 J Clin Aesthet Dermatol. 2021;14(9):50–53.
J Clin Aesthet Dermatol. 2021;14(9):50–53.
by Soheir Ghonemy, MD; Basma Mohamed: MB bch; Kamal Elkashishy, MD; and Al-shimaa M. Ibrahim, MD
All authors are with the Faculty of Medicine, Zagazig University in Zagazig, Egypt.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Squamous cell carcinoma antigens (SCCA1, SCCA2) are members of the ovalbumin serpin family that have been described as biomarkers of squamous cell carcinomas. Different studies to date have stated the involvement of SCCA in the pathogenesis of certain immunological diseases, such as asthma and atopic dermatitis.
Objective. We sought to assess the expression of SCCA2 in the skin of patients with chronic plaque psoriasis and to detect its correlation with the clinical severity of psoriasis and with the density of inflammatory infiltrates in the skin lesions.
Methods. Skin biopsies were taken from 24 patients with psoriasis vulgaris and 24 healthy controls by 5-mm punches. Tissues were stained with hematoxylin and eosin to confirm the diagnosis and to assess the grade of inflammation. The expression level of SCCA2 in the skin was assessed by immunohistochemical analysis.
Results. The tissue SCCA2 level was significantly higher in psoriatic patients than controls and correlated positively with the severity of psoriasis. In addition, the dermal SCCA2 expression correlated positively with the density of dermal inflammatory infiltrates.
Conclusion. SCCA2 could be a useful marker of the clinical severity and the grade of inflammation of psoriasis.
Keywords: Psoriasis, immunology, squamous cell carcinoma antigen
Psoriasis is a T-cell-mediated autoimmune skin disease characterized by keratinocyte hyperproliferation and abnormal differentiation mediated by inflammatory cells such as T helper (Th) 17 and Th 1 cells, macrophages, dendritic cells, and their cytokines.1,2
Squamous cell carcinoma antigen (SCCA), expressed in the granular and prickle cell layers of the normal squamous epithelium, is part of the serine protease inhibitor family that was firstly identified as a serological marker for squamous cell carcinomas. To date, different studies have reported the presence of elevated levels of SCCA in patients with allergic diseases like asthma and atopic dermatitis.3,4 Two isoforms exist, SCCA1 and SCCA2, which are nearly homologous at the amino acid level but active against different proteases.5
The biological functions of SCCA are diverse, including defending against parasitic and bacterial proteases, inhibiting cell apoptosis, stimulating cell proliferation, and suppressing the immune defense against tumors.6
Interleukin (IL)-17 and IL-22 play a crucial role in the pathogenesis of psoriasis, with both stimulating SCCA2 production, so SCCA has been suggested as one of the markers of Th17-mediated inflammation. In addition, SCCA plays an important role in suppressing keratinocyte apoptosis, leading to the keratinocyte hyperproliferation and abnormal differentiation that manifests in psoriasis.7 Previous studies have recorded the presence of elevated levels of SCCA2 in the serum and skin lesions of psoriatic patients.6,8
Methods
This study included 24 patients with psoriasis vulgaris and 24 healthy volunteers who were recruited from the outpatient clinic of the dermatology department of Zagazig University Hospital from October 2018 to January 2019. Informed consent was collected from every participant before starting the study and after obtaining approval from the institutional review board.
Subjects who presented with allergic diseases or other dermatological diseases or had a history of squamous cell carcinoma were excluded from this study. Participants were required to stop any treatment for psoriasis for at least two months before the study.
All patients were subjected to full history-taking, including personal history; family history; and present history, including the onset, course, duration, and previous treatment of psoriasis. In addition, general and full dermatological examinations to assess the severity of psoriasis using the Psoriasis Area and Severity Index (PASI) score were performed.9
One skin biopsy was taken from every participant via 5-mm disposable punches under complete sterile precautions. Skin biopsies from psoriatic patients were collected from lesional skin.
Biopsy handling. Each specimen in both groups was fixed in formalin and embedded in paraffin to form paraffin blocks. Serial sections were obtained from each block and stained with hematoxylin and eosin (H&E) for histological diagnosis. Stained sections were examined under a light microscope. To confirm the diagnosis of psoriasis, the grade of inflammation and the density of dermal inflammatory infiltrate were classified as follows Grade 0, no infiltrate; Grade 1, mild; Grade 2, moderate; or Grade 3, marked infiltrate.10
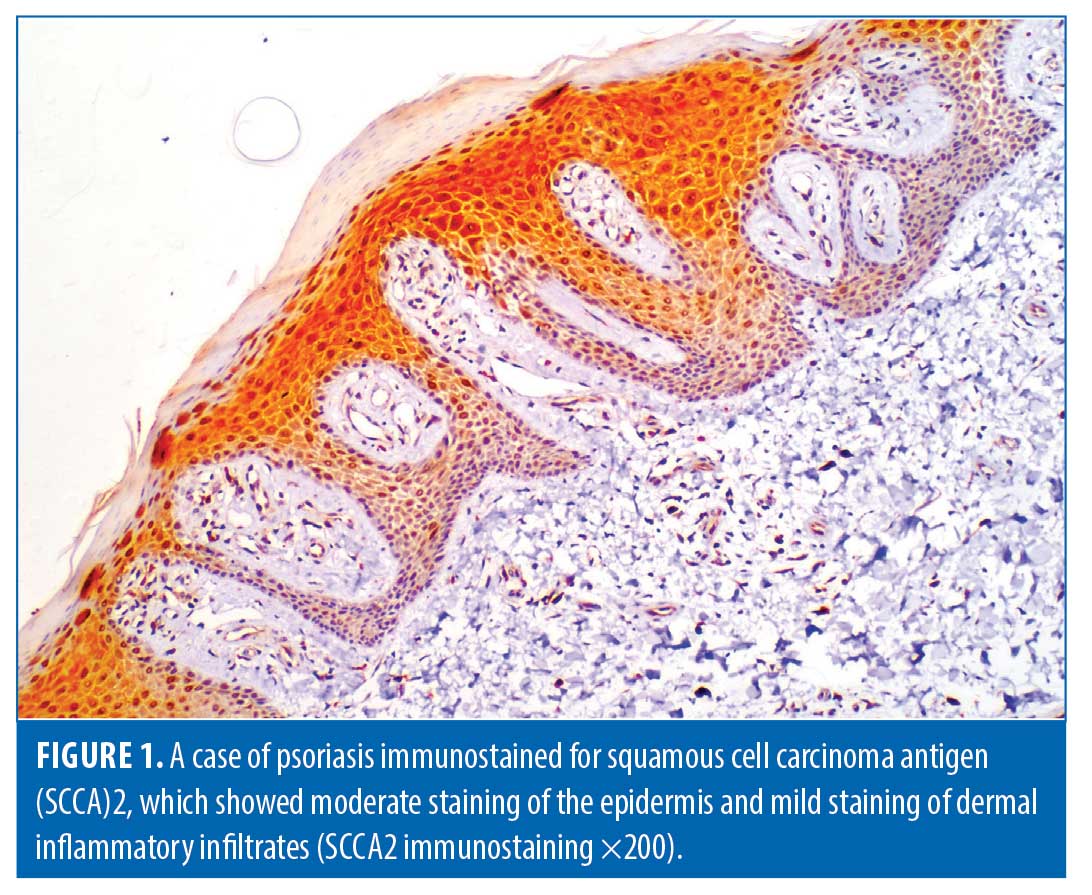
Immunohistochemical staining. We used monoclonal antibodies to SCCA2 (antibodies to SCCA2 (Asn2~Pro390)-DAKO, Rabbit PAb RB-9097-R1 53, Fermont, CA, USA). Immunohistochemical staining was performed according to the method described by Shi et al.11 The pattern of immunostaining for SCCA2 was evaluated in both the epidermis and dermis of psoriatic lesions. The immunohistochemical score (IHC) range was as follows: 0: negative expression of SCCA2; 1+: weak expression of SCCA2; 2+: moderate expression of SCCA2; and 3+: strong expression of SCCA2.8
Statistical analysis. All data were collected, tabulated, and statistically analyzed using the Statistical Package for the Social Sciences version 20.0 for Windows (IBM Corporation, Armonk, NY, USA). Quantitative data were expressed as mean ± standard deviation (minimum–maximum) values, while qualitative data were expressed as absolute frequency (number) and relative frequency (percentage) values. Continuous data were checked for normality by using the Shapiro-Wilk test; the Mann–Whitney U test was used for non-normally distributed variables.
The two dependent groups sign test or Wilcoxon signed-rank test was used when appropriate. Percentages of categorical variables were compared using the chi-squared test or Fisher’s exact test where appropriate. Spearman’s rank correlation coefficient was calculated to assess the relationship between various study variables, where the (+) sign indicated direct correlation and the (-) sign indicated an inverse correlation; also, values close to 1 indicated a strong correlation and those close to 0 indicated a weak correlation. All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant, a p-value of less than 0.001 was considered highly statistically significant, and a p-value of 0.05 or greater was considered statistically insignificant.
Results
Participants. The patient group included 24 individuals with psoriasis vulgaris, including eight female participants (33.3%) and 16 male participants (66.7%), with an age range of 10 to 73 years (38 ± 20 years). The duration of psoriasis in this group ranged from two months to 15 years. Eight patients (33.3%) had a positive family history, and, according to their PASI scores, six patients (25%) had mild psoriasis, 10 patients (41.7%) had moderate psoriasis, and eight patients (33.3%) had severe psoriasis. The control group similarly included 24 healthy individuals, including 11 female participants (46%) and 13 male participants (54%), with an age range of 12 to 73 years (39 ± 18 years). There was no statistically significant difference between these groups regarding age or sex.
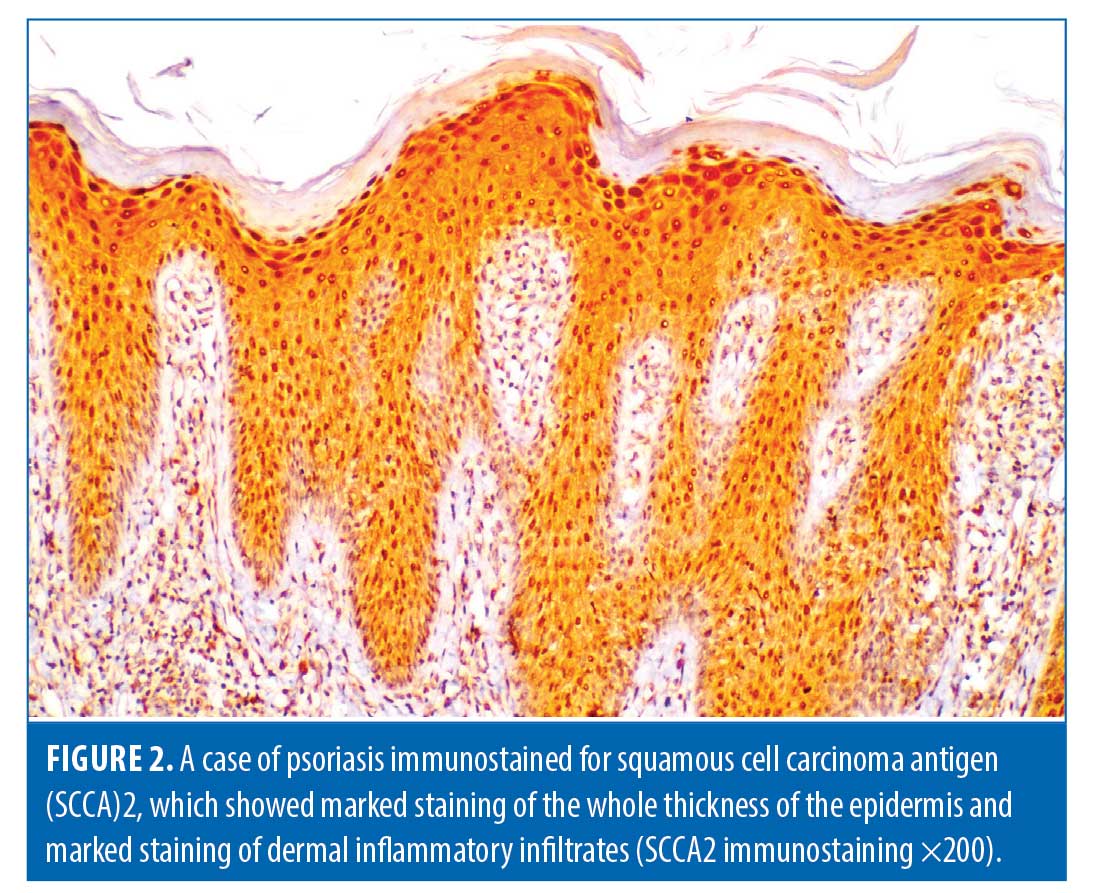
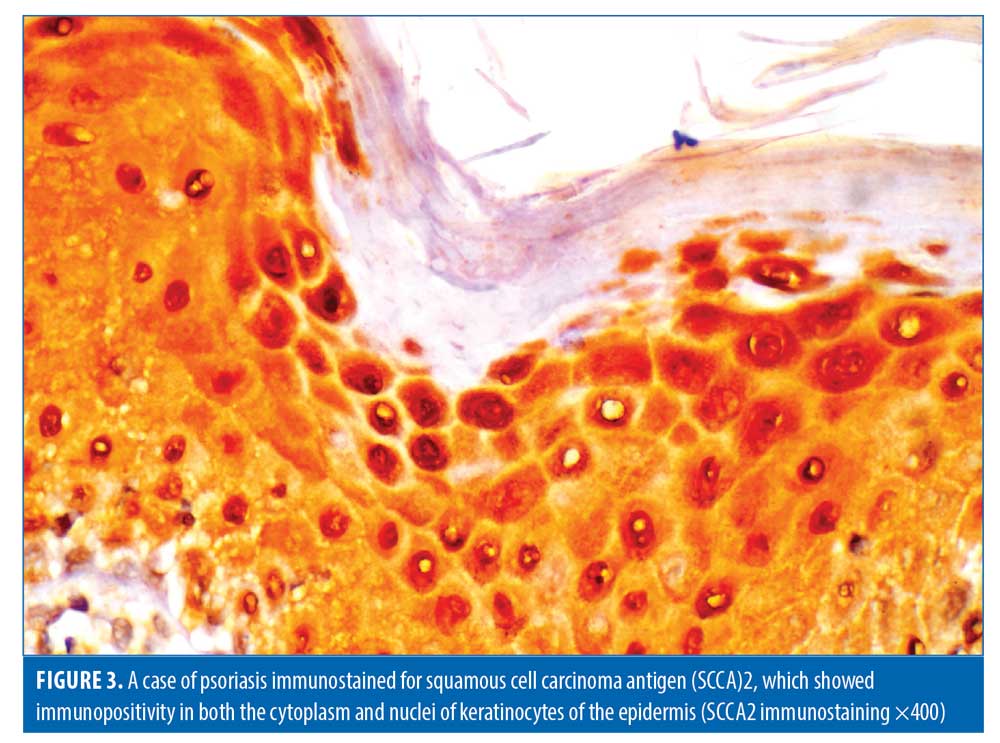
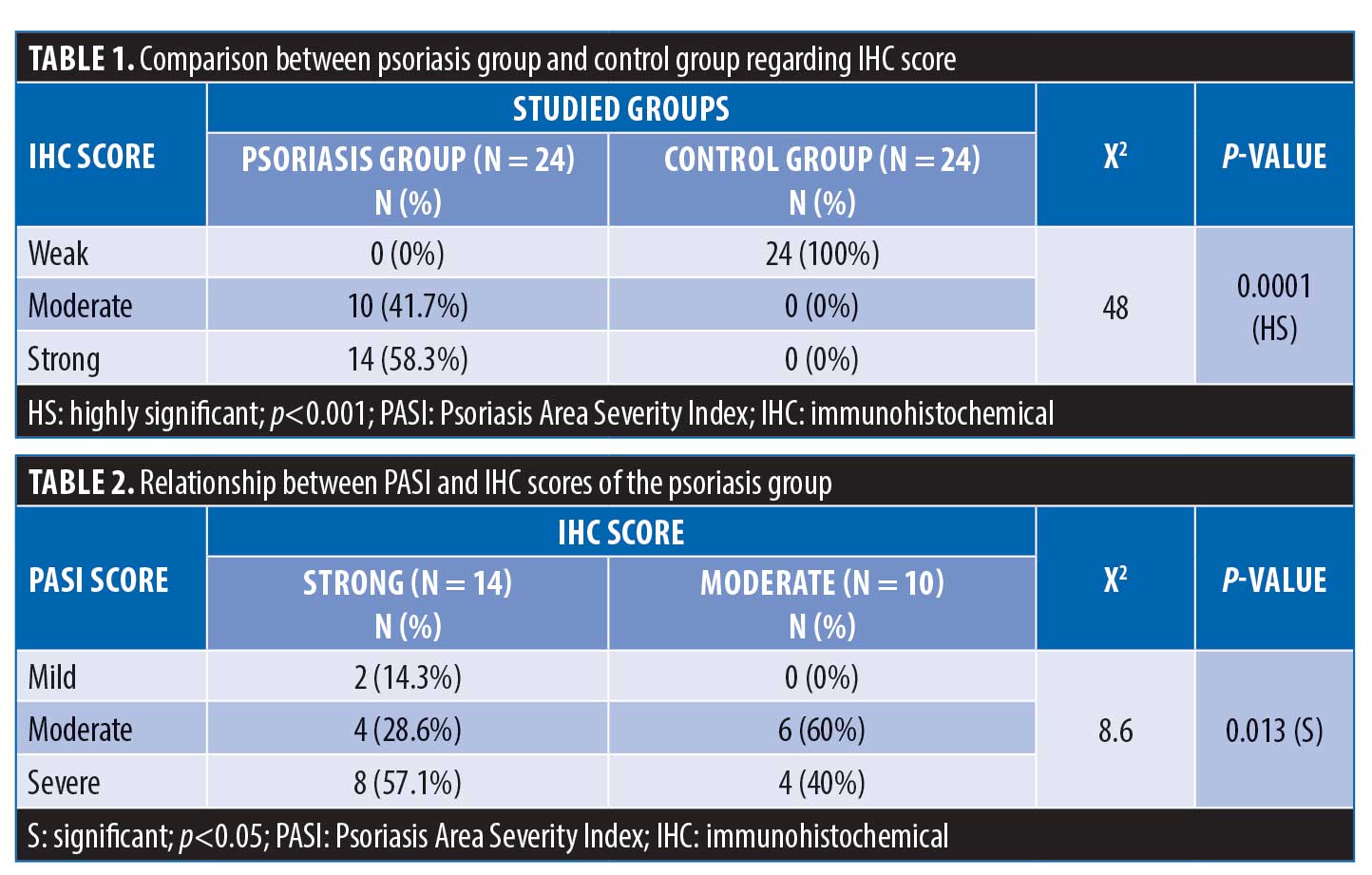
Histopathological evaluation. There was a high statistically significant difference in the density of dermal inflammatory infiltrates in psoriatic patients versus controls per hematoxylin and eosin staining (p<0.001). All control subjects showed no dermal inflammatory infiltrates (Grade 0); meanwhile, eight psoriatic patients showed mild inflammatory infiltrates, 10 patients showed moderate inflammatory infiltrates, and six patients showed marked inflammatory infiltrates. Regarding SCCA2 expression in the layers of the epidermis (Figure 1), 10 psoriatic patients (41.7%) had moderate IHC scores, with diffuse positive SCCA2 staining in the granular and spinous layers of epidermis, while 14 patients (58.3%) showed strong IHC scores with positive SCCA2 staining through the whole thickness of epidermis, mainly in the spinous and granular layers, with weak staining of the basal layer (Figure 2); additionally, SCCA2 staining was positive in the cytoplasm and nuclei of keratinocytes (Figure 3) with presence of SCCA2-positive staining in cells of elongated rete ridges. The IHC scores of psoriatic patients were significantly higher than those of control group as all subjects in the control group showed weak IHC scores (limited scattered staining in the granular layer) (Table 1). There was a significant positive correlation between SCCA2 expression and the severity of psoriasis as assessed by PASI score (Table 2).
Regarding the expression of SCCA2 in the dermis, 8.3% of patients showed marked dermal SCCA expression, while 58.3% of patients showed moderate SCCA expression and 33.3% of patient had mild SCCA expression. There was a significant positive correlation between SCCA expression in the dermis and the density of dermal inflammatory infiltrates (Table 3).
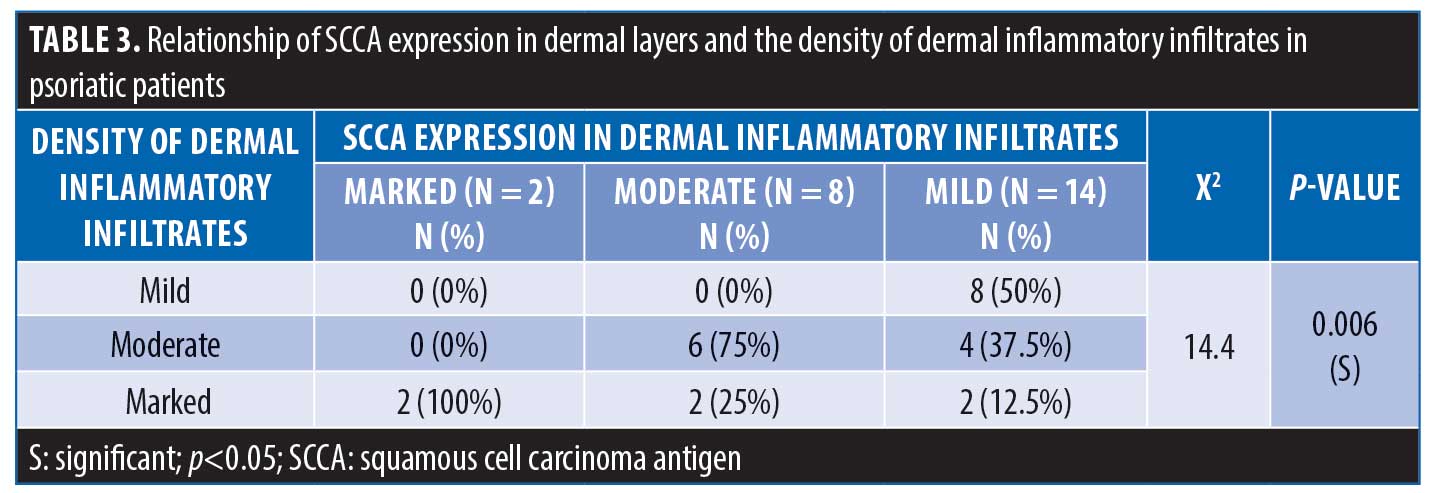
There was no statistically significant relation between SCCA expression in the epidermis and its expression in dermal inflammatory infiltrates in psoriatic patients (p>0.05); also no statistically significant relationship between SCCA expression in the epidermis and the density of dermal infiltrates was observed (p>0.05).
Discussion
Psoriasis is a chronic immunologic disease that affects multiple body systems, of which the skin is one, due to the interaction between immunoinflammatory cells, mainly Th1 and Th17, and their cytokines with keratinocytes in genetically predisposed individuals in response to certain environmental triggers.12
The histopathology of psoriasis is characterized by inordinate hyperproliferation and abnormal differentiation of keratinocytes. The inflammatory cascade of psoriasis begins when certain antigens activate dendritic cells and neutrophils in the skin, which release cytokines, including tumor necrosis factor alpha, IL-23, and IL-12.13
The two homologs of SCCA, SCCA1 and SCCA2, are related to the serine proteinase inhibitor superfamily; however, they have different activities against proteinases. SCCA1 differs from other serpins due to its ability to inhibit the papain-like cysteine proteinases, such as the cathepsins K, L, and S; meanwhile, SCCA2, like other serpins, has the ability to inhibit chymotrypsin-like serine proteinases. Previous studies have discussed the involvement of SCCA in the pathogenesis of certain immunological diseases such as asthma and atopic dermatitis.14–16
Th17 and Th22 and their cytokines have active roles in the pathogenies and severity of psoriasis and have been found to induce the expression of SCCA1 and SCCA2. As such, SCCA may be used as a marker of the activity of Th17- and Th22-mediated inflammatory cascade.6,8,17
The goal of this study was to assess the expression of SCCA2 in the skin of patients with chronic plaque psoriasis and compare the results with expression levels of the same in the skin of healthy controls to detect its correlation with the clinical severity of psoriasis and with the density of inflammatory infiltrates in hematoxylin and eosin-stained sections of psoriasis lesions.
The present immune-histochemical study included 24 patients with chronic plaque psoriasis and 24 healthy controls. There was a high statistically significant difference in SCCA expression in the skin of psoriatic patients versus in that of controls (p<0.05). We reported that SCCA expression in the epidermal layers of psoriatic lesions positively correlated with psoriasis severity as assessed by the PASI score. In moderate cases, there was staining of the granular and spinous layers of the epidermis (IHC score: 2+), while, in severe cases, there was positive SCCA expression throughout the whole thickness of epidermis, albeit mainly in the spinous and granular layers, with weak staining of the basal layer (IHC score: 3+). In controls, we reported scattered weak SCCA2 expression in the granular layer (IHC score: 1+).These results are in agreement with those of Takeda et al,16 Watanabe et al,8 and Izuhara et al,6 who also reported elevated levels of SCCA2 in the skin of psoriatic patients that correlated positively with their serum levels and with the severity of psoriasis and reflected treatment efficacy.
In our study, we assessed the grade of inflammation in psoriasis by counting the numbers of inflammatory cells in hematoxylin and eosin-stained sections. The density of dermal inflammatory infiltrates correlated positively with the severity of psoriasis and with SCCA dermal expression. Takeda et al16 previously reported the presence of SCCA in limited dermal cells of psoriatic lesions.
There was a positive expression of SCCA2 in the cytoplasm as well as the nuclei of epidermal cells of psoriatic lesions, and similar findings were reported by Watanabe et al8 and Sun et al18 Immunohistochemical examinations of skin taken from healthy subjects have previously revealed the presence of SCCA in the cytoplasm of keratinocytes only, with negative staining of nuclei. Katagiri et al19 had suggested that, after exposure of the skin to certain triggers like ultraviolet irradiation, SCCA may travel from the cytoplasm to the nucleus. Research also suggests the transfer of SCCA to the nucleus occurs after its binding to c-Jun NH2-terminal kinase 1 (JNK1) to protect cells from apoptosis via the inhibition of c-Jun NH2-terminal kinase.20,21
Tian et al22 and Izuhara et al6 have suggested the increased levels of SSCA in psoriasis to be linked to its role in the protection of cells against viral and bacterial proteases and in the prevention of keratinocyte apoptosis. In addition, it has been found that keratinocytes secrete excess levels of IL-6 and IL-1alpha, leading to the stimulation of IL-23–/Th17-mediated inflammation as the key factor in the pathogenesis of psoriasis.8,23
Conclusion
Based on our findings, we conclude that SCCA2 could be a useful biomarker of the clinical severity of psoriasis and a marker of the grade of inflammation in psoriasis.
References
- Alatas ET, Kalayci M, Kara A, Dogan G. Association between insulin resistance and serum and salivary irisin levels in patients with psoriasis vulgaris. Dermatologica Sinica. 2017;35(1):12–15.
- Lee YH, Song GG. Association between circulating prolactin levels and psoriasis and its correlation with disease severity: a metaanalysis. Clin Exp Dermatol. 2018;43(1):27–35.
- Chechlinska M, Kowalewska M, Brzoska-Wojtowicz E, et al. Squamous cell carcinoma antigen 1 and 2 expression in cultured normal peripheral blood mononuclear cells and in vulvar squamous cell carcinoma. Tumour Biol. 2010;31(6):559–567.
- Piruzian ES, Sobolev VV, Abdeev RM, et al. Study of molecular mechanisms involved in the pathogenesis of immune-mediated inflammatory diseases, using psoriasis as a model. Acta Naturae. 2009;1(3):125–135.
- Okawa T, Yamaguchi Y, Kou K, et al. Serum levels of squamous cell carcinoma antigens 1 and 2 reflect disease severity and clinical type of atopic dermatitis in adult patients. Allergol Int. 2018;67(1):124–130.
- Izuhara K, Yamaguchi Y, Ohta S, et al. Squamous cell carcinoma antigen 2 (SCCA2, SERPINB4): an emerging biomarker for skin inflammatory diseases. Int J Mol Sci. 2018;19(4):1102.
- Suminami Y, Kishi F, Sekiguchi K, Kato H. Squamous cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem Biophys Res Commun. 1991;181(1):51–58.
- Watanabe Y, Yamaguchi Y, Komitsu N, et al. Elevation of serum squamous cell carcinoma antigen 2 in patients with psoriasis: associations with disease severity and response to the treatment. Br J Dermatol. 2016;174(6):1327–1336.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area and severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26(5):851–856.
- Rana S, Zeeba JS, Sujata J, Madhur K. A. A comparative study of psoriasis and psoriasiform lesion on basis of CD4 and CD8 cell infiltration. Our Dermatol Online. 2012;3(4).
- Shi SR, Gu J, Kalra KL, et al. Antigen retrieval technique: a novel approach to immunohistochemistry on routinely processed tissue sections. In: Analytical Morphology. Boston, MA: Birkhäuser; 1996: 1–40.
- Wong Y, Nakamizo S, Tan KJ, Kabashima K. An update on the role of adipose tissues in psoriasis. Front Immunol. 2019;10:1507.
- Brandon A, Mufti A, Sibbald RG. Diagnosis and management of cutaneous psoriasis: a review. Adv Skin Wound Care. 2019;32(2):58–69.
- Holdenrieder S, Molina R, Qiu L, et al. Technical and clinical performance of a new assay to detect squamous cell carcinoma antigen levels for the differential diagnosis of cervical, lung, and head and neck cancer. Tumor Biol. 2018;40(4):1010428318772202.
- Schick C, Kamachi Y, Bartuski AJ, et al. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J Biol Chem. 1997;272(3):1849–1855.
- Takeda A, Higuchi D, Takahashi T, et al. Overexpression of serpin squamous cell carcinoma antigens in psoriatic skin. J Invest Dermatol. 2002;118(1):147–154.
- Karaaslan C, Birben E, Keskin O, et al. The role of SCCA1 in asthma related physiological events in the airway epithelium and the effect of promoter variants on asthma and gene function. Respir Med. 2013;107(3):368–379.
- Sun Z, Shi X, Wang Y, Zhao Y. Serum squamous cell carcinoma antigen in psoriasis: a potential quantitative biomarker for disease severity. Dermatology. 2018;234(3–4):120–126.
- Katagiri C, Nakanishi J, Kadoya K, Hibino T. Serpin squamous cell carcinoma antigen in-hibits UV-induced apoptosis via suppression of c-JUN NH2-terminal kinase. J Cell Biol. 2006;172(7):983–990.
- Suminami Y, Nagashima S, Vujanovic NL, et al. Inhibi¬tion of apoptosis in human tumour cells by the tumour-associated serpin, SCC antigen-1. Br J Cancer. 2000;82(4):981–989.
- Haider AS, Peters SB, Kaporis H, et al. Genomic analysis de¬fines a cancer-specific gene expression signa¬ture for human squamous cell carcinoma and distinguishes malignant hyperproliferation from benign hyperplasia. J Invest Dermatol. 2006;126(4):869–881.
- Tian S, Krueger JG, Li K, et al. Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PloS One. 2012;7(9):e44274.
- Fujishima S, Watanabe H, Kawaguchi M, et al. Involvement of IL-17F via the induction of IL-6 in psoriasis. Arch Dermatol Res. 2010;302(7):499–505.

