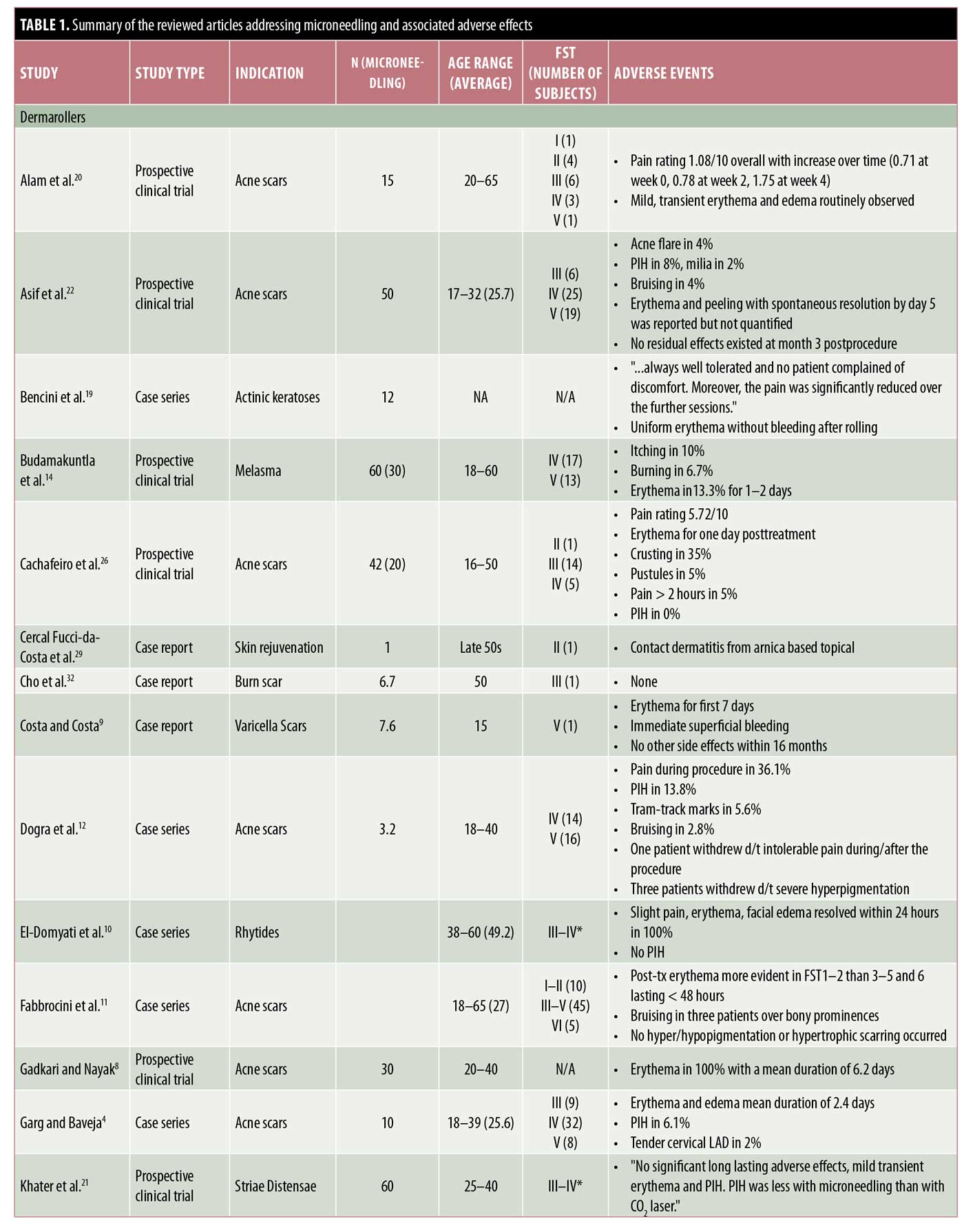
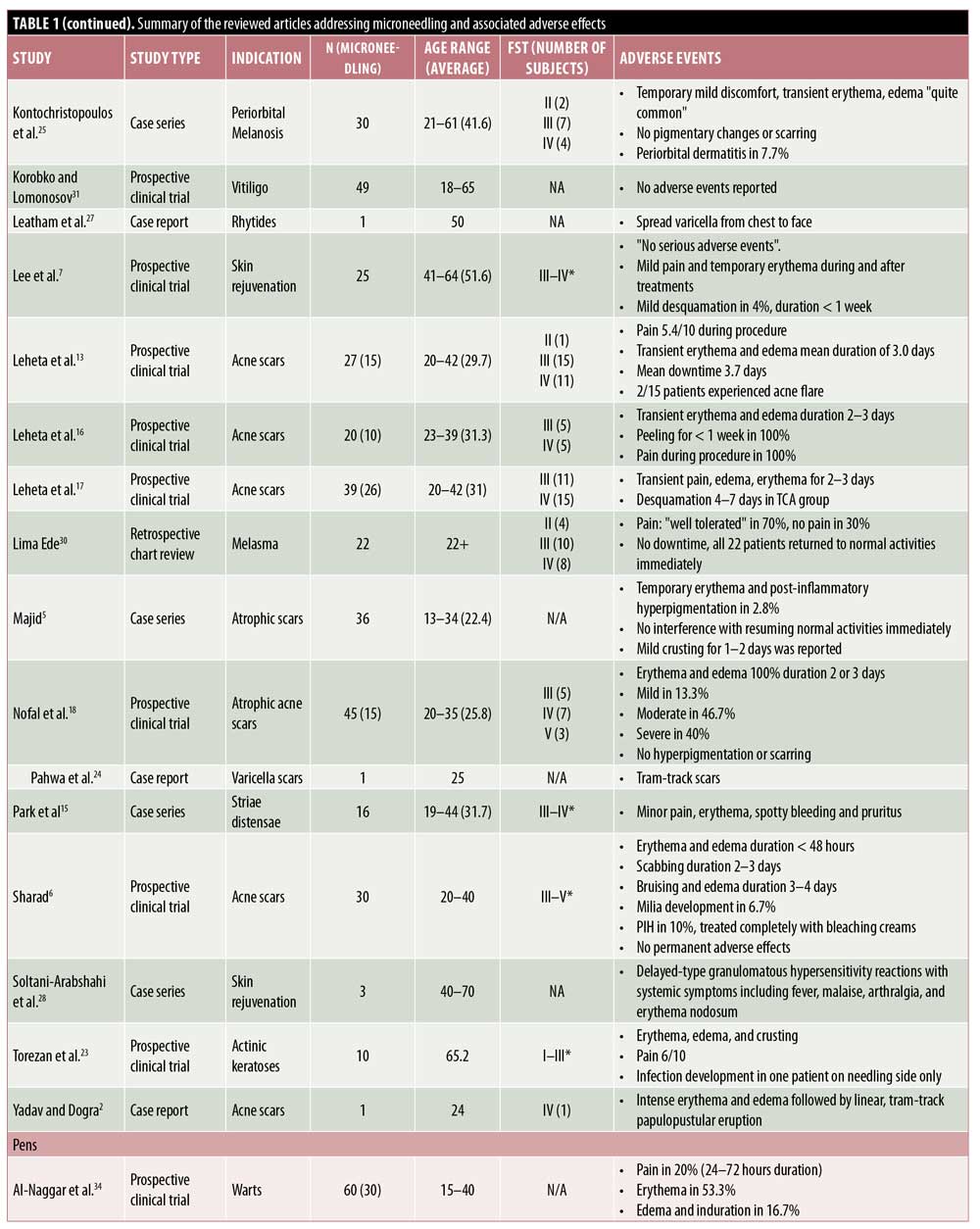
RMN devices. RMN devices are among the most commonly used MN devices and a frequent therapeutic endpoint of this and other modalities of MN is the presence of pinpoint bleeding in the treatment areas. Thus, postprocedure erythema is an expected and frequently encountered side effect.3–6 Transient erythema with RMN may last for a day and, if prolonged, often self-resolves within three to seven days.5–22
Other common AEs of MN include mild pain, edema, and variable postinflammatory dyspigmentation.6,7,10,12,13,15,19,20,22 Patients in one study reported increasing pain with each successive RMN treatment,20 whereas another reported decreasing pain.19 In one study, 15 subjects treated with RMNs reported an average pain score of 5.4 out of 10 points during the procedure.13 Multiple studies also have reported edema as an AE,20,23,24 which often resolves spontaneously in 24 hours10,25 but which can last up to two to three days.2,6,12,13,16,17 Postinflammatory hyperpigmentation (PIH) is a common concern among patients and may resolve with bleaching creams.6 In some patients, PIH may occur after multiple MN sessions.4–6,13,21,24 Rarely, patients may find the pain or hyperpigmentation of MN intolerable, as evidenced by five patients who withdrew from study participation because of these AEs.12
Pinpoint bleeding triggered by MN is desired for optimal results; however, in one instance of RMN, this progressed to spotty bleeding that self-resolved within several days.15 Ecchymosis may be observed with RMN even in patients without a history of bleeding disorders.11,12 For instance, after three monthly RMN sessions, three of 60 patients, none of whom had bleeding disorders, collagen vascular diseases, or were on anticoagulant treatments, developed faint ecchymotic lesions over bony prominences, without residual dyspigmentation.11
Other side effects reported, albeit rare, after the use of RMN include tram-track scarring,12,24 milia,6,22 and pruritus.15 Pustules and crusting also have been noted with RMN.5,22,26 In patients treated with RMN for acne scars, recurrence or flares of acne are possible.13,22 RMN may contain nickel and various other metals; thus, contact allergies may occur. Yadav and Dogra2 discuss the risk of hypersensitivity reactions and suggest a trial procedure be performed in nonvisible body areas prior to treatment on exposed sites. As with any procedure that penetrates the skin barrier, infection is a risk that can be seen with MN as well. One woman who self-treated acne-like eruptions on her chest and face with a home-use RMN was later found to have spread varicella from her chest to her face.27
Combination treatment with roller MN devices and other therapies. MN has also been studied as a combination treatment with various topical agents. Trichloroacetic acid (TCA) is frequently used to complement the effects of MN by inducing exfoliation and neocollagenesis and has been assessed in several studies to date.4,16,17,25 The combination of RMN and TCA appears to lead to pain, erythema, and edema, all of which may be expected with either treatment modality alone. In one study, when patients were treated with subcision followed by 15% TCA peel and RMN alternating every two weeks, erythema and edema occurred and lasted for one to four days.4 Another evaluation of 10 patients with acne scars who had four RMN sessions with 20% TCA every six weeks reported erythema and edema lasting two to three days, transient pain, and skin desquamation for one week.16 Similar findings were observed when this combination treatment was administered six times at monthly intervals.17 Rare AEs reported with this combination treatment include PIH, which was seen in three of 49 patients, and mild cervical lymphadenopathy in one patient that subsided after three weeks.4 In another report, one of 19 patients treated for infraorbital dark circles with RMN and 10% TCA peels developed periorbital dermatitis.25
Another combination treatment studied is RMN followed by tranexamic acid injections for the treatment of melasma. In one study, four of 30 patients treated with this combination three times at monthly intervals reported erythema, itching, and/or burning lasting for 24 to 48 hours after the procedure.14
The combination of intradermal injections of platelet-rich plasma (PRP) with RMN was studied by Nofal et al.,18 who reported variable degrees of erythema and edema lasting to to three days in all 15 subjects treated with this combination.
Infection, which can be seen with RMN alone, can also be seen with combination therapy. In one instance, a patient developed a superficial infection of the face seven days after combination treatment with RMN with photodynamic therapy.23
Systemic reactions are extremely rare with MN; however, they have been reported in three patients who received RMN after pretreatment with topical medication and subsequently developed a delayed-type hypersensitivity granulomatous reaction. Specifically, these patients developed fever, malaise, arthralgia, and erythema nodosum. Two of the three cases were considered to be due to pretreatment with vitamin C serum.28 In another report, a woman received RMN with hyaluronic acid on the dorsal hands and inadvertently applied an arnica-based cream on the treatment areas at home; subsequently, the involved areas evolved into a dermatitis-like reaction with erythema and yellow papules.29 The topicals being used, however, were not necessarily intended for use in conjunction with MN or for delivery into the dermis.
Although MN with the roller device alone and in combination with other therapies have been associated with AEs, subjects in several studies reported no side effects. In a retrospective chart review, 22 subjects who underwent two monthly sessions of RMN followed by a topical depigmentation formula and sunscreen had no significant AEs or downtime.30 In another pilot study, 24 patients with nonsegmental vitiligo vulgaris received four weekly sessions of RMN and topical tacrolimus or latanoprost, followed by three months of narrowband ultraviolet B therapy every other day, with no AEs reported by subjects.31 Similarly, a patient treated with five sessions of CO2 laser followed by RMN for a burn scar and subsequent contracture developed no side effects.32
Stamps. Very few reports are available concerning AEs of stamp MN devices. Only mild side effects including temporary erythema, edema, pain, and xerosis have been reported.3,33 As compared with the fractional-erbium laser, patients reported shorter periods of erythema and edema (p<0.001), but greater pain (p<0.001) with the electric dermastamp (Table 2).33
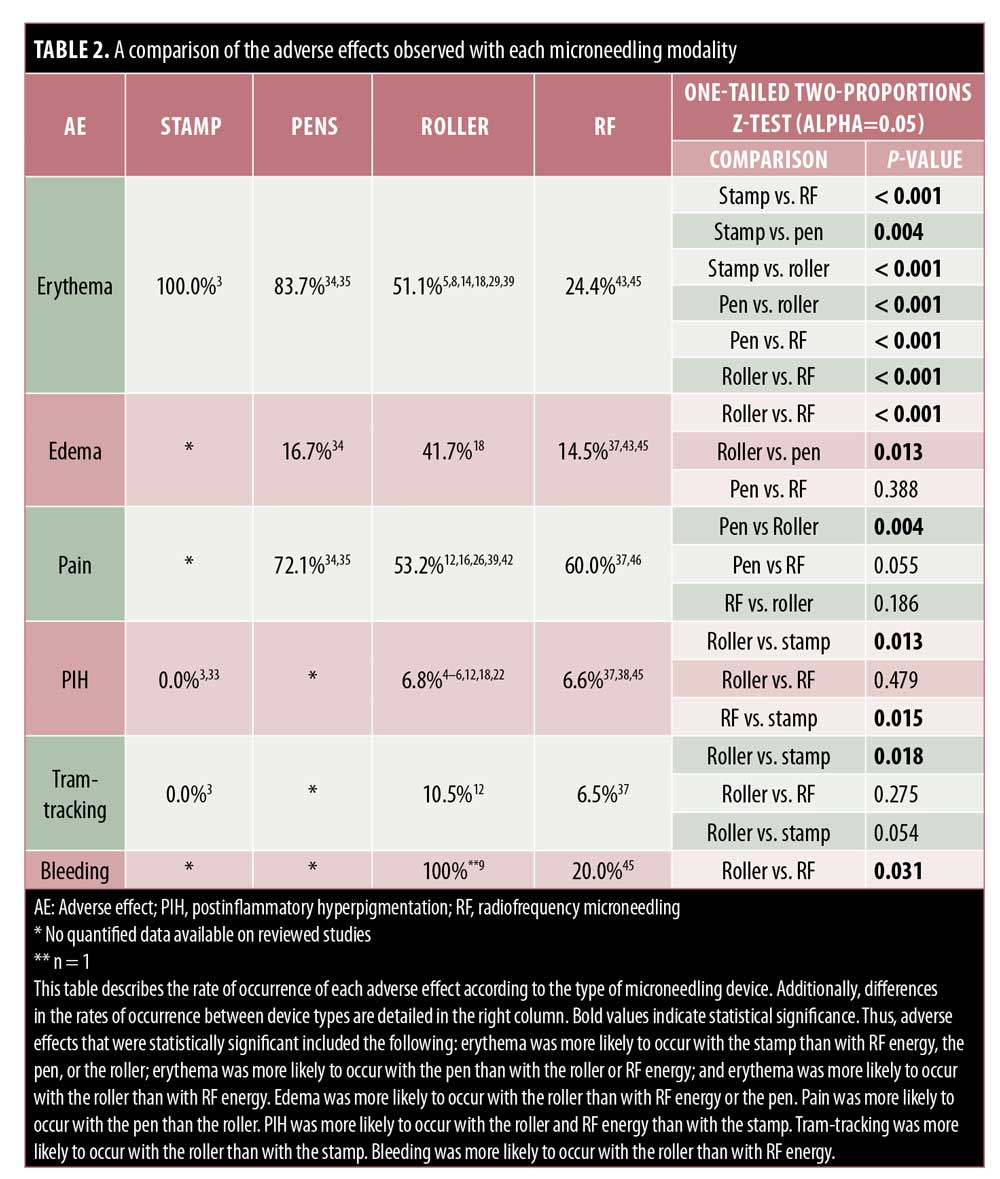
Pens. PNM devices are electric MN devices that resemble a pen and operate on rechargeable batteries and disposable cartridges made of nine to 12 oscillating needles. Few reports discuss the AEs of the pen-type devices, yet erythema remains the most common.34–36 Pain appears variable, with 100% of 56 patients reporting varying severities of pain in one study, whereas 20% of 30 patients reported pain in another study.34,35 Pinpoint bleeding also may occur and last up to 24 hours, comparable to that associated with the use of other MN devices.35
While studying the benefits of combination therapy with the PNM and topical bleomycin, AEs of minimal pain, erythema, and edema were commonly seen. In one study, of the 30 subjects who received PNM followed by topical bleomycin spray, only 20% reported pain within 72 hours as compared with 100% of individuals who received injections.34 Within the MN group, 53.3% experienced erythema and 16.7% developed edema and induration.34 Likewise, Konicke and Olasz36 only reported minimal pain in patients who received PNM to treat plantar warts pretreated with salicylic acid, which was then followed by the intermittent application of topical bleomycin. Signs of neither ischemic changes nor necrosis, both of which are common concerns with intralesional bleomycin injections, were noted.36
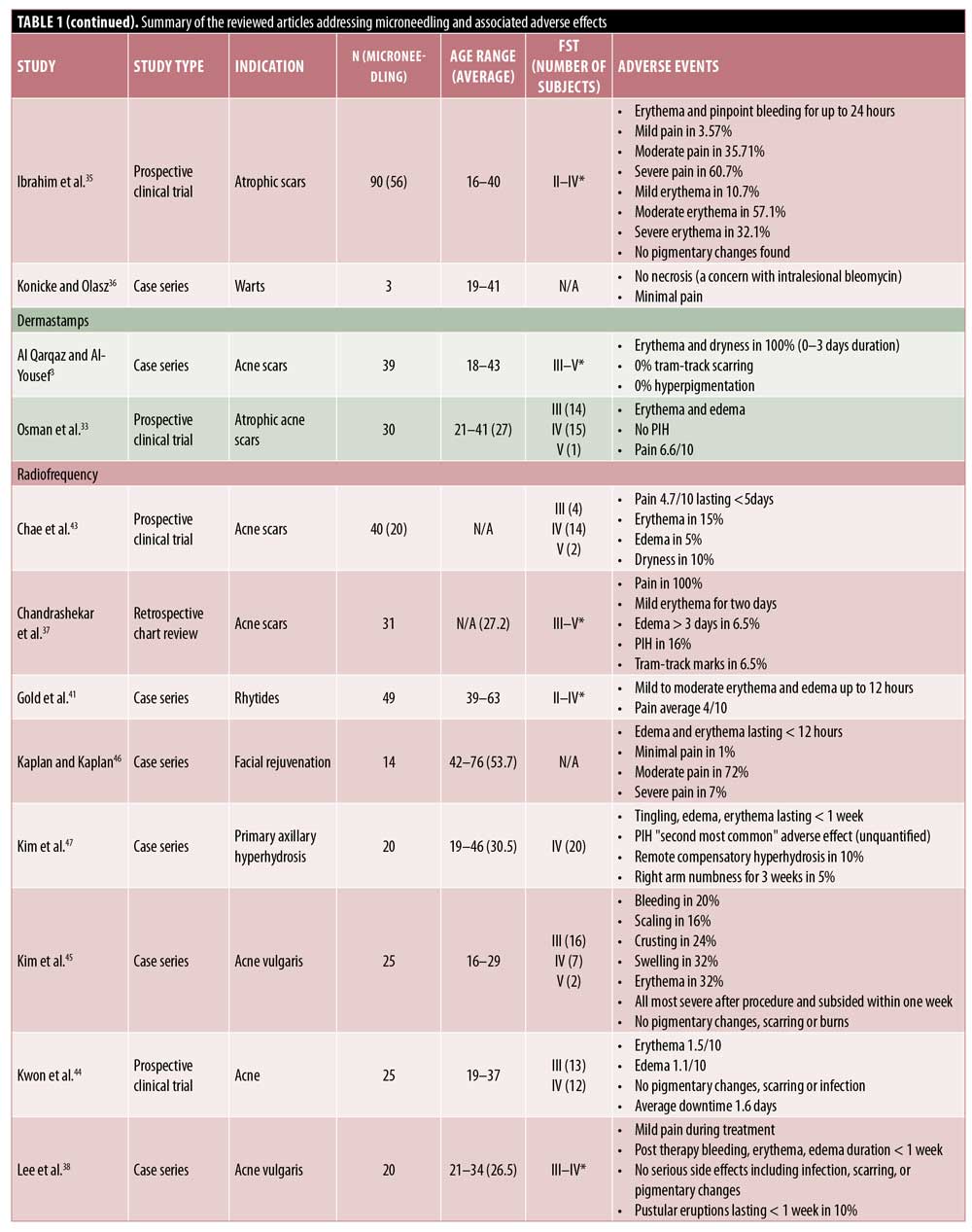
Fractional radiofrequency. RFMN devices use thermal energy that is delivered to the dermal tissue in a controlled manner, with minimal injury to the epidermis.37 In addition to the physical trauma to the tissue caused by the needles, the heat causes trauma that extends past the level of the needle into the deeper dermis. For this reason, RFMN is often used for treating acne scars and rhytides, diseases with etiologies that are dermal in nature.
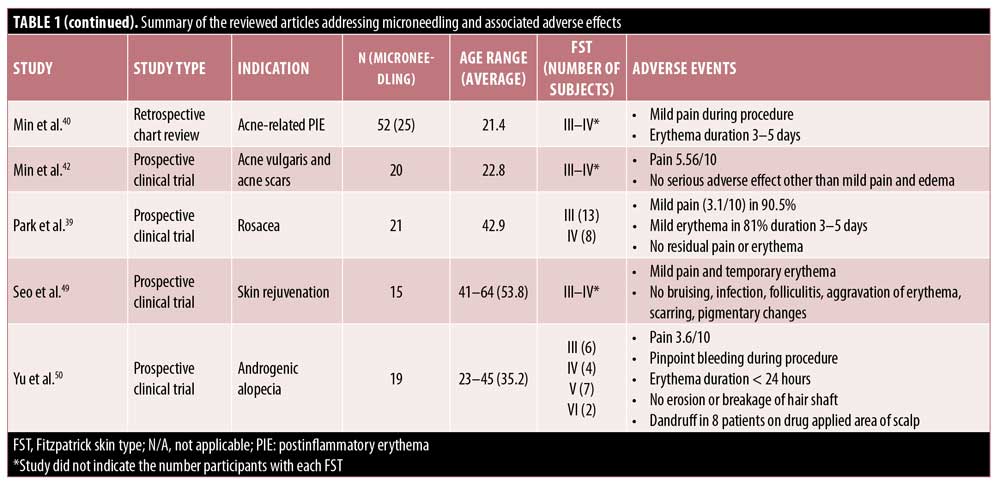
In line with the other MN modalities, RFMN is commonly associated with side effects such as pain, erythema, edema, and bleeding. The pain associated with RFMN is mild and similar to that reported when using a rolling MN device, achieving an average pain score of 5.56 points for RFMN versus 5.4 points for RMN.13,37–42 Additionally, pain with RFMN may be lower than that associated with the fractional laser.43 Erythema seen with RFNM may generally last anywhere from 12 hours to three to five days postprocedure or, occasionally, more than 5 days.37–41,43–45 Edema after RFMN is common and appears to last up to 12 hours after treatment,41–44,46 though some reports suggest that it may last a few days or more and generally lasts longer with RFMN than with other MN modalities.37,38,44,45,47 Likewise, pinpoint bleeding is to be expected with RFMN and may last longer than that following the use of other modalities, with some reports citing bleeding occurring for up to one week.45,38 Tram-track scarring was reported in two patients who were treated for acne or varicella scars with RFMN.37 Remote compensatory hyperhidrosis occurred in two patients after receiving RFMN for axillary hyperhidrosis.47 In the same study, dysesthesia was also reported, including one case of numbness lasting three weeks.47 Xerosis, scaling, and crusting are rare with RFMN and have been reported in two, four, and six patients, respectively.43,45 Pustule development is rare but has also been reported.26,38 Importantly, PIH has been reported to occur with RFMN; however, the incidence of pigmentary alteration is low following RFMN, which may be a preferred treatment in individuals with higher Fitzpatrick skin types or with a history of PIH.3,37,47
Discussion
MN is an increasingly common modality used to treat a variety of dermatologic diseases. This paper reviews the reported literature regarding AEs observed with all four modalities of MN, which reveals a few common and relatively minor side effects with minimal risk of serious AEs (Table 1).
Roller MN devices appear to be the most frequently studied device in the MN literature. The RMN devices are typically rolled with light to medium pressure over the treatment areas multiple times in all directions until pinpoint bleeding is appreciated, to achieve the desired effect. Thus, as expected, erythema and pinpoint bleeding were frequently reported.3–6 The duration of erythema varies from a matter of hours to nearly seven days.7–22 Erythema was mild in most patients; however, patients should be educated to monitor their skin and return for follow-up reassessment to ensure that typical erythema is not a sign of infection. Given the likelihood of erythema, photoprotection is advised for at least a week prior to MN.48
Some complications of RMN could be attributed to operator-related factors, such as the pressure applied. For instance, ecchymoses was seen with RMN even in patients without prior history of bleeding disorders.8,11,12,17 One study reported a patient who withdrew from the study due to pain from RMN, which could be attributed to techniques that are user-dependent.12 Thus, other AEs such as edema and PIH may also have an unpredictable pattern of occurrence. Pahwa et al.24 attribute the development of “tram-track scarring” with RMN in their study to the use of excessive pressure over a bony prominence. On the other hand, Yadav and Dogra2 attribute this finding to nickel-contact dermatitis caused by exposure to RMN devices. Of note, the patients with tram-tracking had no history of scarring disorders; however, careful examination of patients’ existing scars and discussion about the potential for scarring is encouraged.2,12,24 Providers or patients with concerns of scarring can consider a trial of MN in an area of skin that is less visible. Notably, most studies in this review excluded patients with a personal or family history of keloidal or hypertrophic scarring tendencies and, thus, the general risk of severe scarring is difficult to assess.
Overall, only two reports of infection were identified in this review and both were associated with RMN.23,27 It is unclear why infection was seen primarily with RMN, but it may be related to RMN being the most studied modality overall and, thus, an increased likelihood of infections being reported. Also, it is important to note that one of the two cases reported was seen in a patient who used a RMN device at home.27 This draws attention to the dangers of self-administered therapies and highlights the potential for self-inoculation in infected individuals.
Several studies have reported AEs seen with combination treatment with RMN and other topical agents or therapies. Such combination treatments should be considered carefully, as risks for compounded AEs exist with combined regimens. Furthermore, it is difficult to ascertain which AEs can be attributed to a specific treatment modality with confidence, especially when multiple modalities, such as subcision, can yield similar or more serious AEs as compared with MN.4
Minimal side effects have been reported with dermastamp MN. Similar to with the more common RMN devices, dermastamps can lead to temporary postprocedure side effects such as erythema, edema, and xerosis.3,33 Pain can also be expected given the nature of MN and skin trauma. However, the few number of studies evaluating the safety of dermastamps limits the ability to appropriately address and anticipate any associated AEs. Like the RMN devices, dermastamps are also available over the counter; thus, despite MN being a minimally invasive procedure, there may be an increased risk of AEs with dermastamps if consumers are not familiar with proper safety techniques.
Few reports have discussed the AEs associated with PNM devices, with erythema, pain, edema, and mild bleeding being among the most common.34–36 The few AEs seen with pen-type devices may be attributed to the automated features of these electric devices that allow for varying speeds of penetration, while the pressure and depth are controlled and uniform. This may ultimately reduce the likelihood of operator-related side effects. Additionally, the disposable needles confer the advantage of reducing infection and cross-contamination after use.
As with the aforementioned MN devices, RFMN most frequently causes temporary pain, erythema, and edema, despite the technology of thermal energy used in the process.13,37–44,46,49,50 In comparison with other modalities, edema and bleeding seen with RFMN can rarely persist longer.37,38,44,45,47 Dry skin, PIH, and crusting were among the less common side effects.3,37,43,45,47 Rarely, patients developed track marks and pinpoint pustules.26,37,38 Dysesthesia, which is often self-limited, can be seen with RFMN and may be related to heat-induced nerve injury.47 Patients who complain of such sensory changes should be assessed for possible contact dermatitis or infection, which may present similarly. Although the use of heat may be concerning, burns have not been reported as an AE. RFMN does not typically cause hypopigmentation and, therefore, for people of darker skin color desiring MN or those with a history of PIH, it may be a safer option.3,37,47
With the above most common AEs in mind, this review proposes that MN is a relatively safe procedure with minimal side effects for numerous dermatologic conditions. Most patients experienced minimal downtime, with some reporting up to an average of 3.7 days of downtime.13 Appropriate postprocedure care such as sun-protective measures and avoidance of exposure to chemical-based irritants may help to limit skin inflammation; however, some mild side effects may be unavoidable. The possibility of unforeseen AEs should be addressed prior to the use of MN with other therapies.
With all devices, procedural pain appears and erythema and edema seem to be nearly universal, with variations in the severity and duration. It is important to note for clinical application and extrapolation of these data that most studies use topical lidocaine prior to MN, thus mitigating associated pain.2,6,7,9,10,18,20,22,26,28,32,33,43–47,50 While pinpoint bleeding is often a desired endpoint, the risk of bruising and bleeding may be lower in those patients who have not been on blood thinners. Overall, this review did not discover excessive bleeding to be a side effect of MN. Patients with a history of bleeding disorders, coagulopathies, or those on anticoagulation medications were excluded from most studies.6,8,11,12,17,18,22,26,35,41,46
One concern, particularly in individuals with darker skin types, is the risk of PIH. In this review, PIH was found to be of justifiable concern, though it is difficult to draw definite conclusions. Multiple studies report pigmentary changes as side effects of RMN and RFMN;12,24,37,47 however, multiple studies have also reported no observations of pigmentary change with MN in a variety of skin types.11,35,44,45 Many studies that reported hyperpigmentation noted its resolution either spontaneously or with the aid of topical medications, such as bleaching creams.6,33 Despite this, PIH should be considered as a realistic potential side effect, particularly in patients with darker skin. To reduce the risk of PIH, MN should be avoided in patients with obvious signs of sun of exposure.51
Despite MN being a generally safe procedure, proper safety techniques are imperative. The availability of devices such as RMN and dermastamp devices over the counter require that consumers are familiar with proper safety techniques. Infection was rarely noted as a complication of MN in the literature, which some attribute to rapid closure of the microchannels created by the needles,52 but also may be due to the use of prophylactic or postprocedure antibiotics and/or antivirals in many studies.6,8,18,20,24,33,40 This method of infection control was inconsistently used and may confound the true infectious potential of MN. Furthermore, active infections are a contraindication to MN and, thus, many studies excluded patients with active infections.4–6,12,15,17,18,21–23,26,33,35,38 Media has recently brought attention to reports of human immunodeficiency virus transmission to patients treated with MN with PRP.53 This underscores the obligation of physicians to educate patients and the public on strategies to prevent postprocedure infections. Patients and physicians should be aware of the potential risk of infection transmission and take precautionary measures to ensure MN is performed safely and properly. Patient education through patient–physician discussions and/or pamphlets can improve expectations and possibly avoid some of the AEs.
Conclusion
MN is a new and highly requested therapeutic modality in dermatology due to its various clinical applications and nonsinvasive nature. This review of AEs concludes that, with the available data in mind, MN appears to be a safe treatment option for many patients and mild AEs such as erythema and pain can be expected, while more severe AEs are relatively rare.
References
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21(6):543–549.
- Yadav S, Dogra S. A cutaneous reaction to microneedling for postacne scarring caused by nickel hypersensitivity. Aesthet Surg J. 2016;36(4):NP168–170.
- Al Qarqaz F, Al-Yousef A. Skin microneedling for acne scars associated with pigmentation in patients with dark skin. J Cosmet Dermatol. 2018;17(3):390–395.
- Garg S, Baveja S. Combination therapy in the management of atrophic acne scars. J Cutan Aesthet Surg. 2014;7(1):18–23.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2(1):26–30.
- Sharad J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin. J Cosmet Dermatol. 2011;10(4):317–323.
- Lee HJ, Lee EG, Kang S, et al. Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: a randomized, controlled, blinded split-face study. Ann Dermatol. 2014;26(5):584–591.
- Gadkari R, Nayak C. A split-face comparative study to evaluate efficacy of combined subcision and dermaroller against combined subcision and cryoroller in treatment of acne scars. J Cosmet Dermatol. 2014;13(1):38–43.
- Costa IM, Costa MC. Microneedling for varicella scars in a dark-skinned teenager. Dermatol Surg. 2014;40(3):333–334.
- El-Domyati M, Barakat M, Awad S, et al. Multiple microneedling sessions for minimally invasive facial rejuvenation: an objective assessment. Int J Dermatol. 2015;54(12):1361–1369.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatolog Treat. 2014;25(2):147–152.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13(3):180–187.
- Leheta T, El Tawdy A, Abdel Hay R, Farid S. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37(2):207–216.
- Budamakuntla L, Loganathan E, Suresh DH, et al. A randomised, open-label, comparative study of tranexamic acid microinjections and tranexamic acid with microneedling in patients with melasma. J Cutan Aesthet Surg. 2013;6(3):139–143.
- Park KY, Kim HK, Kim SE, et al. Treatment of striae distensae using needling therapy: a pilot study. Dermatol Surg. 2012;38(11):1823–1828.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial. J Dermatolog Treat. 2014;25(2):130–136.
- Leheta TM, Abdel Hay RM, Hegazy RA, El Garem YF. Do combined alternating sessions of 1540 nm nonablative fractional laser and percutaneous collagen induction with trichloroacetic acid 20% show better results than each individual modality in the treatment of atrophic acne scars? A randomized controlled trial. J Dermatolog Treat. 2014;25(2):137–141.
- Nofal E, Helmy A, Nofal A, et al. Platelet-rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: a comparative study. Dermatol Surg. 2014;40(8):864–873.
- Bencini PL, Galimberti MG, Pellacani G, Longo C. Application of photodynamic therapy combined with pre-illumination microneedling in the treatment of actinic keratosis in organ transplant recipients. Br J Dermatol. 2012;167(5):1193–1194.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150(8):844–849.
- Khater MH, Khattab FM, Abdelhaleem MR. Treatment of striae distensae with needling therapy versus CO2 fractional laser. J Cosmet Laser Ther. 2016;18(2):75–79.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15(4):434–443.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39(8):1197–1201.
- Pahwa M, Pahwa P, Zaheer A. “Tram track effect” after treatment of acne scars using a microneedling device. Dermatol Surg. 2012;38(7 Pt 1):1107–1108.
- Kontochristopoulos G, Kouris A, Platsidaki E, et al. Combination of microneedling and 10% trichloroacetic acid peels in the management of infraorbital dark circles. J Cosmet Laser Ther. 2016;18(5):289–292.
- Cachafeiro T, Escobar G, Maldonado G, et al. Comparison of nonablative fractional erbium laser 1,340 nm and microneedling for the treatment of atrophic acne scars: a randomized clinical trial. Dermatol Surg. 2016;42(2):232–241.
- Leatham H, Guan L, Chang ALS. Unintended widespread facial autoinoculation of varicella by home microneedling roller device. JAAD Case Rep. 2018;4(6):546–547.
- Soltani-Arabshahi R, Wong JW, Duffy KL, Powell DL. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150(1):68–72.
- Cercal Fucci-da-Costa AP, Reich Camasmie H. Drug delivery after microneedling: report of an adverse reaction. Dermatol Surg. 2018;44(4):593–594.
- Lima Ede A. Microneedling in facial recalcitrant melasma: report of a series of 22 cases. An Bras Dermatol. 2015;90(6):919–921.
- Korobko IV, Lomonosov KM. A pilot comparative study of topical latanoprost and tacrolimus in combination with narrow-band ultraviolet B phototherapy and microneedling for the treatment of nonsegmental vitiligo. Dermatol Ther. 2016;29(6):437–441.
- Cho SB, Lee SJ, Kang JM, et al. The treatment of burn scar-induced contracture with the pinhole method and collagen induction therapy: a case report. J Eur Acad Dermatol Venereol. 2008;22(4):513–514.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43 Suppl 1:S47–S56.
- Al-Naggar MR, Al-Adl AS, Rabie AR, et al. Intralesional bleomycin injection vs microneedling-assisted topical bleomycin spraying in treatment of plantar warts. J Cosmet Dermatol. 2019;18(1):124–128.
- Ibrahim ZA, El-Ashmawy AA, Shora OA. Therapeutic effect of microneedling and autologous platelet-rich plasma in the treatment of atrophic scars: a randomized study. J Cosmet Dermatol. 2017;16(3):388–399.
- Konicke K, Olasz E. Successful treatment of recalcitrant plantar warts with bleomycin and microneedling. Dermatol Surg. 2016;42(8):1007–1008.
- Chandrashekar BS, Sriram R, Mysore R, et al. Evaluation of microneedling fractional radiofrequency device for treatment of acne scars. J Cutan Aesthet Surg. 2014;7(2):93–97.
- Lee KR, Lee EG, Lee HJ, Yoon MS. Assessment of treatment efficacy and sebosuppressive effect of fractional radiofrequency microneedle on acne vulgaris. Lasers Surg Med. 2013;45(10):639–647.
- Park SY, Kwon HH, Yoon JY, et al. Clinical and histologic effects of fractional microneedling radiofrequency treatment on rosacea. Dermatol Surg. 2016;42(12):1362–1369.
- Min S, Park SY, Yoon JY, et al. Fractional microneedling radiofrequency treatment for acne-related post-inflammatory erythema. Acta Derm Venereol. 2016;96(1):87–91.
- Gold M, Taylor M, Rothaus K, Tanaka Y. Non-insulated smooth motion, micro-needles RF fractional treatment for wrinkle reduction and lifting of the lower face: international study. Lasers Surg Med. 2016;48(8):727–733.
- Min S, Park SY, Yoon JY, Suh DH. Comparison of fractional microneedling radiofrequency and bipolar radiofrequency on acne and acne scar and investigation of mechanism: comparative randomized controlled clinical trial. Arch Dermatol Res. 2015;307(10):897–904.
- Chae WS, Seong JY, Jung HN, et al. Comparative study on efficacy and safety of 1550 nm Er:glass fractional laser and fractional radiofrequency microneedle device for facial atrophic acne scar. J Cosmet Dermatol. 2015;14(2):100–106.
- Kwon HH, Park HY, Choi SC, et al. Novel device-based acne treatments: comparison of a 1450-nm diode laser and microneedling radiofrequency on mild-to-moderate acne vulgaris and seborrhoea in Korean patients through a 20-week prospective, randomized, split-face study. J Eur Acad Dermatol Venereol. 2018;32(4):639–644.
- Kim ST, Lee KH, Sim HJ, et al. Treatment of acne vulgaris with fractional radiofrequency microneedling. J Dermatol. 2014;41(7):586–591.
- Kaplan H, Kaplan L. Combination of microneedle radiofrequency (RF), fractional RF skin resurfacing and multi-source non-ablative skin tightening for minimal-downtime, full-face skin rejuvenation. J Cosmet Laser Ther. 2016;18(8):438–441.
- Kim M, Shin JY, Lee J, et al. Efficacy of fractional microneedle radiofrequency device in the treatment of primary axillary hyperhidrosis: a pilot study. Dermatology. 2013;227(3):243–249.
- Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg. 2009;2(2):110–111.
- Seo KY, Kim DH, Lee SE, et al. Skin rejuvenation by microneedle fractional radiofrequency and a human stem cell conditioned medium in Asian skin: a randomized controlled investigator blinded split-face study. J Cosmet Laser Ther. 2013;15(1):25–33.
- Yu AJ, Luo YJ, Xu XG, et al. A pilot split-scalp study of combined fractional radiofrequency microneedling and 5% topical minoxidil in treating male pattern hair loss. Clin Exp Dermatol. 2018;43(7):775–781.
- Alster TS, Graham PM. Microneedling: a review and practical guide. Dermatol Surg. 2018;44(3):397–404.
- O’Mahony C, Pini F, Blake A, et al. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sensor Actuat A-Phys. 2012;186:130–136.
- Howard J. Vampire facial at New Mexico spa tied to 2 HIV cases, health officials say. Available at: https://www.cnn.com/2019/04/30/health/vampire-facial-hiv-cases-new-mexico-bn/index.html. Accessed April 30, 2019.

