J Clin Aesthet Dermatol. 2021;14(10):42–47.
 by Lawrence F. Eichenfield, MD; Pearl Kwong, MD, PhD; Mercedes E. Gonzalez, MD; Albert Yan, MD; Pieter d’Arnaud, MS; Patrick Burnett, MD, PhD;
by Lawrence F. Eichenfield, MD; Pearl Kwong, MD, PhD; Mercedes E. Gonzalez, MD; Albert Yan, MD; Pieter d’Arnaud, MS; Patrick Burnett, MD, PhD;
and Melissa Olivadoti, PhD
Dr. Eichenfield is with Rady Children’s Hospital and the University of California, San Diego, Medical School in San Diego, California. Dr. Kwong is with Solutions Through Advanced Research in Jacksonville, Florida. Dr. Gonzalez is with the University of Miami Medical School in Miami, Florida. Dr. Yan is with the Children’s Hospital of Philadelphia and the Perelman School of Medicine, University of Philadelphia in Philadelphia, Pennsylvania. Mr. D’Arnaud is with Instat Consulting Inc. in Chatham, New Jersey. Drs. Burnett and Olivadoti are former employees of Verrica Pharmaceuticals Inc. in West Chester, Pennsylvania.
FUNDING: Funding for the described research as well as for figure and table creation was provided by Verrica Pharmaceuticals.
DISCLOSURES: Dr. Eichenfield received funding to his institution for the described research, has received consulting fees for and stock from Verrica Pharmaceuticals for activities unrelated to this publication, and is a member of the Board of Directors of Verrica Pharmaceuticals. Dr. Kwong has received consulting fees and research funding from Verrica Pharmaceuticals. Dr. Gonzalez has received research funding for the described research and consulting fees from Verrica Pharmaceuticals unrelated to this publication. Dr. Yan has received consulting fees from Verrica Pharmaceuticals for activities unrelated to this publication. Mr. d’Arnaud was a contractor for Verrica Pharmaceuticals. Dr. Burnett was employed by Verrica Pharmaceuticals and holds company stock. Dr. Olivadoti is an employee of Verrica Pharmaceuticals
Trial Registration: ClinicalTrials.gov identifier nos. NCT03377790 (for CAMP-1) and NCT03377803 (for CAMP-2).
ABSTRACT: Background.VP-102 is drug–device combination product containing cantharidin (0.7% w/v) and has undergone Phase III clinical trials for the treatment of molluscum contagiosum (molluscum). Efficacy and safety may differ by body region due to variable skin anatomy.
Objective. We investigated the pooled safety and efficacy of VP-102 by affected body region.
Methods. Individuals at least two years of age with molluscum were randomized to topical VP-102 or vehicle once every 21 days until clear (maximum of four applications). Participants were assigned to body region groups where lesions were present at baseline. Body region lesion counts were recorded at each visit. Efficacy was measured by the percentage of participants with complete clearance of lesions by region. Pre-specified adverse events (AEs) were analyzed for those treated in the region on that visit.
Results. Participants had a mean of two regions affected at baseline. Complete clearance was significantly higher in the VP-102–treated group than with vehicle application in all regions at the last visit (P<0.01 for each region). The incidence of pre-specified AEs was consistent across regions. However, these analyses were post hoc, and individual lesions were not tracked for efficacy.
Conclusion. VP-102 treatment shows consistent safety and efficacy across molluscum body regions.
Key words: Child, molluscum, cantharidin, molluscum contagiosum, incidence, topical, VP-102, infectious disease, lesion, sensitive skin, pediatric
______________________________________________________________________________
Molluscum contagiosum (molluscum) is a common viral skin infection that primarily affects children, the immunocompromised, and sexually active adults.1 Molluscum accounts for roughly 1% of all diagnosed skin disorders, and ranks in the top 50 most common diseases.2 Epidemiological data are limited; however, molluscum appears to be gender-neutral and its incidence peaks around the age of six years,3 with reported prevalence rates ranging from 5.1% to 11.5% among children younger than 16 years.4
Molluscum lesions can be widespread or confined to a single body region, and, in the immunocompetent person, typically range in number from 20 to 30 lesions.5 Approximately one-third of patients with molluscum experience molluscum dermatitis, furthering the burden of disease.6,7 Studies have been conducted assessing the distribution of lesions on the body, but have small sampling sizes.8–10 These studies suggested that lesions can present anywhere on the body; however, lesion distribution typically follows patterns that depend on the patient’s age. In children, lesions are most often located on regions of exposed skin like the trunk, limbs, and face, as well as intertriginous areas such as the axillae.9 In adults, most cases are transmitted via sexual contact; thus, lesions typically present in the abdomen, inner thighs, genitals, and perianal area. In children, genital and perianal lesions are most often due to autoinoculation.11 Atypical locations for lesions, regardless of age, include palms, soles, nipples, areolae, eyelids and conjunctiva, oral mucosa, lips, and scalp.11,12 If left untreated, molluscum can cause pain13 and scarring.14 Experts suggest treating molluscum to reduce the impact on the patient and caregiver and decrease the risk of spread of the disease.15
Cantharidin, a topically applied vesicant, has been used to treat molluscum for decades, but, until recently, had limited supportive clinical data in large-scale controlled trials.16 Safety information is likewise limited for compounded cantharidin in large-scale controlled trials, and concern for pain or large blisters, especially in sensitive skin areas, such as the face and groin, often preclude its use. Compounded cantharidin application methods employ rudimentary tools15 that may leave the patient at risk for application to uninfected skin, and the compound may change in concentration, leading to variable outcomes.11
Multiple treatment reviews for molluscum advise that compounded cantharidin should not be used in sensitive skin areas, such as the face or groin.7,11,17–21 It is hypothesized that the intraepidermal blistering induced by compounded cantharidin may go too deep into skin layers, producing pain and scarring, thus indicating that compounded cantharidin should not be used in areas of thin skin17; in addition, cantharidin-induced blisters can increase the risk of bacterial superinfection.11 Randomized, controlled, blinded clinical studies with a consistent shelf-stable cantharidin formulation with a consistent and targeted application technique are necessary to elucidate the distribution of molluscum and uncover any potential differences in efficacy or safety due to anatomical region when treating with a topical cantharidin product.
VP-102 is a drug–device combination product with cantharidin (0.7%, w/v) included in a topical, film-forming solution contained in a single-use applicator. The topical formulation, contained in a glass ampule inside the single-use applicator, is shelf-stable at room temperature for storage, and is manufactured under Good Manufacturing Practices. The cantharidin in the topical solution is more than 99% pure. VP-102 has completed Phase III clinical trials (Cantharidin Application in Molluscum Patients 1 and 2, or CAMP-1 and CAMP-2) in 528 participants aged two years or older with clinically diagnosed molluscum.22 These studies showed significantly higher complete clearance rates of lesions in VP-102–treated participants compared to vehicle-treated participants at the end of study (EOS) Visit/Day 84 in both CAMP-1 (46.3% vs. 17.9%; P<0.0001) and CAMP-2 (54.0% vs. 13.4%; P<0.0001).22 Given the differences in skin anatomy throughout the body as well as past concerns with using cantharidin in sensitive skin areas (i.e., face/groin), the efficacy and safety of VP-102 in different areas of the body remain of interest. Herein, we present post hoc analyses of pooled data from two randomized, double-blind, vehicle-controlled, Phase III clinical trials (CAMP-1 and CAMP-2) segmented by six body regions to determine the potential efficacy and safety of VP-102 by body region compared to vehicle.
Methods
Study drug. Subjects were randomized to receive either VP-102 or vehicle for treatment. VP-102 is a drug–device combination product that contains cantharidin (0.7%w/v) in a film-forming topical solution within a single-use applicator. The topical formulation also contains gentian violet (a surgical dye meant to facilitate the distinction of treated lesions) as well as denatonium benzoate (a bittering agent meant to deter oral ingestion). The solution is contained inside a glass ampule in a single-use applicator device. When the ampule is crushed, the solution flows through a filter and into the tip for precision application to lesions. The vehicle treatment was identical to VP-102 in all aspects (including solution and applicator) with the omission of the active ingredient, cantharidin.
Study design. The design for the CAMP-1 and CAMP-2 trials is illustrated in Figure 1. A detailed description of trial methodology and approval by local institutional review boards were previously published.22 The CAMP-1 and -2 studies assessed the efficacy and safety of VP-102 vs. vehicle when topically applied to all baseline and newly emergent treatable lesions every 21±4 days until complete lesion clearance or a maximum of four treatment applications (Visit 1/Day 1, Visit 2/Day 21, Visit 3/Day 42, and Visit 4/Day 63). At Day 84, an EOS visit was conducted to perform a final safety evaluation of the response to treatment and to assess the presence or absence of lesions for efficacy. Lesion counts of six body regions were completed in the trials at each study visit: head/neck, chest/abdomen, back/buttocks, groin, upper extremities, and lower extremities at study visits prior to treatment (if applicable). Delineations of regions were decided upon by the investigators. Lesion locations occurring at baseline were used to assign a participant to a body region and lesion count by region was tracked throughout the study. Participants could present with multiple body regions and be included in more than one body region group. If lesions spread to a new body region after baseline assessments, these regions were not included in the efficacy analyses.
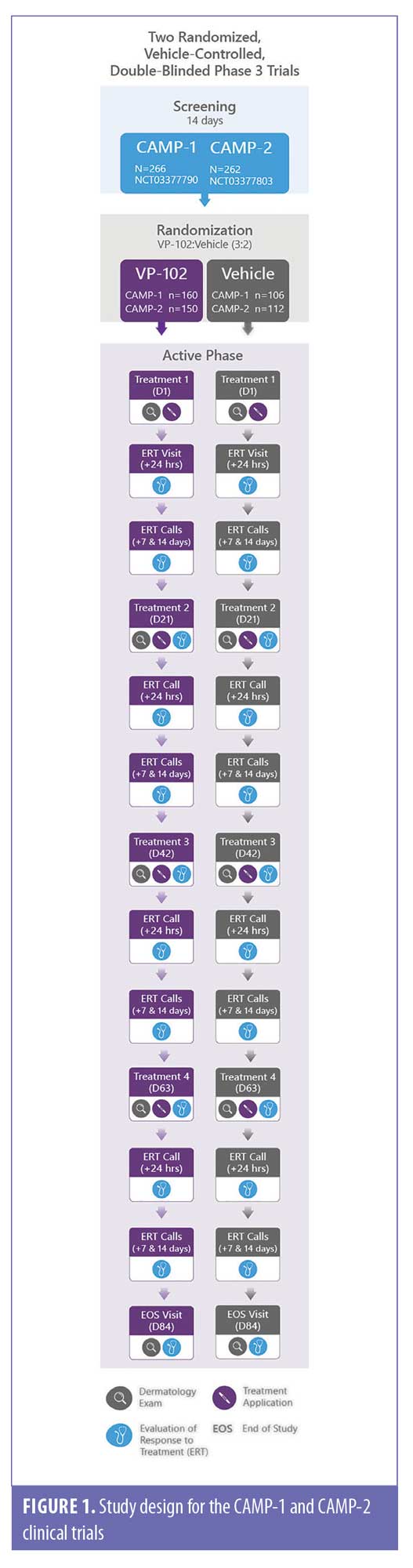
The study drug (VP-102 or vehicle) was applied at each study visit where lesions were present, and participants were instructed to wash off the agent 24 hours after application or earlier if significant blistering or adverse events (AEs) occurred.
Participants. Eligible participants were healthy males and females, at least two years old, with a clinical diagnosis of molluscum. Diagnosis, inclusion, and exclusion criteria are fully reported in the primary publication.22 Briefly, participants who were systemically immunosuppressed or were receiving treatments such as chemotherapy or other non-topical immunosuppressive agents were excluded. Participants with active atopic dermatitis, non-mucosal lesions, and inflamed lesions were allowed to participate in the study. Participants with lesions in sensitive skin areas, such as the face and groin, were included if they were deemed treatable. The decision on treatability of lesions (within 10mm of mucosal openings) was determined by the investigator, and all subjects were required to abstain from treatment for molluscum within two weeks of the baseline visit, as well as during the study period.
Efficacy and safety assessments. A post hoc analysis of efficacy was conducted by evaluating the complete clearance of baseline and new lesions in the identified region; individual lesions were not tracked. The proportion of participants with complete clearance in each region was compared between those who received VP-102 and those who received the vehicle at each visit.
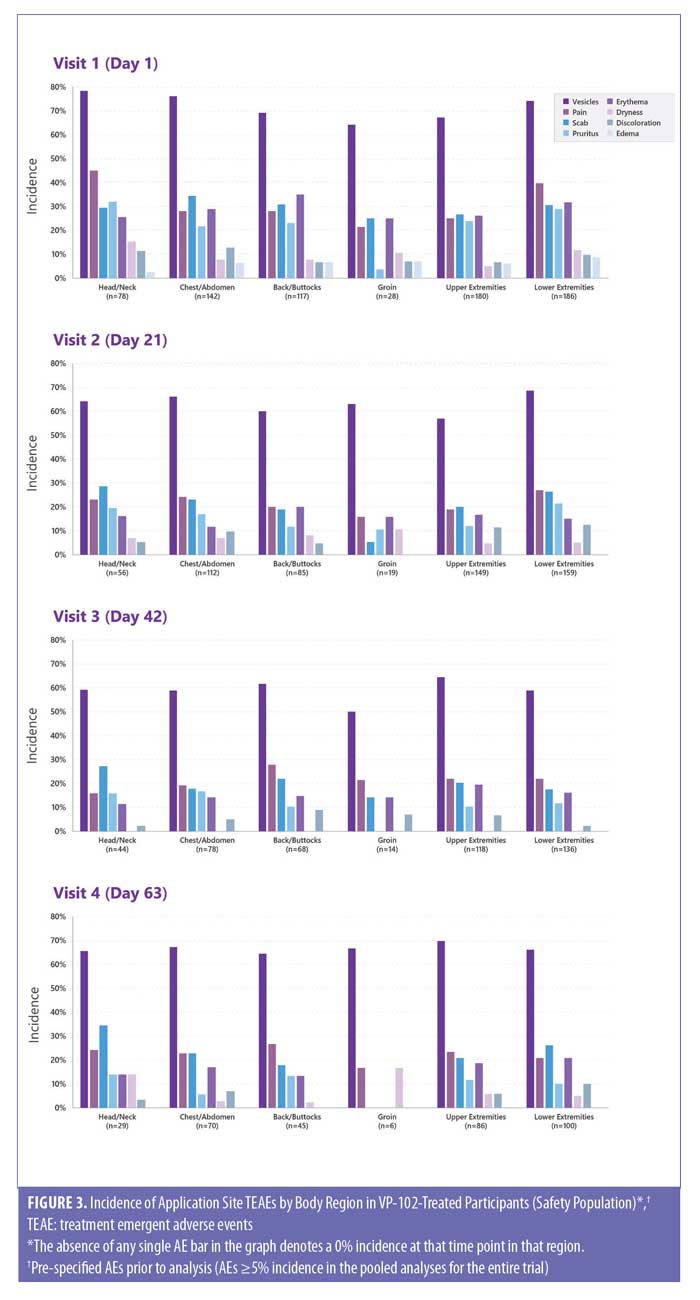
Safety and tolerability were analyzed in the safety population (i.e., participants who received at least one treatment application), and assessments included monitoring of treatment-emergent AEs (TEAEs), which were any adverse event occurring during or after each individual treatment application and prior to the next visit; physical examinations; and rates and types of concomitant medication usage. TEAEs were documented throughout the study with a specific focus on local skin reactions (LSRs), which were expected due to the pharmacodynamic action of cantharidin as a vesicant and attributed to the body region. The most common AEs (greater than or equal to 5%) in overall pooled safety data were pre-selected for this safety analyses, including application site vesicles, erythema, pain, dryness, scab, discoloration, pruritus, and edema. The safety analyses included participants who received a treatment in the particular body region on the specified treatment visit until just before the next visit. As such, different populations were included in the efficacy analyses (any participant with lesions in the region at baseline, no new regions were tracked) versus the safety analyses (any participant who received study drug at that specific visit in that body region).
Statistical analysis. In assessing efficacy by body region, for all efficacy variables, data were summarized using percentages for the pooled intent-to-treat (ITT) population (i.e., all randomized participants) by body region or total body results for proportion of participants with complete clearance.22 Binary endpoints were tested with Pearson’s chi-squared test. All statistical tests were two-sided, with a significance level of alpha=0.05. Participants with missing clearance data at day 84 were considered as not having achieved complete clearance. Statistical analyses were performed using the SAS version 9.3 software program (SAS Institute Inc., Cary, North Carolina).
In assessing safety by body region, the incidence of pre-specified TEAEs is expressed in percentage. Any occurrence of a pre-specified TEAE for any treated lesion(s) during the time period described were included. Discontinuations are expressed as a percentage of the safety population.
Results
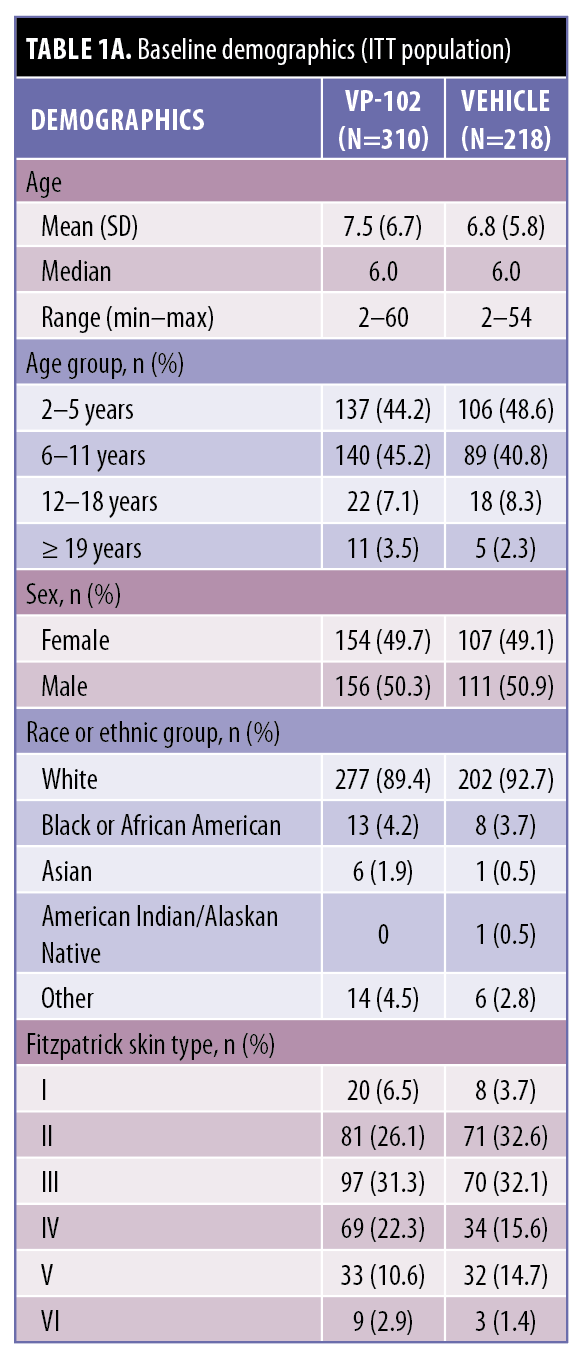
Baseline characteristics. A total of 528 individuals were enrolled and randomized in the CAMP trials (n=310 VP-102–treated cases and n=218 vehicle-treated cases; ITT population). Most participants (472/528, 89.9%) were 2 to 11 years old, with mean ages of 7.5 and 6.8 years for the pooled VP-102 and vehicle groups, respectively (Table 1). The mean time since the clinical diagnosis of molluscum was 122.9 days for the VP-102 group (range, 1–1247 days) and 126.2 days for the vehicle group (range, 1–1302 days) (Table 1B). Most participants (89.1% of the VP-102 group and 92.7% of the vehicle group) were Caucasian, yet the trial population included representatives from each Fitzpatrick scale group for skin tone (see Table 1A for details).
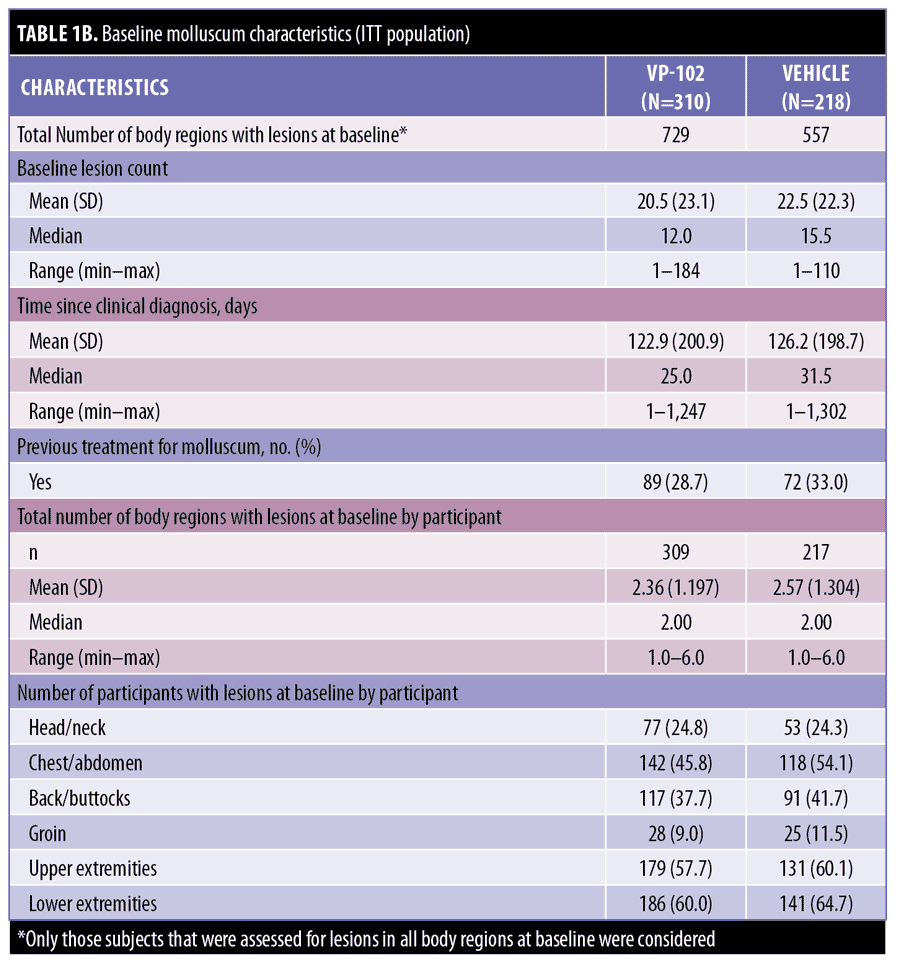
At baseline, participants who received VP-102 or vehicle presented with a mean number of 2.36 and 2.57 body regions with molluscum, respectively. In order of highest to lowest, the numbers of VP-102- and vehicle-treated participants with lesions at baseline in the respective body region were as follows: lower extremities (60.0% and 64.7%), upper extremities (57.7% and 60.1%), chest/abdomen (45.8% and 54.1%), back/buttocks (37.7% and 41.7%), head/neck (24.8% and 24.3%), and groin (9.0% and 11.5%) (Table 1).
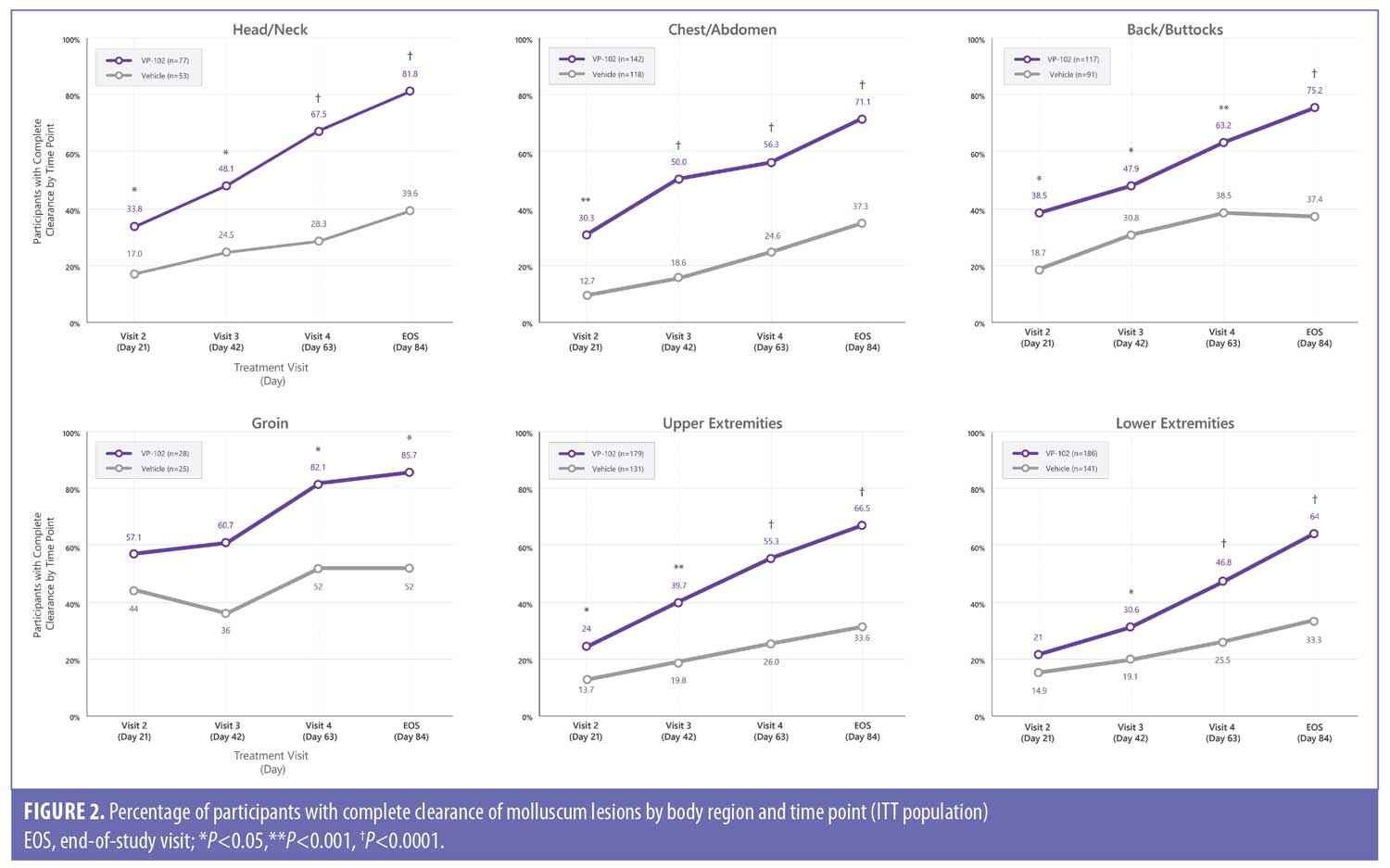
Efficacy outcomes. The percentage of participants with complete clearance in each body region for VP-102 and vehicle at EOS were as follows: head/neck (81.8% vs. 39.6%; P<0.0001), back/buttocks (75.2% vs. 37.4%; P<0.0001), chest/abdomen (71.1% vs. 37.3%; P<0.0001), groin (85.7% vs. 52.0%; P=0.0076), upper extremities (66.5% vs. 33.6%; P<0.0001), and lower extremities (64.0% vs. 33.3%; P<0.0001). For the head/neck, chest/abdomen, back/buttocks, and upper extremities, the difference in favor of VP-102 treatment was statistically significant at all time points/visits following the first treatment visit. For the lower extremities, the difference was significant beginning at the second treatment (Day 42/Visit 3). For the groin, the difference was significant at the Day 63/Visit 4 and Day 84/EOS visit (Figure 2).
Safety outcomes. The trials were completed by 93.6% (291/311) of VP-102–treated participants and 95.4% (206/216) of vehicle-treated participants (safety population). The proportion of participants with at least one TEAE leading to study drug discontinuation was 1.9% (6/311) for the VP-102 group and 0.5% (1/216) for the vehicle group (safety population).22 Most participants (4/6) who discontinued the study medication in the VP-102 group had more than one region affected by molluscum at discontinuation.
The percentage of participants reporting TEAEs was numerically higher among VP-102–treated participants than vehicle-treated ones. The incidence of TEAEs was relatively consistent across body regions in the VP-102–treated group and decreased with later visits (Figure 3). Scarring did not meet the 5% minimum requirement for presentation in these analyses but was documented in 2% of both VP-102–treated and vehicle-treated participants in the pooled data (safety population).
Discussion
There are no treatments approved by the United States Food and Drug Administration for molluscum, and many treatments that are utilized have limitations due to side effects of pain and poor tolerability, especially in sensitive skin areas (i.e., face or groin), where molluscum can often be found.12,17 In addition, aspects of the skin anatomy (e.g., thickness, layers, nerve innervation, water content, etc.) vary by location,8,17 so the efficacy and safety of treatments may differ by body region. The results of these post hoc analyses provide much-needed data about the natural distribution of lesions as well as the efficacy and safety of VP-102, a cantharidin-containing drug–device product, in different body regions.
The most common regions of lesions in participants in this study at baseline were the chest/abdomen and the upper and lower extremities, which align with findings of previous studies.8–10 Efficacy and safety were similar across regions in those treated with VP-102. In all body regions, participants treated with VP-102 had statistically significantly higher efficacy compared to the vehicle group in each body region at the EOS visit. Statistically significant differences between groups emerged after the first treatment in the upper extremities, chest/abdomen, and face/neck and after the second treatment in the lower extremities and groin, persisting to the EOS visit in those treated with VP-102 versus vehicle.
Higher clearance rates were identified in these post hoc analyses for individual regions than for the rate of complete body clearance as reported by Eichenfield et al. (46.3% for VP-102–treated subjects in CAMP-1 and 54.0% for the same in for CAMP-2).22 Individual participants had a median of two affected body regions at baseline, which suggests that it may be easier to achieve clearance in a region than the entire body when multiple body regions are affected. Treating prior to the spread of the virus to new body regions may also be beneficial for patient outcomes. These findings support the results of a retrospective review conducted by Jahnke et al. that advised that cantharidin is safe to use on sensitive areas such as the face.19
In CAMP-1 and -2, TEAEs were primarily of mild to moderate intensity.22 The incidence and types of TEAEs reported in those treated with VP-102 were similar across body regions, and the incidence was higher after the first treatment application of VP-102 than later visits (Figure 3). This could be due to a decrease in reporting, a reduction in the number of lesions treated, or a reduction in AEs. Application site vesicles were the most commonly reported TEAE for all body regions, which is expected due to cantharidin’s pharmacodynamic action as a vesicant. The incidence of TEAEs was similar across the body regions. One exception of interest was the groin region, for which lower rates of TEAEs were reported. In addition, pain emerged as numerically higher in the head/neck and the lower extremities. There was no single body region affected in common among the six subjects that discontinued the study drug due to an AE.
Limitations. Lesions within 10 mm of a mucosal opening may have been limited in the trials, and immunocompromised patients were excluded from participation. Lesions that occurred in new body regions after baseline were not able to be included in the efficacy analyses. In addition, the safety and efficacy populations may have differed, as the efficacy population only included participants who had lesions in specific area(s) at baseline, and the safety population included anyone who had a lesion that was treated at the specific visit, regardless of baseline location status. Additional studies could elucidate more information about body regions that had a lower number of participants in our trials, including the groin, or in adult populations where the data are limited.
The appearance of new lesions can occur from one to 50 days after inoculation6; thus, it is likely that new lesions arose after therapy had been initiated. Individual body regions had differences in the number of treatment visits to reach significance in those participants treated with VP-102 compared to vehicle. It is not possible to know the true rate of individual lesion clearance at each study time point because new (untreated) lesions may have developed between clinic visits in new body regions or may have undergone variable amounts of treatment, and lesions that occurred in new areas after baseline were not included in the efficacy analyses. Additional studies are needed to know the exact amount of treatments to clear an individual lesion. Treatments were also limited to a maximum of four with a defined study period of 84 days, so it is not possible to know if further treatments would allow participants to achieve complete clearance.
As AE incidence was only counted once per participant per time frame, information is not available for TEAEs that arose or changed in severity because of repeated treatments and, perhaps, whether certain areas of the body are more sensitive than others to repeated applications of VP-102. However, the incidence of TEAEs was similar across treatments. Our post hoc analyses were not powered for the endpoints presented, and more research is warranted to make strong conclusions about outcomes of VP-102 treatment in specific regions of the body.
Conclusion
The physical distribution of lesions found in our pooled analyses is consistent with previous findings from retrospective studies.8–10 Complete clearance was significantly higher in those treated with VP-102 compared to vehicle, and efficacy and safety outcomes were relatively consistent across body regions.
References
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13(10):877–888.
- Leung AKC. The natural history of molluscum contagiosum in children. Lancet Infect Dis. 2015;15(2):136–137.
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Dermatol Rep. 2020; 9(1):83–92.
- Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130–136.
- Badri T, Gandhi GR. Molluscum Contagiosum. In: StatPearls. Treasure Island, Florida: StatPearls Publishing LLC; 2020: 6.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104(5):301.
- Piggott C, Fallon Friedlander S, Tom W. Chapter 195. Poxvirus Infections. In: Medicine FsDiG, ed. Fitzpatrick’s Dermatology in General Medicine. New York, New York: McGraw-Hill Education; 2019: 63.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54(1):47–54.
- Berger EM, Orlow SJ, Patel RR, Schaffer JV. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148(11):1257–1264.
- Osio A, Deslandes E, Saada V, et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: a prospective study in 661 patients. Dermatology. 2011;222(4):314–320.
- Meza-Romero R, Navarrete-Dechent C, Downey C. Molluscum contagiosum: an update and review of new perspectives in etiology, diagnosis, and treatment. Clin Cosmet Investig Dermatol. 2019;12:373–381.
- Leung AK, Barankin B, Hon KL. Molluscum contagiosum: an update. Recent Pat Inflamm Allergy Drug Discov. 2017;11(1):22–31.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104(5):301–305;E301;E302.
- Weller R, O’Callaghan CJ, MacSween RM, White MI. Scarring in Molluscum contagiosum: comparison of physical expression and phenol ablation. BMJ. 1999;319(7224):1540.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5(8):505–512.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum Contagiosum: Review and Update on Management. Pediatr Dermatol. 2017;34(5):504–515.
- Nelson KC, Morrell DS. Spreading bumps: molluscum contagiosum in the pediatric population. Pediatr Ann. 2007;36(12):814, 816–818.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9(2):2.
- Jahnke MN, Hwang S, Griffith JL, Shwayder T. Cantharidin for treatment of facial molluscum contagiosum: a retrospective review. J Am Acad Dermatol. 2018;78(1):198–200.
- Moye V, Cathcart S, Burkhart CN, Morrell DS. Beetle juice: a guide for the use of cantharidin in the treatment of molluscum contagiosum. Dermatol Ther. 2013;26(6):445–451.
- Nguyen HP, Tyring SK. An update on the clinical management of cutaneous molluscum contagiosum. Skin Therapy Lett. 2014;19(2):5–8.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary drug–device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020; 156(12):1315–1323.

