J Clin Aesthet Dermatol. 2021;14(10):E53–E65.
 by Nathalie C. Zeitouni, MDCM, FRCPC; Neal Bhatia, MD; Roger I. Ceilley, MD; Joel L. Cohen, MD; James Q. Del Rosso, DO; Angela Y. Moore, MD; Gilly Munavalli, MD, MHS, FACMS; David M. Pariser, MD, FACP, FAAD; Todd Schlesinger, MD; Daniel M. Siegel, MD, MS; Andrea Willey, MD; and Mitchel P. Goldman, MD
by Nathalie C. Zeitouni, MDCM, FRCPC; Neal Bhatia, MD; Roger I. Ceilley, MD; Joel L. Cohen, MD; James Q. Del Rosso, DO; Angela Y. Moore, MD; Gilly Munavalli, MD, MHS, FACMS; David M. Pariser, MD, FACP, FAAD; Todd Schlesinger, MD; Daniel M. Siegel, MD, MS; Andrea Willey, MD; and Mitchel P. Goldman, MD
Dr. Zeitouni is with Medical Dermatology Specialists, University of Arizona COM Phoenix in Phoenix, Arizona. Dr. Bhatoa is with Therapeutics Clinical Research in San Diego, California. Dr. Ceilley is with Dermatology PC in West Des Moines, Iowa. Dr. Cohen is with AboutSkin Dermatology and DermSurgery in Greenwood Village, Colorado. Dr. Del Rosso is with JDR Dermatology Research in Las Vegas, Nevada. Dr. Moore is with Arlington Research Center in Arlington, Texas, and Baylor University Medical Center in Dallas, Texas. Dr. Munavalli is with Dermatology, Laser, & Vein Specialists of the Carolinas in Charlotte, North Carolina. Dr. Pariser is with the Department of Dermatology, Eastern Virginia Medical School and Virginia Clinical Research, Inc. in Norfolk, Virginia. Dr. Schlesinger is with the Dermatology and Laser Center of Charleston and the Clinical Research Center of the Carolinas in Charleston, South Carolina, and the Department of Dermatology, SUNY Downstate Health Sciences University and the Brooklyn VA Medical Center in Brooklyn, New York. Dr. Siegel is with Long Island Skin Cancer and Dermatologic Surgery in New York, New York. Dr. Willey is with Surgical and Aesthetic Dermatology in Sacramento, California. Dr. Goldman is with Cosmetic Laser Dermatology, A West Dermatology Company in San Diego, California.
FUNDING: An expert board meeting was organized and funded by an unrestricted grant from Biofrontera. This publication, however, was initiated by the authors, who developed the recommendations and drafted, edited, and approved the final version.
DISCLOSURES: Dr. Zeitouni is an investigator, speaker, and scientific advisory board member for Biofrontera. Dr. Bhatia has affiliations with Almirall, Biofrontera, Galderma, Ortho, Pharmaderm, and SunPharma. Dr. Ceilley is a consultant to Biofrontera and SunPharma. Dr. Cohen is a on the scientific advisory board and has participated in a clinical trial for Biofrontera. Dr. Del Rosso is a consultant for Biofrontera. Dr. Moore is a consultant and investigator for and has received grants and honoraria from Biofrontera. Dr. Schlesinger is a consultant and investigator for Biofrontera; a consultant, investigator, and on the speaker bureau for Almirall; a consultant for SunPharma; and a consultant and investigator for Galderma. Dr. Siegel is a consultant to Biofrontera, SunPharma, and Almirall. Dr. Willey holds patent PCT/US 2018/042505. Dr. Goldman is a clinical investigator for Biofrontera.
ABSTRACT: Photodynamic therapy (PDT) can be an effective treatment for actinic keratosis (AK) as well as selected non-melanoma skin cancers (NMSCs), such as Bowen’s disease and superficial basal cell carcinoma. PDT has also demonstrated effectiveness in the management of acne vulgaris. Results from controlled clinical trials have shown the safety and efficacy of PDT for these conditions with the use of different photosensitizers and a wide range of light sources. PDT has been employed effectively as monotherapy and in combination with other topicals and alternate light or laser energy therapies. This article provides expert practical guidance for the use of the newest 5-aminolevulinic acid (ALA) product (ALA 10% gel) plus red light as monotherapy for AKs, NMSC, and acne. Here, information from clinical guidelines and a summary of supporting evidence is provided for each cutaneous condition. The authors also provide detailed guidance for employing ALA 10% gel, a photosensitizer precursor, for each of these applications.
Key words: Photodynamic therapy, 5-aminolevulinic acid gel, red light, actinic keratosis, acne, non-melanoma skin cancer, BF-200 ALA, Ameluz, BF-RhodoLED
________________________________________________________________________________
Photodynamic therapy (PDT) is a widely used therapeutic modality in dermatology.1–5 The procedure is most often carried out in the office and it requires three elements6: a photosensitizer, a light source, and tissue oxygen. The therapeutic effect is achieved by light activation of a photosensitizing agent, resulting in the aerobic formation of reactive oxygen species (ROS), which irreversibly oxidize essential cellular components, causing apoptosis and necrosis as well as cell death secondary to increased autophagy.6,7
Multiple photosensitizers have been used for PDT, with the most common being the photosensitizer prodrugs 5-aminolevulinic acid (ALA) and methyl-5-aminolevulinate (MAL). ALA and MAL have been used extensively for lesion- and field-directed treatment in patients with AKs,4,8–11 Bowen’s disease, superficial and nodular basal cell carcinoma (BCC), and acne vulgaris.6,12,13 There are noteworthy differences among the branded photosensitizer prodrugs for PDT, mostly regarding the active ingredient’s stability and epidermal penetration.14 ALA is prone to degradation, and particularly older ALA preparations (e.g., ALA compounded in alcohol, topical creams, or ointments) have a very limited stability. ALA methyl ester (MAL) is less susceptible to degradation, but the ester must be cleaved before ALA can enter the heme biosynthesis pathway to be metabolized to the photosensitizer protoporphyrin IX (PpIX). As a result, MAL induces less PpIX compared to ALA after the same incubation time. In common practice, ALA and MAL are referred to as photosensitizers, and thus the same descriptive term is used in this article. A more recent photosensitizer technology stabilizes ALA within a nanoscale lipid–vesicle gel formulation (BF-200 ALA nanoemulsion gel; Biofrontera Bioscience GmbH, Leverkusen, Germany).15 This BF-200 10% ALA-HCl nanoemulsion gel formulation remains stable over 24 months. Research has also shown that nanoemulsion BF-200 can enhance the penetration of ALA through the stratum corneum.14 The BF-200 formulation of ALA (referred to hereafter to ALA 10% gel) was granted marketing authorization by the European Medicines Agency in December 2011 for the treatment of mild and moderate AKs on the face and scalp; the indication was extended to include field cancerization in 2016, superficial and nodular BCC in 2017, and AK on the extremities and trunk/neck in 2020.16 ALA 10% gel was approved in combination with the BF-RhodoLED® red light lamp (~635nm) (Biofrontera Bioscience GmbH) for treatment of lesion- and field-directed PDT of AKs on the face and scalp by the United States (US) Food and Drug Administration (FDA) in 2016.17
Given the widespread use of PDT, both on- and off-label, and the varying protocols regarding its clinical use, it is important for clinicians to understand best practices for its use (for different indications). Dermatology offices often develop custom PDT protocols, including various preparation procedures, debridement measures, photosensitizer incubation, illumination methods, and postcare protocols. Protocols are also often customized according to the various indications (e.g., AK, NMSC, acne) to achieve optimal outcomes in clearing lesions. The goal of this article is to provide detailed guidance, including practice tips and clinical pearls, for the use of ALA 10% gel monotherapy with red-light illumination in patients with AK, NMSC, and/or acne.
Actinic Keratosis
Treatment of AK includes lesion- and field-directed approaches. Lesion-directed treatments target individual visible lesions of atypical keratinocytes, while field-directed treatments treat subclinical surrounding atypical keratinocytes in chronic sun-damaged skin.18 Field cancerization was initially described in 1953 and has been applied to numerous epithelial tissues, including the skin.19,20 The goal of treatment with field cancerization is to reduce the risk of keratinocyte carcinoma development.21 Patients with extensive field cancerization can benefit from a combination of field- and lesion-directed treatments.21 Field-directed therapy is important for treating AKs because of the potential risk of developing cutaneous squamous cell carcinomas (SCC) in the surrounding skin of lesion-directed AK treatments.21,23 Lesion-directed therapies for AKs include cryotherapy (lesion-directed liquid nitrogen), laser therapy, and curettage. Field-directed therapies include PDT, 5-fluorouracil (5-FU), nonsteroidal anti-inflammatory drugs (diclofenac sodium, piroxicam), chemical peels, and immunomodulators such as imiquimod.18,24 Ingenol mebutate is no longer marketed in the US. A new topical agent, tirbanibulin, was FDA-approved in the US in December 2020.
Importantly, clinically visible and subclinical lesions may lead directly to SCC, and research indicates that field-directed therapy should be delivered early.24 Additionally, AKs have been observed to exhibit abnormal growth patterns with varying degrees of downward extension; thus it is important that both the photosensitizer and the light source used for PDT penetrate quickly and treat the full thickness of the epidermis, up to the basement membrane. Achieving this deep penetration is particularly important when treating areas with excessive papillary sprouting.25
Existing guidelines. In 2016, a clinical consensus guide stated that PDT for AK is highly effective, that the efficacy for head and neck lesions is similar or exceeds other FDA-approved therapies, and that cosmetic outcomes are superior to those of cryotherapy.3 New guidelines for the treatment of AKs from the American Academy of Dermatology (AAD) were expected in 2020.26 The Evidence- and Consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum recommend PDT for patients with AKs and for field-directed treatment.27 Similarly, the British Association of Dermatologists’ guidelines for the care of patients with AKs state that PDT is an effective treatment for confluent AKs in the absence of invasive disease. Otherwise, confluent AKs in such areas as the scalp are difficult to manage or resistant to treatment. The British Association of Dermatologists also note that PDT has low scarring potential and imparts less risk for poor healing in comparison to other physical therapies in certain sites, such as the lower leg.28 The European Dermatology Forum has provided guidance on the use of PDT for the treatment of AKs with a focus on photosensitizers and light sources and recommends this treatment (level A recommendation with level 1 quality of evidence).13 UpToDate also provides detailed recommendations for the treatment of AKs.29
Efficacy of PDT for the treatment of AKs. Photodynamic therapy with ALA was FDA-approved for lesion-directed AK therapy in 1999.18,30 It has since been used extensively for both lesion- and field-directed therapy of AKs with consistent efficacy demonstrated in controlled clinical trials.9,11,31–36 In a meta-analysis that included 641 participants with a total of 2,174 AKs treated with cryotherapy and 2,170 AKs treated with PDT, participants achieved 14 percent greater complete lesion clearance at three months following treatment with PDT. Short-term complete clearance rates for PDT ranged from 69 to 91 percent versus 63 to 88 percent for cryotherapy in the six studies included in this analysis.37 Results from controlled clinical trials also support the efficacy of PDT as a field-directed therapy for patients with AKs.9,38–40 Results from comparative clinical studies have also shown PDT to be at least as effective as other approaches to field therapy, including imiquimod,41–43 chemical skin peels,44 diclofenac,41 and 5-FU.45 However, a large clinical trial in Europe assessed the effectiveness of 5-FU (4–8 weeks), imiquimod (4–8 weeks), ingenol mebutate (3–6 days), and MAL-PDT (1–2 sessions), finding that the probability of remaining free from treatment failure (greater than or equal to 75% clearance) was significantly higher in patients who had received therapy with 5-FU compared to the other treatments at 12 months post-treatment.46 PDT has also demonstrated rejuvenating benefits on actinic degeneration and other aging effects of sun damage.5,9,47–49
At present, ALA 10% gel is the only photosensitizer approved for both lesion- and field-directed therapy in the US.17 Results from multiple studies have demonstrated the efficacy of ALA 10% gel for both lesion and field use.9,11,50–56 A meta-analysis included different AK treatment modalities (ALA-PDT, MAL-PDT, imiquimod, cryotherapy, diclofenac, 5-FU, and ingenol mebutate) and analyzed a total of 25 randomized controlled trials (5,562 patients) with the primary endpoint parameter “complete patient clearance.”57 PDT with ALA 10% gel was found to be the most effective treatment in both naïve and network meta-analyses.57
Another meta-analysis compared PDT with ALA 10% gel to MAL for lesion-directed therapy. The meta-analysis included 5,988 AK lesions from five randomized controlled trials (with 2,953 patients treated with ALA 10% gel and 3,035 patients treated with MAL).58 ALA gel 10% achieved significantly higher overall complete clearance rates (P=0.01) and three-month complete clearance rates (P<0.00001) compared to MAL. The pooled relative risk for recurrence at 12 months for ALA 10% gel was 0.67 (P=0.01).58
PDT has been combined effectively with other topical agents for field-directed therapy of AKs.59–62 Meta-analyses that included results from 10 randomized controlled trials in which PDT was combined with imiquimod, 5-FU, ingenol mebutate, tazarotene, or calcipotriol indicated that the combination of PDT with another topical drug improved AK clearance rates compared to PDT or topical monotherapy.63 PDT has also been employed for the prevention of AKs and NMSC in organ-transplant recipients. In a small pilot study, 12 high-risk patients were treated with cyclic ALA-PDT at 4- to 8-week intervals for two years. Median reductions in the development of SCCs at 12 and 24 months were 79 percent and 95 percent, respectively.64 Repeated PDT treatment has also been used as primary prevention for skin dysplasia in renal transplant recipients. In a randomized split-side study of 25 patients with clinically normal skin who received MAL-PDT at six-month intervals for five years, a 63-percent decrease in new AKs was observed in treated skin versus 28 percent in untreated skin at three years of follow-up.65 More recently, consecutive treatments of daylight PDT showed the potential for preventing new AK and keratinocyte carcinoma in transplant patients. Field cancerization-treated areas showed significantly fewer new lesions with a higher patient preference compared to the cryotherapy control.66 A recently published systematic review and meta-analysis of 12 studies in transplant patients favored the use of PDT as a preventive measure for both AK and SCC, with PDT demonstrating a lower incidence of new lesions.67
Practical guidance for the use of ALA-PDT in the treatment of AKs. A summary of the following guidance for the use of ALA 10% gel with red light in the treatment of AKs is provided in Table 1. Pain management is summarized in Table 2.
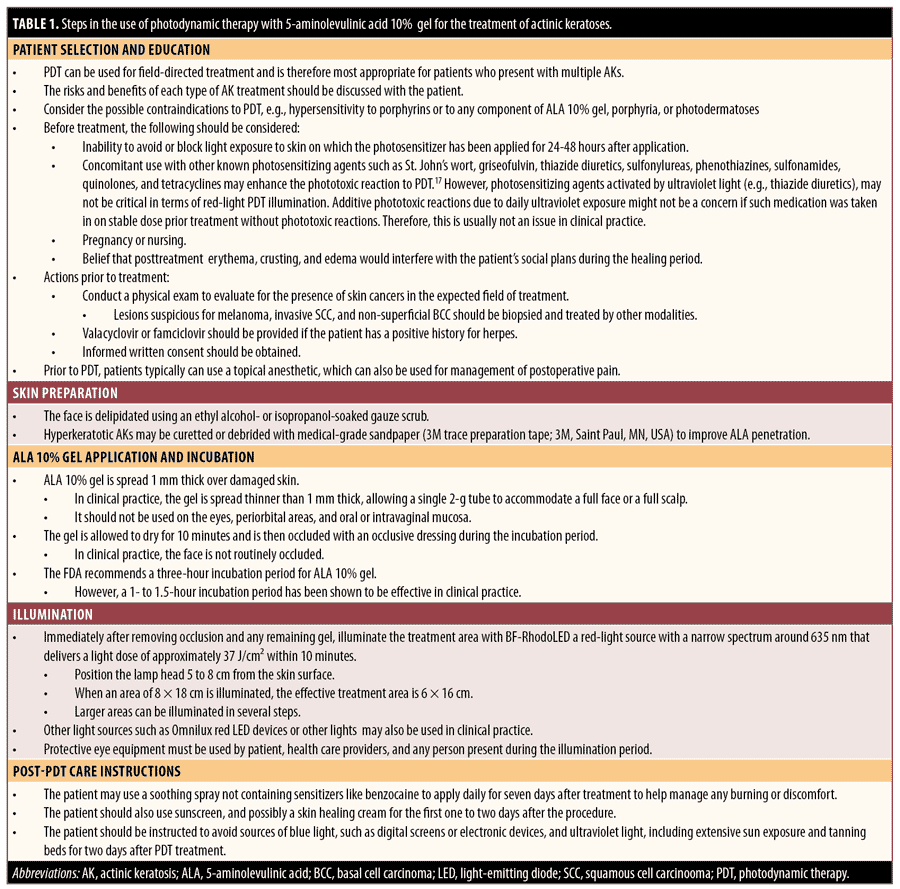
Step 1: Patient selection and education. Most often, PDT treats a field of skin and is therefore most appropriate for patients who present with multiple AKs. The risks and benefits of each type of AK treatment should be discussed with the patient, and the choice of treatment made on a case-by-case basis. Generally, the advantages of PDT include negligible long-term side effects, reproducible outpatient efficacy, noninvasive procedures, irrelevant patient adherence, insurance coverage by Medicare and other payers, and treatment of subclinical lesions. Potential risks include increased sensitivity of the skin to light for 24 to 48 hours after treatment and possible side effects in the PDT treatment area for approximately two weeks, including short-term swelling of the skin, scaliness, crusts, blisters, itching, stinging or burning, and (rarely) skin infections.68
Practitioners should also consider possible contraindications of PDT before prescribing treatment, including hypersensitivity to porphyrins or to any component of ALA 10% gel, porphyria, or photodermatoses.17 In addition, practitioners should consider the following before treatment:
- An inability to avoid or block light exposure to skin on which the PDT drug has been applied for approximately 24 to 48 hours following incubation. Post-PDT, the photosensitizer PpIX may be still activated by the visible spectrum of natural sunlight, thereby photosensitizing effects may proceed after illumination until excessive PpIX is eliminated.
- Concomitant use with other known photosensitizing agents. Such agents, including St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulfonamides, quinolones and tetracyclines, may enhance the phototoxic reaction to PDT.17 However, photosensitizing agents activated by ultraviolet (UV) light (e.g., thiazide diuretics) may not be critical in terms of red-light PDT illumination. Additive phototoxic reactions due to daily UV-exposure might not be a concern if such medication was taken at a stable dose and as prior treatment without phototoxic reactions. Therefore, this is usually not an issue in clinical practice.
- During pregnancy or nursing
- Concerns about temporary post-treatment erythema, crusting, and edema interfering with social plans during the healing period
Before starting PDT, a physical exam should be performed to evaluate the presence of skin cancers in the expected field of treatment. In addition to AKs of Grades 1 (mild) or 2 (moderate), Grade 3 (hyperkeratotic) AKs, biopsy-proven SCC in situ (SCCIS), or superficial BCC (sBCC) can also be treated with PDT. Lesions suspicious for melanoma, invasive SCC, or nonsuperficial BCC should be biopsied and treated using other modalities. The presence or absence of a history of herpes simplex should be confirmed, and valacyclovir or famciclovir should be provided in advance of PDT if the patient has a positive history for herpes.
Step 2. Education. Before patients are treated with PDT, they should be thoroughly educated about the procedure and provided with instructions or an educational handout. The concept of subclinical lesions should also be explained, emphasizing that if the patient can see four lesions, there may be 20 more that they cannot see.69 The diagnosis for treatment, the area to be treated, and correct preparation should all be confirmed. The area to be treated can be photodocumented, and written informed consent should be obtained. Patients should be informed that they will be photoreactive for 24 to 48 hours and that they should avoid any blue light and sunlight. A list of possible sources of blue light and UV light should be provided as part of preoperative/postoperative instructions. Patients should also be advised not to schedule any social events and to avoid excessive exposure to the sun for 1 to 2 days after their procedure. It may also be useful to have the patient initiate an antihistamine regimen (e.g., cetirizine or another nonsedating agent) two days prior to treatment ALA-PDT has been shown to promote the release of histamine.70 Pretreatment with retinoids or topical 5-FU may be used days before the treatment day.60,71,72
Step 3. Skin preparation. Before applying the ALA 10% gel, the face should be degreased using an ethanol- or isopropanol-soaked gauze scrub. Hyperkeratotic AKs may be curetted or debrided (e.g., with medical-grade sandpaper) to improve ALA penetration. Care should be taken to avoid causing bleeding while preparing the skin.5 Fractionated lasers, microneedling, and microdermabrasion could be used during skin preparation to increase the absorption of the photosensitizer.73–75
Step 4. ALA 10% gel application and incubation. ALA 10% gel can be dispensed from the tube directly to the skin, indirectly with a spatula, or indirectly with a gloved hand. It should not be used on the skin of the eyelids, periorbital areas, and oral or intravaginal mucosa. Per the US prescribing information, the gel is applied 1mm thick, allowed to dry for 10 minutes, and then occluded with a light-blocking dressing during the incubation period.17 In clinical practice in the US, ALA 10% gel is generally spread thinner than 1mm, allowing a single 2g tube to accommodate a full face or a full scalp. It should be noted that the individual Healthcare Common Procedure Coding System for ALA 10% gel is based on 10mg (which equals 1 unit). One tube of ALA 10% gel contains 2,000mg, so when using an entire tube, 200 units have to be reported in the claim form.76
The FDA approved a three-hour incubation period for ALA 10% gel. However, a 1- to 1.5-hour incubation has been shown to be effective in clinical practice but represents an off-label use of the product. A rigid comparison study of the effect of incubation time with ALA 10% gel is needed. In clinical practice in the US, the face is not routinely occluded, and occlusion of the scalp, chest, arms, legs, hands, and back is at the physician’s discretion. Longer incubation times for 2 to 3 hours and/or an occlusive dressing may be employed for the extremities or for harder-to-treat areas.18 Occlusion during incubation may be beneficial due to the creation of a moist chamber effect and possible increase in local temperature.77 Warming past physiological temperature increases apoptosis, which might decrease incubation time by up to one-third. Porphyrin synthesis is profoundly temperature-sensitive, and research has shown that, with ALA-PDT, increasing the temperature of healthy skin by approximately 10°C during ALA-PDT for AKs increases porphyrin production and improves outcomes for patients being treated for AKs.78,79
Step 5. Illumination. Immediately after removing the occlusive dressing and any remaining gel, the treatment area should be illuminated with a light source that activates ALA 10% gel. The FDA-approved light source is BF-RhodoLED, a red-light source with a narrowband wavelength spectrum around 635 nm that delivers a light dose of approximately 37J/cm2 within 10 minutes. Calibration by the operator is not needed; the illumination time is calculated automatically. First, the lamp head should be positioned 5 to 8cm from the skin surface. When an area of 8×18cm is illuminated, the effective treatment area is 6×16cm. Larger areas can be illuminated in several steps. In clinical practice, the lamp head is pulled back 4 to 6 inches so that half a face can be treated in one illumination and the other half can be addressed in a second illumination. When treating the scalp, pulling the lamp back may allow for a single illumination versus two. However, by pulling the lamp back, the light energy dose will be reduced. To avoid eye irritation, glare, or injury, protective eye equipment must be used by the patient, healthcare providers, and any person present during the illumination period. Do not stare into the light source. The operator and other persons present must wear specific wavelength–protective glasses with a visible light transmission of approximately 10 percent. The patient must wear specific-wavelength eye protection, such as disposable eye protection pads or eye caps, with an optical density for visible light of 6 or higher. Both options are effective and comfortable for use during treatment.
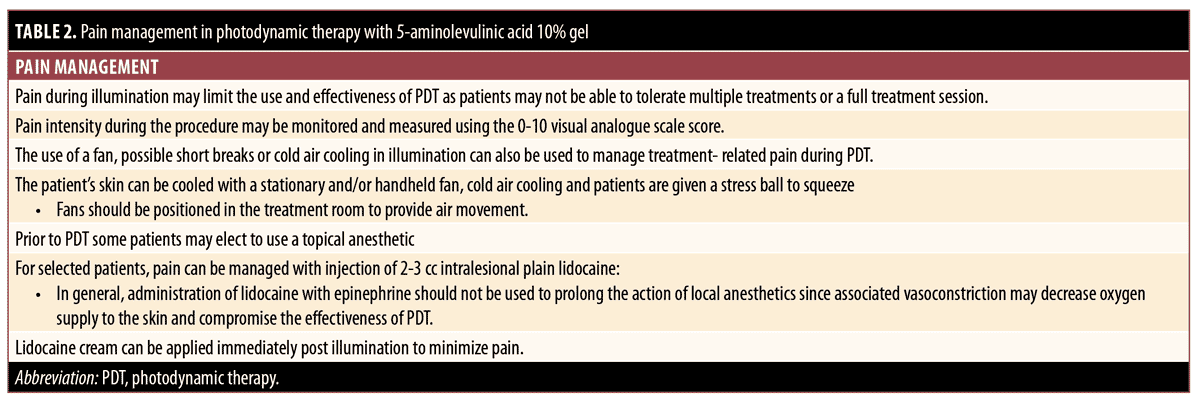
For pain management, the patient’s skin can be cooled with a stationary and/or handheld fan and cool water in the form of a mist or intermittent compress or with cold air cooling; patients can be given a stress ball to squeeze. Fans should be positioned in the treatment room to provide air movement. Illumination may be paused if the patient is too uncomfortable. Prior to PDT, patients typically can use a topical anesthetic, which can also be used for the management of postoperative pain.
As per the Ameluz prescribing information, lesions that have not completely resolved 12 weeks after the initial treatment should be retreated. As noted above, patients who have received an organ transplant may benefit from cyclic treatment at a shorter interval.64,66,67,80,81
Step 6. Post-PDT care instructions. A postcare sheet should be given to patients to read during the incubation period. The patient should be instructed to avoid blue light sources, such as digital screens and electronic devices, and UV light sources, such as extensive sun exposure and tanning beds, for two days after PDT treatment. Suggested components of postoperative care are varied. One suggestion includes a soothing spray to apply daily for seven days after treatment to help manage any burning or discomfort.69 Thermal spring water, which is a low-mineral-content spring water, can also be useful after ALA-PDT and is effective for reducing postprocedure cutaneous inflammation and patient discomfort.82 Each provider may recommend their choice of soothing creams and/or sunscreens. Adverse events that have occurred in 10 percent of patients with AKs treated with ALA 10% gel include transient application site erythema, pain/burning, irritation, edema, pruritus, exfoliation, scab, induration, and vesicles.17
Non-melanoma Skin Cancer
PDT is an established treatment for superficial and thin nodular BCC but remains an off-label therapy in the US. In general, PDT should not be used on thick BCC lesions or more aggressive basosquamous, morpheaform, or infiltrating subtypes. PDT has demonsetrated efficacy in the treatment of Bowen’s disease/SCCIS and is recommended for managing large lesions in cosmetically sensitive areas and sites with the potential for poor healing.13 Treatment of both BCC and SSCIS with PDT is approved in the European Union and frequently used off-label in the US.
Exisiting guidelines. A summary of international guidelines supports the following consensus recommendations regarding the use of PDT in NMSC:13 ALA-PDT is effective for the treatment of selected BCC, offering an advantage in the treatment of large or multiple superficial BCC lesions or thin nodular BCC. Good cosmetic results have been noted with PDT for BCC. There are only limited data regarding the efficacy of PDT for primary cutaneous invasive SCC, but guidelines typically advise against its use. PDT is also an effective therapy for Bowen’s disease/SCCIS.83 PDT is considered to have the efficacy equivalent to that of cryotherapy and equivalent or superior efficacy to that of topical 5-FU. The cosmetic outcome is considered superior to that seen with standard therapy. PDT may be considered for treatment of large NMSC lesion areas and lesions located on cosmetically sensitive areas, at poor healing sites, or for which surgery is considered inappropriate.13 PDT is also a treatment option for patients with multiple lesions, such as organ-transplant recipients,67 or for patients with comorbidities that may prevent ideal healing of large closures and skin grafts.
Evidence supporting PDT for the treatment of NMSC. PDT has been shown to be generally more effective for superficial BCC than for nodular BCC or smaller lesions measuring less than 2cm.84 In a recent randomized, Phase III trial that included 281 patients, 138 were treated with ALA 10% gel and 143 were treated with MAL and red-light PDT. Patients received two PDT sessions one week apart, and any remaining lesions after 12 weeks were retreated with a third PDT session. In the ALA 10% gel group, 93.4 percent were complete responders compared to 91.8 percent in the MAL group. Overall, treatment of nonaggressive BCC with ALA 10% gel was shown to be highly effective and well tolerated, with low recurrence rates at one year of follow, and it was found to be noninferior to MAL-PDT.85 In the longest follow-up of any study to date, which spanned 10 years, the overall complete response rate was 75 percent for all subtypes of BCC treated with ALA-PDT, with a 60-percent complete response after one treatment and 87-percent response after two treatments.86 Other studies have shown ALA gel 10% PDT to be a safe and noninvasive treatment option for both SCCIS and for sBCC and that it provides better results than MAL-PDT.87–89 Another recent study in which patients were treated with ALA 20% solution for PDT of SCCIS reported that longer incubation times with ALA, smaller tumor diameters, and location on the face were associated with increased efficacy.90
Practical guide for the use of ALA-PDT in the treatment of NMSC. A summary of the following guidance for the use of ALA 10% gel PDT in the treatment of NMSC is provided in Table 3.
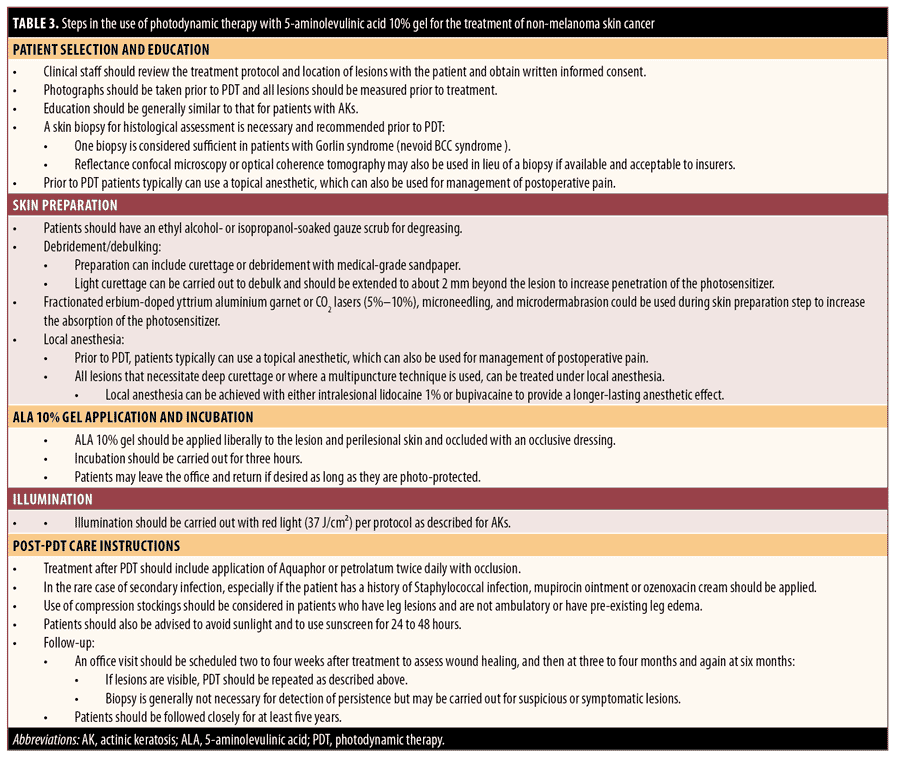
Step 1. Patient selection and patient education. Clinical staff should review the treatment protocol and location of lesions with the patient and obtain written informed consent. Photographs can be taken prior to PDT, and all lesions should be measured prior to treatment. Education is similar to patients with AKs.
A skin biopsy for histological assessment is necessary and recommended prior to PDT. One biopsy is considered sufficient in patients with Gorlin syndrome (nevoid BCC syndrome).
Step 2. Skin preparation. Patients should have an ethanol- or isopropanol-soaked gauze scrub for degreasing. Preparation can include curettage or debridement with medical-grade sandpaper tape. Light curettage or microdermabrasion may be carried out for debulking so that the surface of the tumor is equivalent to the surface of healthy skin to increase penetration of the photosensitizer. Fractional erbium-doped yttrium aluminium garnet laser, CO2 lasers (5%–10%), or microneedling may be employed as an alternative to curettage on areas such as the dorsal foot or leg, which may heal slowly after curettage.
All lesions that necessitate deep curettage or where multiple puncture techniques are used can be treated under local anesthesia. Local anesthesia can be achieved with either intralesional lidocaine 1% or bupivacaine to provide a longer-lasting anesthetic effect.
Step 3. ALA 10% gel application and incubation. ALA 10% gel should be applied liberally (~1 mm thickness) to the lesion and perilesional skin and covered with an occlusive dressing. Incubation should be carried out for three hours for NMSCs. Patients may leave the office and return, if desired, so long as they are photoprotected.
Step 4. Illumination. Illumination should be carried out with red light (37 J/cm2) per protocol as described above. Red and blue light in combination with a pulsed-dye laser and intense pulsed laser (IPL) also have been shown to be effective energy sources for treating NMSC with PDT.91 Eye protection should be used as described above for AKs.
For pain management, a topical anesthetic can typically be applied before and after to PDT. For selected patients, pain can be managed with injections of 2 to 3cc of intralesional plain lidocaine. The administration of lidocaine with epinephrine should not be used to prolong the action of local anesthetics during illumination since associated vasoconstriction may decrease oxygen supply to the skin and compromise the effectiveness of PDT.92 The use of a fan, cold air cooling, and short breaks in illumination can also be used to manage treatment-related pain during PDT. Pain during illumination may limit the use and effectiveness of PDT as patients may not be able to tolerate multiple treatments or a full treatment session. Pain intensity during the procedure may be monitored and measured using a 10-point visual analog scale score.
Regarding frequency of treatment, in clinical studies, treatment with ALA-PDT for NMSC has been administered as a single session or two treatments spaced 7 to 30 days apart. Retreatment intervals ranged from six weeks to three months.93
Step 5. Post-PDT care instructions. Patients should also be advised to avoid sunlight and to use sunscreen for 24 to 48 hours. Treatment after PDT should include the application of petrolatum ointment twice daily with occlusion. In the rare case of secondary infection, especially if the patient has a history of Staphylococcal infection, mupirocin ointment or ozenoxacin cream should be applied. Use of compression stockings should be considered in patients who have leg lesions and are not ambulatory or who have pre-existing leg edema.
Follow-up can be carried out by phone in the immediate post-treatment period, and an office visit should be scheduled two to four weeks after treatment to assess wound healing, then at three to four months and again at six months. If lesions are visible, PDT should be repeated as described above. Biopsy is generally not necessary for the detection of persistence but may be carried out for suspicious or symptomatic lesions. Patients should be followed closely for at least five years.
Acne
PDT is a useful treatment alternative for patients with mild, moderate, and severe acne who have not responded to standard topical and oral medications and who may not be candidates for isotretinoin.94 PDT has been widely studied for the management of inflammatory acne and has also been shown to improve comedonal acne.94 This modality can be used in conjunction with long-term topical acne therapies (interrupted during PDT) and may be an optimal alternative for patients who cannot tolerate systemic therapies.94
PDT has several actions that may contribute to its efficacy in the treatment of acne. ALA-PDT directly targets multiple pathophysiologic factors in acne, including destruction of sebaceous gland lobules, sebocyte death, and downregulation of Toll-like receptors (TLR2, TLR4) that trigger inflammation, as well as the inhibition of sebaceous gland function and increased keratinocyte shedding in the pilosebaceous unit involved in comedogenesis.95–97 PDT may also have antimicrobial effects, but a reduction in Cutibacterium acnes has not been consistently observed with this treatment.98–102
Exisiting guidelines. PDT is not currently approved by the FDA for the treatment of acne. Nevertheless, the most recently published American Academy of Dermatology acne guidelines notes that this treatment approach shows great promise and suggests that additional studies are needed to determine the optimal photosensitizer, incubation time, and light source.103 European evidence–based (S3) guidelines for the treatment of acne state that, due to a lack of sufficient evidence, it is currently not possible to make a recommendation for or against maintenance treatment with red light, blue light, IPL, or PDT.104 It should also be noted that generally accepted guidelines for PDT treatment of pediatric patients with acne are lacking.
Evidence supporting ALA-PDT for the treatment of acne. Light therapy and lasers have been used together with various photosensitizers for the treatment of acne.47,94,105–107 The combination of ALA and red- or blue-light irradiation, with either lasers or other light sources, has been repeatedly demonstrated to be effective for acne treatment.47,95,98,108–112 Red light may be more beneficial over blue light given the increased depth of penetration with red light and the need to reach the pilosebaceous unit to clear acne lesions.6 Studies have also demonstrated that PDT using a photosensitizer is superior to light therapy alone for the treatment of acne.113,114
There is also evidence that PDT with ALA and red-light irradiation is significantly superior to oral doxycycline (100mg/day) plus adapalene gel 0.1% for decreasing inflammatory and total lesions in patients with moderate inflammatory facial acne.115 The addition of PDT to minocycline treatment has been shown to significantly reduce the number of inflammatory and noninflammatory lesions, increase the percentage of patients achieving an Investigator Global Assessment score of less than two points (mild), and improve patient quality of life as assessed by the Dermatology Life Quality Index versus minocycline alone.116 It should also be noted that ALA can simply be combined with exposure to daylight for the treatment of acne.117 At present, there are no published results describing the efficacy and safety of ALA 10% gel for the treatment of acne.
Practical guidance for the use of ALA-PDT in the treatment of acne. Some clinicians use ALA-PDT as first-line therapy in the treatment of moderate-to-severe inflammatory acne vulgaris, while others adopt this therapy if routine medical care has failed or is inadequate to achieve acne clearance.118,119 It is also a useful alternative for patients who cannot tolerate commonly used topical or systemic acne treatments,120 those who do not want to take antibiotics or isotretinoin, and women wanting to become pregnant. Numerous investigations have shown that blue-light sources, potassium titanyl phosphate lasers, pulsed-dye lasers, IPL sources, and red light at 630nm can all be used to activate ALA.121 A summary of the following guide for the use of PDT with ALA 10% gel in the treatment of acne is provided in Table 4.
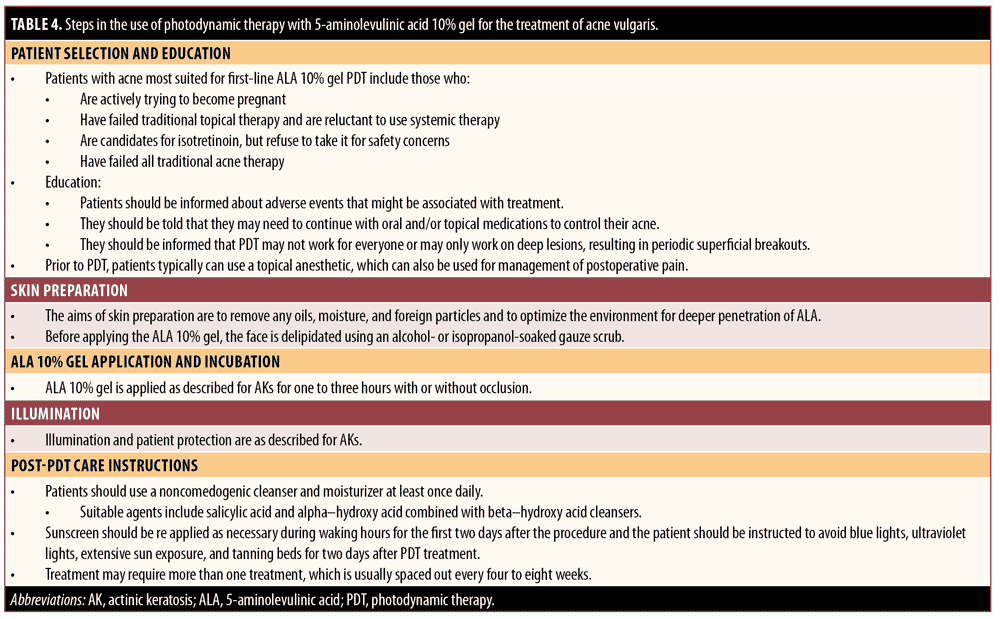
Step 1. Patient selection and education. Patients with acne most suited for first-line ALA 10% gel PDT include those actively trying to become pregnant, patients who have failed traditional topical therapy and are reluctant to use systemic therapy, patients who are candidates for isotretinoin but who refuse to take it for safety concerns, and patients who have failed traditional acne therapies.
Patients should be informed about potential adverse events and that they may have to continue with oral and/or topical medications to control their acne. In addition, they should be informed that PDT may not work for everyone or may only work on deep lesions, resulting in periodic superficial breakouts.
Step 2. Skin preparation. The aim of skin preparation is to remove any oils, moisture, and foreign particles from the skin surface, optimizing the environment for deeper penetration of ALA.96 Before applying the ALA 10% gel, the face should be degreased using an ethanol- or isopropanol-soaked gauze scrub.
Step 3. ALA 10% gel application incubation. ALA 10% gel is applied as described for AKs for 1 to 3 hours with or without occlusion.
Step 4. Illumination. Illumination and patient protection are performed as described for AKs.
Regarding pain management, younger patients are likely to have less UV-associated skin damage than older individuals, making pain less of an issue.122 The use of a fan, cold air cooling, and possible short breaks in illumination can also be used to manage treatment-related pain during PDT. Some patients may not be able to tolerate multiple treatments or a full treatment session due to pain during illumination, which can limit the use and effectiveness of PDT. Pain intensity during the procedure can be monitored and measured using a10-point visual analog scale score. Topical anesthetic typically can be applied before and after PDT. Results from one recent small-scale study that used a 5% ALA cream in acne patients showed that shortening the incubation time from 90 to 30 minutes did not adversely affect efficacy and resulted in “nearly painless” treatment.120
Regarding frequency of treatment, some patients achieve significant efficacy with a single treatment, while others require additional sessions. There is no specific guidance regarding the optimal interval between treatments, but intervals ranging from weekly to once every 4 to 8 weeks have been employed in clinical trials.6
Step 5. Post-PDT care instructions. Patients should use a noncomedogenic cleanser and moisturizer at least once daily. Suitable agents include salicylic acid and alpha-hydroxy acid combined with beta-hydroxy acid cleansers. Sunscreen should be used and reapplied as necessary during waking hours for the first two days after the procedure, and the patient should be instructed to avoid souces of blue light, such as digital screens and electronic devices, or UV light sources, including extensive sun exposure and tanning beds, for two days post-treatment. Adverse events associated with ALA-PDT for acne may include moderate-to-severe pain, acneiform eruptions (starting about 2 days after treatment and lasting about 3 days), erythema, edema, crusting, transient acne flares, hyperpigmentation, exfoliation, and rare blistering or contact hypersensitivity.95
Conclusion
PDT is an effective modality for the treatment of AK, NMSC, and acne; however, while PDT has received FDA-approval for the treatment of AK, it remains off-label for NMSC and acne. PDT can be delivered with any of several different photosensitizers, light sources, and in combination with a wide range of other topical treatments. While the use of ALA-PDT for the AK, NMSC, and acne is well-supported by controlled clinical trials, there is a paucity of detailed guidance on how to employ this therapy in clinical practice. This article focused on providing such guidance for ALA 10% gel PDT monotherapy. We hope that our advice will encourage dermatologists to consider employing this therapeutic alternative in appropriately selected patients.
Acknowledgments
The authors wish to acknowledge AraMed Strategies for medical writing support in preparing this manuscript.
References
- Kostovic K, Pastar Z, Ceovic R, et al. Photodynamic therapy in dermatology: current treatments and implications. Coll Antropol. 2012;36(4):1477–1481.
- Kim M, Jung HY, Park HJ. Topical PDT in the treatment of benign skin diseases: principles and new applications. Int J Mol Sci. 2015;16(10):23259–23278.
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804–827.
- Wen X, Li Y, Hamblin MR. Photodynamic therapy in dermatology beyond non-melanoma cancer: an update. Photodiagnosis Photodyn Ther. 2017;19:140–152.
- Moy LS, Frost D, Moy S. Photodynamic therapy for photodamage, actinic keratosis, and acne in the cosmetic practice. Facial Plast Surg Clin North Am. 2020;28(1):135–148.
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145–163.
- Sparsa A, Bellaton S, Naves T, et al. Photodynamic treatment induces cell death by apoptosis or autophagy depending on the melanin content in two B16 melanoma cell lines. Oncol Rep. 2013;29(3):1196–1200.
- Lee PK, Kloser A. Current methods for photodynamic therapy in the US: comparison of MAL/PDT and ALA/PDT. J Drugs Dermatol. 2013;12(8):925–930.
- Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz (®)) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED (®) lamp. Br J Dermatol. 2016;175(4):696–705.
- Philipp-Dormston WG. Photodynamic therapy for aesthetic-cosmetic indications. G Ital Dermatol Venereol. 2018;153(6):817–826.
- Dirschka T, Ekanayake-Bohlig S, Dominicus R, et al. A randomized, intraindividual, non-inferiority, phase III study comparing daylight photodynamic therapy with BF-200 ALA gel and MAL cream for the treatment of actinic keratosis. J Eur Acad Dermatol Venereol. 2019;33(2):288–297.
- Buggiani G, Troiano M, Rossi R, Lotti T. Photodynamic therapy: off-label and alternative use in dermatological practice. Photodiagnosis Photodyn Ther. 2008;5(2):
134–138. - Morton CA, Szeimies R-M, Basset-Seguin N, et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: treatment delivery and established indications—actinic keratoses, Bowen’s disease and basal cell carcinomas. J Eur Acad Dermatol Venereol. 2019;33(12):2225–2238.
- Reinhold U. A review of BF-200 ALA for the photodynamic treatment of mild-to-moderate actinic keratosis. Future Oncol. 2017;13(27):2413–2428.
- Maisch T, Santarelli F, Schreml S, et al. Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Exp Dermatol. 2010;19(8):e302–e305.
- Ameluz®. Summary of Product Characteristics: Ameluz 78 mg/g gel. Biofrontera Pharma GmbH. Updated February 2021.
- Ameluz®. Full Prescribing Information Ameluz® (aminolevulinic acid hydrochloride) gel, 10%: Ameluz. Biofrontera Pharma GmbH. Updated August 2020.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59(6):677–684.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968.
- Cornejo CM, Jambusaria-Pahlajani A, Willenbrink TJ, et al. Field cancerization: treatment. J Am Acad Dermatol. 2020;83(3):719–730.
- Jetter N, Chandan N, Wang S, Tsoukas M. Field cancerization therapies for management of actinic keratosis: a narrative review. Am J Clin Dermatol. 2018;19(4):543–557.
- Stockfleth E. The importance of treating the field in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31 Suppl 2:8–11.
- Huang A, Nguyen JK, Austin E, et al. Updates on treatment approaches for cutaneous field cancerization. Curr Dermatol Rep. 2019;8(3):122–132.
- Fernandez-Figueras MT, Carrato C, Saenz X, et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29(5):
991–997. - Schmitz L, Gambichler T, Gupta G, et al. Actinic keratoses show variable histological basal growth patterns—a proposed classification adjustment. J Eur Acad Dermatol Venereol. 2018;32(5):745–751.
- American Accademy of Dermatology. Clinical guidelines. Available at: https://www.aad.org/member/clinical-quality/guidelines. Accessed March 23, 2021.
- Werner RN, Stockfleth E, Connolly SM, et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—short version. J Eur Acad Dermatol Venereol. 2015;29(11):2069–2079.
- Berker D de, McGregor JM, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):
20–43. - Berman B. Treatment of actinic keratosis. Available at: https://www.uptodate.com/contents/treatment-of-actinic-keratosis. Accessed February 25, 2021.
- Stritt A, Merk HF, Braathen LR, von, Felbert, V. Photodynamic therapy in the treatment of actinic keratosis. Photochem Photobiol. 2008;84(2):388–398.
- Smith S, Piacquadio D, Morhenn V, et al. Short incubation PDT versus 5-FU in treating actinic keratoses. J Drugs Dermatol. 2003;2(6):
629–635. - Alexiades-Armenakas MR, Geronemus RG. Laser-mediated photodynamic therapy of actinic cheilitis. J Drugs Dermatol. 2004;3(5):548–551.
- Gholam P, Bosselmann I, Enk A, Fink C. Impact of red versus blue light on tolerability and efficacy of PDT: a randomized controlled trial. J Dtsch Dermatol Ges. 2018;16(6):711–717.
- Zaar O, Sjöholm Hylén A, Gillstedt M, Paoli J. A prospective, randomized, within-subject study of ALA-PDT for actinic keratoses using different irradiation regimes. Photodermatol Photoimmunol Photomed. 2018;34(5):
338–342. - Gutiérrez G-RC, Pellegrini C, Piccioni A, et al. Single versus two-treatment schedule of methyl aminolevulinate daylight photodynamic therapy for actinic keratosis of the face and scalp: an intra-patient randomized trial. Photodiagnosis Photodyn Ther. 2019;27:100–104.
- Brian Jiang SI, Kempers S, Rich P, et al. A randomized, vehicle-controlled phase 3 study of aminolevulinic acid photodynamic therapy for the treatment of actinic keratoses on the upper extremities. Dermatol Surg. 2019;45(7):890–897.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150(12):1281–1288.
- Wennberg AM, Stenquist B, Stockfleth E, et al. Photodynamic therapy with methyl aminolevulinate for prevention of new skin lesions in transplant recipients: a randomized study. Transplantation. 2008;86(3):423–429.
- Apalla Z, Sotiriou E, Chovarda E, et al. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study. Br J Dermatol. 2010;162(1):171–175.
- Wollina U, Gaber B, Koch A. Photodynamic treatment with nanoemulsified 5-aminolevulinic acid and narrow band red light for field cancerization due to occupational exposure to ultraviolet light irradiation. Georgian Med News. 2018;(274):138–143.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170(5):1143–1150.
- Sotiriou E, Apalla Z, Vrani F, et al. Photodynamic therapy vs. imiquimod 5% cream as skin cancer preventive strategies in patients with field changes: a randomized intraindividual comparison study. J Eur Acad Dermatol Venereol. 2015;29(2):325–329.
- Genovese G, Fai D, Fai C, et al. Daylight methyl-aminolevulinate photodynamic therapy versus ingenol mebutate for the treatment of actinic keratoses: an intraindividual comparative analysis. Dermatol Ther. 2016;29(3):191–196.
- Holzer G, Pinkowicz A, Radakovic S, et al. Randomized controlled trial comparing 35% trichloroacetic acid peel and 5-aminolaevulinic acid photodynamic therapy for treating multiple actinic keratosis. Br J Dermatol. 2017;176(5):1155–1161.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156(2):320–328.
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380(10):935–946.
- Gold MH, Goldman MP. 5-aminolevulinic acid photodynamic therapy: where we have been and where we are going. Dermatol Surg. 2004;30(8):1077–1083.
- Tarstedt M, Rosdahl I, Berne B, et al. A randomized multicenter study to compare two treatment regimens of topical methyl aminolevulinate (Metvix)-PDT in actinic keratosis of the face and scalp. Acta Derm Venereol. 2005;85(5):424–428.
- Morton CA. Can photodynamic therapy reverse the signs of photoageing and field cancerization? Br J Dermatol. 2012;167(1):2.
- Szeimies RM, Radny P, Sebastian M, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br J Dermatol. 2010;163(2):386–394.
- Dirschka T, Radny P, Dominicus R, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol. 2012;166(1):137–146.
- Dirschka T. Follow-up analysis of the efficacy of photodynamic therapy in actinic keratosis: reply from the authors. Br J Dermatol. 2013;169(5):1156–1157.
- Neittaanmäki-Perttu N, Karppinen TT, Gronroos M, et al. Daylight photodynamic therapy for actinic keratoses: a randomized double-blinded non-sponsored prospective study comparing BF-200 aminolevulinic acid with methyl-5-aminolaevulinate. Br J Dermatol. 2014;171(5):1172–1180.
- Serra-Guillén C, Nagore E, Bancalari E, et al. A randomized intraindividual comparative study of methyl-5-aminolaevulinate vs. 5-aminolaevulinic acid nanoemulsion (BF-200 ALA) in photodynamic therapy for actinic keratosis of the face and scalp. Br J Dermatol. 2018;179(6):1410–1411.
- Räsänen JE, Neittaanmäki N, Ylitalo L, et al. 5-aminolaevulinic acid nanoemulsion is more effective than methyl-5-aminolaevulinate in daylight photodynamic therapy for actinic keratosis: a nonsponsored randomized double-blind multicentre trial. Br J Dermatol. 2019;181(2):265–274.
- Nestor MS, Berman B, Patel J, Lawson A. Safety and efficacy of aminolevulinic acid 10% topical gel versus aminolevulinic acid 20% topical solution followed by blue-light photodynamic therapy for the treatment of actinic keratosis on the face and scalp: a randomized, double-blind study. J Clin Aesthet Dermatol. 2019;12(3):32–38.
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PloS One. 2014;9(6):e96829.
- Fu C, Kuang BH, Qin L, et al. Efficacy and safety of photodynamic therapy with amino-5-laevulinate nanoemulsion versus methyl-5-aminolaevulinate for actinic keratosis: a meta-analysis. Photodiagnosis Photodyn Ther. 2019;27:408–414.
- Pei S, Kaminska ECN, Tsoukas MM. Treatment of actinic keratoses: a randomized split-site approach comparison of sequential 5-fluorouracil and 5-aminolevulinic acid photodynamic therapy to 5-aminolevulinic acid photodynamic monotherapy. Dermatol Surg. 2017;43(9):1170–1175.
- Maytin EV, Anand S, Riha M, et al. 5-Fluorouracil enhances protoporphyrin IX accumulation and lesion clearance during photodynamic therapy of actinic keratoses: a mechanism-based clinical trial. Clin Cancer Res. 2018;24(13):3026–3035.
- Torezan L, Grinblat B, Haedersdal M, et al. A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br J Dermatol. 2018;179(4):829–835.
- Seo JW, Song KH. Topical calcipotriol before ablative fractional laser-assisted photodynamic therapy enhances treatment outcomes for actinic keratosis in Fitzpatrick grades III-V skin: a prospective randomized clinical trial. J Am Acad Dermatol. 2018;78(4):795–797.
- Heppt MV, Steeb T, Leiter U, Berking C. Efficacy of photodynamic therapy combined with topical interventions for the treatment of actinic keratosis: a meta-analysis. J Eur Acad Dermatol Venereol. 2019;33(5):863–873.
- Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg. 2010;36(5):652–658.
- Togsverd-Bo K, Omland SH, Wulf HC, et al. Primary prevention of skin dysplasia in renal transplant recipients with photodynamic therapy: a randomized controlled trial. Am J Transplant. 2015;15(11):2986–2990.
- Bernad I, Aguado L, Núñez-Córdoba JM, Redondo P. Daylight photodynamic therapy for prevention of new actinic keratosis and keratinocyte carcinomas in organ transplants. A cryotherapy-controlled randomized clinical trial. J Eur Acad Dermatol Venereol. 2020;34(7):1464–1470.
- Liew YCC, De Souza NNA, Sultana RG, Oh CC. Photodynamic therapy for the prevention and treatment of actinic keratosis/squamous cell carcinoma in solid organ transplant recipients: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34(2):251–259.
- Cleveland Clinic. Photodynamic therapy (PDT): risks/venefits. Available at: https://my.clevelandclinic.org/health/treatments/17922-photodynamic-therapy-pdt/risks–benefits. Accessed March 23, 2021.
- Berman B, Bhatia N, Cohen JL, et al. A practical approach: field treatment of AKs with PDT. Available at: https://practicaldermatology.com/articles/2018-sept-insert/a-practical-approach-field-treatment-of-aks-with-pdt. Accessed March 23, 2021.
- Brooke RC, Sinha A, Sidhu MK, et al. Histamine is released following aminolevulinic acid-photodynamic therapy of human skin and mediates an aminolevulinic acid dose-related immediate inflammatory response. J Invest Dermatol. 2006;126(10):2296–2301.
- Galitzer BI. Effect of retinoid pretreatment on outcomes of patients treated by photodynamic therapy for actinic keratosis of the hand and forearm. J Drugs Dermatol. 2011;10(10):1124–1132.
- Galitzer BI. Photodynamic therapy for actinic keratoses of the upper extremities using 10% aminolevulinic acid gel, red light, and adapalene pretreatment. J Clin Aesthet Dermatol. 2021;14(10):19–24.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25(4):417–422.
- Petukhova TA, Hassoun LA, Foolad N, et al. Effect of expedited microneedle-assisted photodynamic therapy for field treatment of actinic keratoses: a randomized clinical trial. JAMA Dermatol. 2017;153(7):637–643.
- Wenande E, Phothong W, Bay C, et al. Efficacy and safety of daylight photodynamic therapy after tailored pretreatment with ablative fractional laser or microdermabrasion: a randomized, side-by-side, single-blind trial in patients with actinic keratosis and large-area field cancerization. Br J Dermatol. 2019;180(4):756–764.
- Biofrontera. Ameluz® (aminolevulinic acid hydrochloride ) gel, 10%. Available at: http://www.biofrontera.us.com/reimbursement/. Accessed March 23, 2021.
- Meierhofer C, Silic K, Urban MV, et al. The impact of occlusive vs non-occlusive application of 5-aminolevulinic acid (BF-200 ALA) on the efficacy and tolerability of photodynamic therapy for actinic keratosis on the scalp and face: A prospective within-patient comparison trial. Photodermatol Photoimmunol Photomed. 2021;37(1):56–62.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40(10):1094–1102.
- Willey A. Thermal photodynamic therapy for actinic keratoses on facial skin: a proof-of-concept study. Dermatol Surg. 2019;45(3):
404–410. - Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172(2):467–474.
- Togsverd-Bo K, Halldin C, Sandberg C, et al. Photodynamic therapy is more effective than imiquimod for actinic keratosis in organ transplant recipients: a randomized intraindividual controlled trial. Br J Dermatol. 2018;178(4):903–909.
- Goldman MP, Merial-Kieny C, Nocera T, Mery S. Comparative benefit of two thermal spring waters after photodynamic therapy procedure. J Cosmet Dermatol. 2007;6(1):31–35.
- O’Connell KA, Okhovat J-P, Zeitouni NC. Photodynamic therapy for Bowen’s Disease (squamous cell carcinoma in situ) current review and update. Photodiagnosis Photodyn Ther. 2018;24:109–114.
- Cohen DK, Lee PK. Photodynamic therapy for non-melanoma skin cancers. Cancers (Basel). 2016;8(10):90.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179(2):309–319.
- Christensen E, Mork C, Skogvoll E. High and sustained efficacy after two sessions of topical 5-aminolaevulinic acid photodynamic therapy for basal cell carcinoma: a prospective, clinical and histological 10-year follow-up study. Br J Dermatol. 2012;166(6):1342–1348.
- Alique-García S, Company-Quiroga J, Sánchez Campos A, et al. Treatment of superficial basal cell carcinoma with photodynamic therapy. Observational study in 22 patients with 5-aminolaevulinic acid and methyl aminolaevulinate. Photodiagnosis Photodyn Ther. 2019;26:190–192.
- Alique-García S, Alique D, Company-Quiroga J, et al. Treatment of Bowen’s disease with photodynamic therapy. Observational study in 171 patients with 5-aminolaevulinic acid (BF-200 ALA) and methyl aminolaevulinate (MAL). Photodiagnosis Photodyn Ther. 2019;28:192–194.
- Navarro-Triviño FJ, Ayén-Rodríguez Á, Llamas-Molina JM, et al. Treatment of superficial basal cell carcinoma with 7.8% 5-aminolaevulinic acid nanoemulsion-based gel (BF-200 ALA) and photodynamic therapy: results in clinical practice in a tertiary hospital. Dermatol Ther. 2021;34(1):e14558.
- Kibbi N, Zhang Y, Leffell DJ, Christensen SR. Photodynamic therapy for cutaneous squamous cell carcinoma in situ: Impact of anatomic location, tumor diameter, and incubation time on effectiveness. J Am Acad Dermatol. 2020;82(5):1124–1130.
- Friedmann DP, Goldman MP, Fabi SG, Guiha I. The effect of multiple sequential light sources to activate aminolevulinic acid in the treatment of actinic keratoses: a retrospective study. J Clin Aesthet Dermatol. 2014;7(9):20–25.
- Gonzalez S, Vibhagool C, Sherwood M, et al. The phototoxicity of photodynamic therapy may be suppressed or enhanced by modulation of the cutaneous vasculature. J Photochem Photobiol B. 2000;57(2-3):142–148.
- Braathen LR, Szeimies RM, Basset-Seguin N, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. J Am Acad Dermatol. 2007;56(1):125–143.
- Boen M, Brownell J, Patel P, Tsoukas MM. The role of photodynamic therapy in acne: an evidence-based review. Am J Clin Dermatol. 2017;18(3):311–321.
- Hongcharu W, Taylor CR, Chang Y, et al. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J Invest Dermatol. 2000;115(2):183–192.
- Sakamoto FH, Torezan L, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part II. Understanding parameters for acne treatment with photodynamic therapy. J Am Acad Dermatol. 2010;63(2):195–211.
- Jeong E, Hong JW, Min JA, et al. Topical ALA-photodynamic therapy for acne can induce apoptosis of sebocytes and down-regulate their TLR-2 and TLR-4 expression. Ann Dermatol. 2011;23(1):23–32.
- Pollock B, Turner D, Stringer MR, et al. Topical aminolaevulinic acid-photodynamic therapy for the treatment of acne vulgaris: a study of clinical efficacy and mechanism of action. Br J Dermatol. 2004;151(3):616–622.
- Horfelt C, Stenquist B, Larko O, et al. Photodynamic therapy for acne vulgaris: a pilot study of the dose-response and mechanism of action. Acta Derm Venereol. 2007;87(4):
325–329. - Choi MS, Yun SJ, Beom HJ, et al. Comparative study of the bactericidal effects of 5-aminolevulinic acid with blue and red light on Propionibacterium acnes. J Dermatol. 2011;38(7):661–666.
- Ma Y, Chen Q, Liu Y, et al. Effects of 5-aminolevulinic acid photodynamic therapy on TLRs in acne lesions and keratinocytes co-cultured with P. acnes. Photodiagnosis Photodyn Ther. 2016;15:172–181.
- Choi SH, Seo JW, Kim KH. Comparative study of the bactericidal effects of indocyanine green- and methyl aminolevulinate-based photodynamic therapy on Propionibacterium acnes as a new treatment for acne. J Dermatol. 2018;45(7):824–829.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne—update 2016—short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261–1268.
- Keyal U, Bhatta AK, Wang XL. Photodynamic therapy for the treatment of different severity of acne: A systematic review. Photodiagnosis Photodyn Ther. 2016;14:191–199.
- Friedmann DP, Goldman MP, Fabi SG, Guiha I. A retrospective study of multiple sequential light and laser sources to activate aminolevulinic acid in the treatment of acne vulgaris. Skinmed. 2017;15(2):105–111.
- Tang X, Li C, Ge S, et al. Efficacy of photodynamic therapy for the treatment of inflammatory acne vulgaris: A systematic review and meta-analysis. J Cosmet Dermatol. 2020;19(1):10–21.
- Goldman MP, Boyce SM. A single-center study of aminolevulinic acid and 417 NM photodynamic therapy in the treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2003;2(4):393–396.
- Mavilia L, Malara G, Moretti G, et al. Photodynamic therapy of acne using methyl aminolaevulinate diluted to 4% together with low doses of red light. Br J Dermatol. 2007;157(4):810–811.
- Yin R, Hao F, Deng J, et al. Investigation of optimal aminolaevulinic acid concentration applied in topical aminolaevulinic acid-photodynamic therapy for treatment of moderate to severe acne: a pilot study in Chinese subjects. Br J Dermatol. 2010;163(5):1064–1071.
- Ma L, Xiang LH, Yu B, et al. Low-dose topical 5-aminolevulinic acid photodynamic therapy in the treatment of different severity of acne vulgaris. Photodiagnosis Photodyn Ther. 2013;10(4):583–590.
- Serini SM, Cannizzaro MV, Dattola A, et al. The efficacy and tolerability of 5-aminolevulinic acid 5% thermosetting gel photodynamic therapy (PDT) in the treatment of mild-to-moderate acne vulgaris. A two-center, prospective assessor-blinded, proof-of-concept study. J Cosmet Dermatol. 2019;18(1):156–162.
- Mei X, Shi W, Piao Y. Effectiveness of photodynamic therapy with topical 5-aminolevulinic acid and intense pulsed light in Chinese acne vulgaris patients. Photodermatol Photoimmunol Photomed. 2013;29(2):90–96.
- Pinto C, Schafer F, Orellana JJ, et al. Efficacy of red light alone and methyl-aminolaevulinate-photodynamic therapy for the treatment of mild and moderate facial acne. Indian J Dermatol Venereol Leprol. 2013;79(1):77–82.
- Nicklas C, Rubio R, Cárdenas C, Hasson A. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—A simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35(1):3–10.
- Xu X, Zheng Y, Zhao Z, et al. Efficacy of photodynamic therapy combined with minocycline for treatment of moderate to severe facial acne vulgaris and influence on quality of life. Medicine. 2017;96(51):e9366.
- Del DE, Manfredini M, Petrini N, et al. Daylight photodynamic therapy with 5-aminolevulinic acid 5% gel for the treatment of mild-to-moderate inflammatory acne. G Ital Dermatol Venereol. 2021 Feb;156(1):46–50.
- Gold MH. Therapeutic and aesthetic uses of photodynamic therapy part two of a five-part series: lasers and light treatments for acne vulgaris promising therapies. J Clin Aesthet Dermatol. 2008;1(3):28–34.
- Wangsuwan S, Meephansan J. Comparative study of photodynamic therapy with riboflavin-tryptophan gel and 13% 5-aminolevulinic acid in the treatment of mild to moderate acne vulgaris. Clin Cosmet Investig Dermatol. 2019;12:805–814.
- Zhang J, Zhang X, He Y, et al. Photodynamic therapy for severe facial acne vulgaris with 5% 5-aminolevulinic acid vs 10% 5-aminolevulinic acid: A split-face randomized controlled study. J Cosmet Dermatol. 2020;19(2):368–374.
- Gold MH. Therapeutic and aesthetic uses of photodynamic therapy part five of a five-part series: ALA-PDT in clinical practice how one clinician performs this procedure. J Clin Aesthet Dermatol. 2009;2(1):32–35.
- Fink C, Enk A, Gholam P. Photodynamic therapy—aspects of pain management. J Dtsch Dermatol Ges. 2015;13(1):15–22.

