J Clin Aesthet Dermatol. 2021;14(10):48–51.
 by Natsinee Tangkijngamvong, MD; Paweena Susantitaphong, MD; Numphung Numkarunarunrote, MD; Onanong Jearnsujitwimol, MD; Pravit Asawanonda, MD, DSc; and Pawinee Rerknimitr, MD, MSc
by Natsinee Tangkijngamvong, MD; Paweena Susantitaphong, MD; Numphung Numkarunarunrote, MD; Onanong Jearnsujitwimol, MD; Pravit Asawanonda, MD, DSc; and Pawinee Rerknimitr, MD, MSc
Tangkijngamvong, Asawanonda, and Dr. Rerknimitr are with the Division of Dermatology, Department of Medicine, Faculty of Medicine, Skin and Allergy Research Unit, Chulalongkorn University in Bangkok, Thailand. Susantitaphong is with the Division of Nephrology, Department of Medicine, Faculty of Medicine, Chulalongkorn University in Bangkok, Thailand. Numkarunarunrote is with the Department of Radiology, Faculty of Medicine, Chulalongkorn University in Bangkok, Thailand. Jearnsujitwimol is with the Division of Nephrology, Department of Medicine, Prapokklao Hospital in Chantaburi, Thailand.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: The use of sodium thiosulfate has emerged as a promising treatment for calciphylaxis, albeit inconclusively in terms of efficacy and variable outcomes. Research in this field has been limited by a paucity of samples due to the rarity of the disease. We herein discuss eight calciphylaxis patients’ responses to STS, the potential predictive factors affecting outcomes and compare our results with previously published literature. We are able to show that lesion severity, concomitant drugs, and dialysis duration may be predictive factors of outcomes. Further, improvement of the wound site may be a clinically relevant prognostic determinant.
Key words: Calciphylaxis, sodium thiosulfate, outcome
________________________________________________________________________________
Calciphylaxis is a rare but catastrophic condition chiefly observed in dialysis patients.1 In current practice, a gold standard and recommended approach are still to be established. Despite a multi-interventional approach, the mortality rate remains high. In a search for a cure, sodium thiosulfate (STS) has been in the spotlight. A proposed action is through reversing calcium phosphate deposition.2 However, because of limited case reports and small case series, the clinical outcome of STS is still inconclusive. In this report, we discuss our patients’ responses to STS and the potential predictive factors affecting outcomes and compare our results with those in the previously published literature.
Medical records of patients who received STS between January 2013 and December 2018 at King Chulalongkorn Memorial Hospital in Bangkok, Thailand were reviewed retrospectively. Patients who received STS for indications other than calciphylaxis were excluded. The diagnosis of calciphylaxis was confirmed by histology and/or radiography reports. A total of eight patients were included for analysis and were categorized as responders and non-responders, respectively, where responders were those whose wound sites showed improvement.
Demographics, clinical features, treatment modalities, and outcomes are listed in Table 1. All patients received STS at a dosage of 12.5g to 25g three times weekly. Of the eight patients, four (50%) were classified as responders. The responder and non-responder groups differed in terms of the cumulative dose (1611.3±560.8 g vs. 456.3±280.9 g) and duration of STS (22.3±6.1 weeks vs. 6.0±3.5 weeks), with both being higher/longer in the responder group. Thus, we believe that STS may influence positive outcomes, although determining the optimal dosage and duration is challenging. The earliest positive response that we observed was as late as eight weeks after the treatment was initiated.
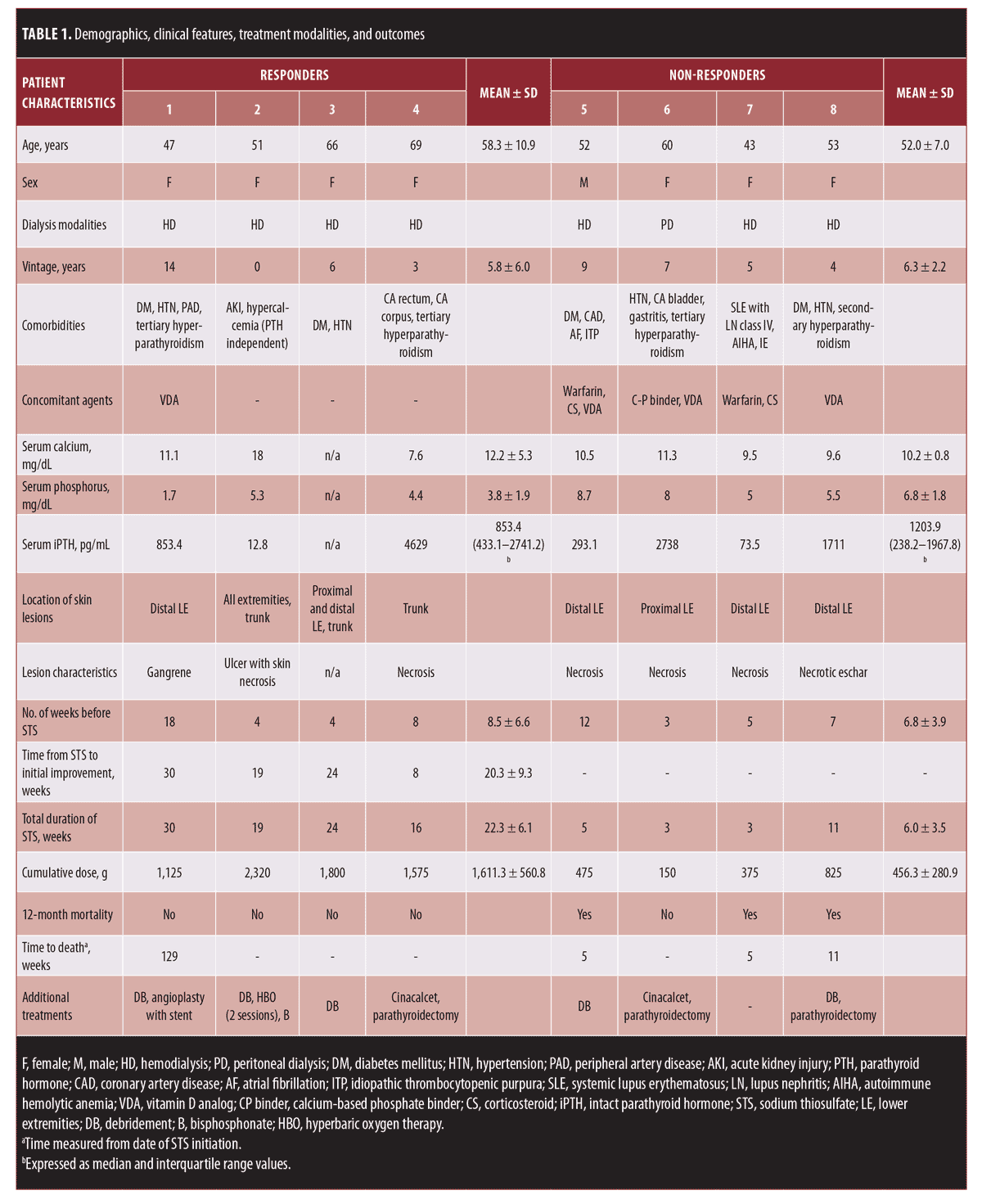
Compared to other published reports, fewer positive outcomes were achieved in this series (Table 2). All of the patients in this study presented with ulcer and necrosis, which were moderate to highly severe, according to the previously defined staging system.3 Two of the non-responders were unable to discontinue warfarin and corticosteroid treatment because of comorbidities. Further, the mean dialysis vintage in this series was longer than that of the others. It is important to note that the non-responders also showed longer dialysis vintage than the responders did (6.3 ± 2.2 years vs. 5.8 ± 6.0 years). It is also possible that these long-standing processes may be associated with poorer wound healing and survival.
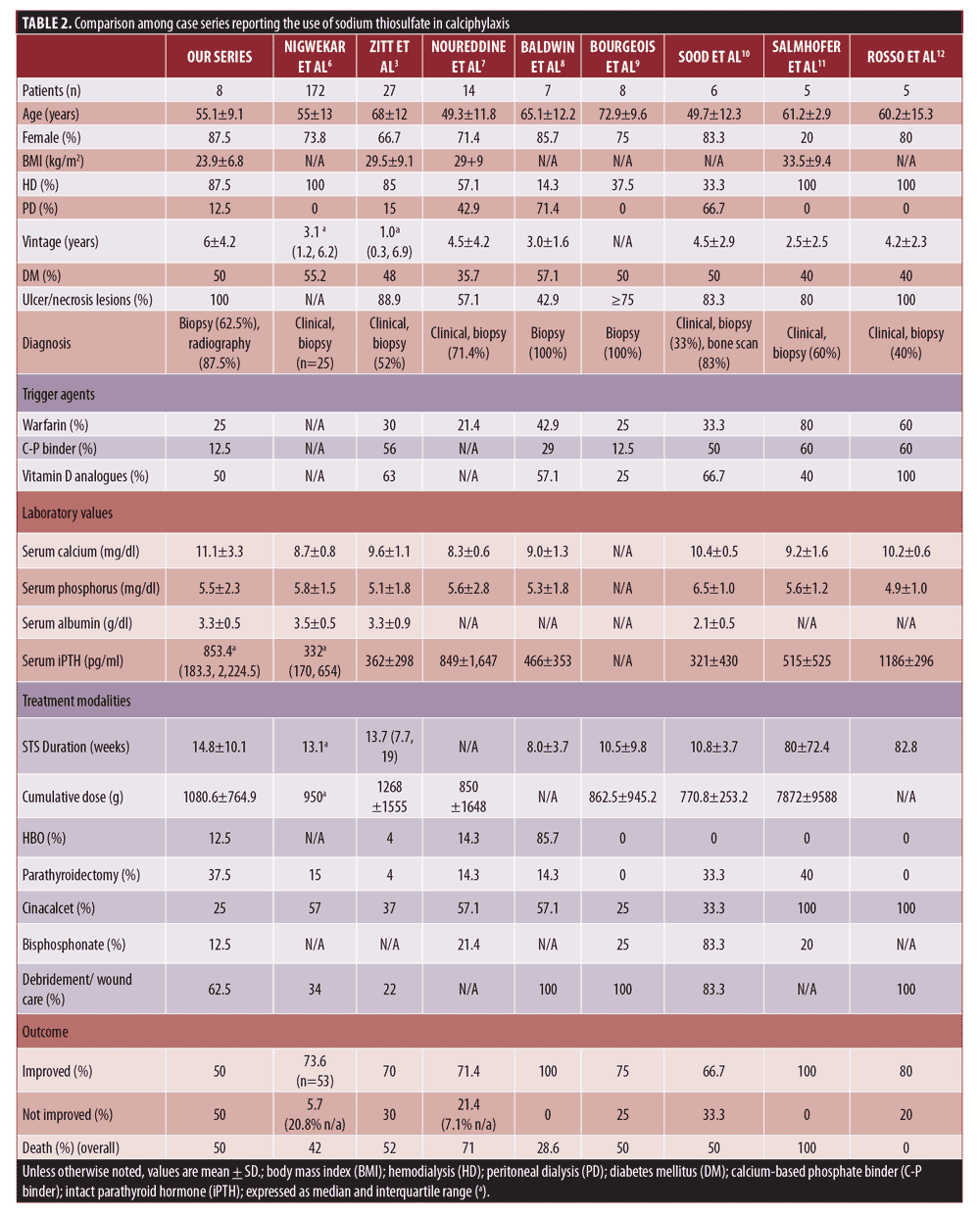
Factors reported to have a hand in predicting poor survival were female sex, higher weight, severe cutaneous lesions, and proximal lesions.3,4 Interestingly, all non-survivors in our series exhibited distal calciphylaxis. Thus, the proximal type might not necessarily dictate such poor outcomes as previously reported. An additional finding observed in this series was that the improvement of the wound sites might be a clinically relevant prognostic determinant. The mortality rate in the responder group at 12 months was 0%, while that of the non-responders was 75%.
Given the small number of study participants, the fact that not all cases had a histologically confirmed diagnosis, and the difference in clinical evaluation times across the studies, determining the clinical outcome of STS is especially challenging. Further studies are warranted which, in the long run, should be beneficial to those who suffer from this deadly disease.
We propose that lesion severity, concomitant drugs, and dialysis duration might be predictive factors of outcomes. In addition, improvement of the wound site may be a clinically relevant prognostic determinant. Given the proposed action of STS, it may take some time before positive responses are observed. Meanwhile, providing specific treatments such as calcimimetics and parathyroidectomy may lower the rates of wound deterioration and mortality for those with tertiary hyperthyroidism.5
Acknowledgments
The authors thank the Skin and Allergy Research Unit at Chulalongkorn University for its support.
References
- Guldbakke KK, Khachemoune A. Calciphylaxis. Int J Dermatol. 2007;46(3):231–238.
- Schlieper G, Brandenburg V, Ketteler M, Floege J. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5(9):539–543.
- Zitt E, Konig M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28(5):1232–1240.
- Lal G, Nowell AG, Liao J, et al. Determinants of survival in patients with calciphylaxis: a multivariate analysis. Surgery. 2009;146(6):1028–1034.
- Udomkarnjananun S, Kongnatthasate K, Praditpornsilpa K, et al. Treatment of calciphylaxis in CKD: a systematic review and meta-analysis. Kidney Int Rep. 2019;4(2): 231–244.
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162–1170.
- Noureddine L, Landis M, Patel N, Moe SM. Efficacy of sodium thiosulfate for the treatment for calciphylaxis. Clin Nephrol. 2011;75: 485–490.
- Baldwin C, Farah M, Leung M, et al. Multi-intervention management of calciphylaxis: a report of 7 cases. Am J Kidney Dis. 2011;58:988–991.
- Bourgeois P, De Haes P. Sodium thiosulfate as a treatment for calciphylaxis: A case series. J Dermatolog Treat. 2016;27:520–524.
- Sood AR, Wazny LD, Raymond CB, et al. Sodium thiosulfate-based treatment in calcific uremic arteriolopathy: a consecutive case series. Clin Nephrol. 2011;75:8–15.
- Salmhofer H, Franzen M, Hitzl W, et al. Multi-modal treatment of calciphylaxis with sodium-thiosulfate, cinacalcet and sevelamer including long-term data. Kidney Blood Press Res. 2013;37:346–359.
- Russo D, Capuano A, Cozzolino M, et al. Multimodal treatment of calcific uraemic arteriolopathy (calciphylaxis): a case series. Clin Kidney J. 2016;9:108–112.

