 by Eman Mostafa Sanad, MD; Asmaa Adel El-fallah, MD; Ahmed Raad Al-doori, Msc; and Rehab Mohammed Salem, MD
by Eman Mostafa Sanad, MD; Asmaa Adel El-fallah, MD; Ahmed Raad Al-doori, Msc; and Rehab Mohammed Salem, MD
Dr. Sanad is Professor of Dermatology and Andrology, Faculty of Medicine, at Benha University in Banha, Egypt. Dr. El-fallah is Lecturer of Chemical and Clinical Pathology, Faculty of Medicine, at Benha University in Banha, Egypt. Dr. Al-doori is a dermatology resident at Baghdad University in Baghdad, Iraq. Dr. Salem is Assistant Professor of Dermatology and Andrology, Faculty of Medicine, at Benha University in Banha, Egypt.
FUNDING: This study was supported by Ortho Dermatologics.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. The relation between zinc and the cytokines involved in vitiligo pathogenesis has not been studied well.
Objective. We aimed to investigate the serum levels of zinc in patients with vitiligo and to assess their relation to serum interleukins (IL)-4, IL-6, and IL-17.
Patients and Methods. The study included 50 patients with active vitiligo and 100 age-, sex-, and BMI-matched healthy volunteers as a control group. Serum zinc levels, IL-4, IL-6, IL-17 were evaluated in all participants.
Results. The mean serum levels of zinc was significantly reduced in patients with vitiligo, while the serum levels of IL-17, IL-4, and IL-6 were significantly elevated in the vitiligo group when compared with the controls (P<0.001). The serum zinc levels showed significant negative correlation with serum IL-6, IL-4. and IL-17 levels (P< 0.05). There was a significant positive correlation between the serum levels of the three studied interleukins (P< 0.001).
Conclusion. Zinc supplementation could potentially be used as a beneficial treatment for vitiligo, but the required dosage needs to be studied further.
KEYWORDS: zinc, cytokines, vitiligo
J Clin Aesthet Dermatol. 2020;13(12 Suppl 1):S29–S33
Vitiligo is a common skin depigmenting disorder resulting from a selective progressive loss of functional epidermal melanocytes.1 Its prevalence in the general population worldwide ranges from 0.06 to 2.28 percent.2 The pathogenesis of the disease is not fully elucidated. It is a multifactorial disorder, and both genetic and nongenetic factors seem to play a role in its pathogenesis. Autoimmune processes, deficiency of melanocyte growth factor, enzymatic self-destruction mechanisms, and abnormal neurogenic stimulus leading to dermatomal melanocyte destruction have all been postulated as possible pathogenetic factors.3
Zinc is an essential trace element that is necessary for growth and development at all stages of life.4 The main dietary sources of zinc include oysters, red meat, poultry, and beans. The current recommended dietary allowance (RDA) varies according to age and sex: 11mg/day and 8mg/day for healthy adult men and women, respectively.5 Zinc plays a key role in the immune system, reproductive health, and sensory functioning, as well as neurobehavioral development.6 It is also an important coenzyme in the reactions of melanin synthesis.7
It has been suggested that zinc supplements are a beneficial adjuvant therapy for patients with vitiligo. This theory is based on zinc’s proposed ability to protect melanocytes via antiapoptotic and antioxidant properties. Zinc can also regulate the melanin production by its role in alpha-melanocyte stimulating hormone release and by precipitating zinc-alpha2-glycoprotein (ZAG) in the tissue; moreover, zinc is one of the nonspecific elements that help regulate cell-mediated immunity.8
All these roles played by zinc makes it a promising adjuvant in managing vitiligo. However, its relation to the cytokines involved in vitiligo pathogenesis has not been well-studied. Interleukin (IL)-4, IL-69, and IL-1710 have been shown to be significantly elevated in patients with vitiligo. These cytokines are also involved in controlling melanocytic proliferation and differentiation and inhibiting melanogenesis.11 An association between zinc serum levels and IL-4, IL-6, and IL-17 has also been observed.12,13
In this study, we investigated the relationship between serum levels of zinc and serum levels of IL-4, IL-6, and IL-17 in patients with vitiligo.
Methods
Inclusion/exclusion criteria. This case control study was approved by the Research Ethical Committee of Benha Faculty of Medicine in Benha, Egypt. An informed consent was obtained from each participant before the collection of blood samples. The study included 50 adult patients (>18 years of age) with active vitiligo and 100 age-, sex-, and body mass index (BMI)-matched healthy volunteers as a control group. Participants were recruited from the outpatient clinic of Dermatology and Andrology Department of Benha University Hospitals from February to December in 2019. Participants who were not using any systemic therapy or phototherapy for vitiligo for at least two months or any topical therapy for at least two weeks prior to enrollment in the study were included in our investigation. Patients were excluded from the study with any of the following characteristics: ongoing treatment for vitiligo or treatment with any drugs or topical preparations that could affect the outcome of the study (e.g., zinc supplements, systemic steroids, hepatic enzyme inducers); pregnancy or lactation; and/or severe systemic diseases (e.g., hepatic, renal, or cardiac dysfunction; autoimmune disorders; infectious or inflammatory cutaneous disorders; malignant tumors).
Patient history and examination. A complete history was taken from each patient in the study, and all participants underwent complete general and dermatological examinations to assess vitiligo severity according to the Vitiligo Area Severity Index (VASI).14
Sampling. Five milliliters of venous blood were collected, for serum separation, in a plain sterile tube (without anticoagulant) from each participant under complete aseptic conditions via clean venipuncture using a disposable plastic syringe. The tube was left at room temperature for 30 minutes until coagulation. It was then centrifuged for 10 minutes at 3000rpm. The resultant serum was stored at -20°C until analysis.
Laboratory investigations. Serum zinc levels were measured by colorimetric method using zinc fluid monoreagent (Centronic GmbH Company , Wartenberg, Germany.) The sensitivity was 2µg/dL and linearity up to 400µg/dL.
Measurement of IL 4, IL-6, and IL-17 were measured using human interleukin 17 ELISA kit Cat. No. E0142Hu, human interleukin 4 ELISA kit Cat. No. E3762Hu, and human interleukin 6 ELISA Kit Cat. No. E0090Hu (Bioassay Technology Laboratory [BT Lab], Shanghai Korain Biotech Co Ltd, Shanghai, China). The analytical sensitivity was 2.49ng/L and assay range 5 to 1500ng/L for IL-4; the analytical sensitivity was 1.03ng/L and assay range 2 to 600ng/L for IL-6; and the analytical sensitivity was 1.06ng/L and assay range 2 to 600ng/L for IL-17. All methods were performed according to the manufacturer’s instructions.
Statistical analysis. Data were statistically described in terms of mean ± standard deviation (± SD), median and range, or frequencies (number of cases), and percentages when appropriated. Comparison of numerical variables between the study groups was done using Student t test (t-test) for independent samples in comparing two groups when normally distributed. For comparing categorical data, Chi square (chi2 test) test was performed. Comparison of numerical variables between more than two groups of normally distributed data was done using one-way analysis of variance (ANOVA) test with post-hoc multiple two-group comparisons. Fisher’s exact test (F-test) was used to determine if the proportions for one variable were different among values of the other variable.
Pearson’s correlation coefficient (r-test) was used to measure the statistical relationship, or association, between two continuous variables. P values less than 0.05 were considered statistically significant. Receiver operating characteristic (ROC) curve was used to show the connection/trade-off between clinical sensitivity and specificity for every possible cut-off for a test or a combination of tests. All statistical calculations were performed using SPSS (Statistical Package for the Social Science; SPSS Inc. Chicago, Illinois) Version 15 for Microsoft Windows.
Results
There was insignificant difference between the patients and control groups regarding age (35.360±11.412 years, 36.167± 9.61 years, respectively; p=0.747) and sex (50% of both groups are male patients).
The mean disease duration was 12.3±9.654 years, while the mean VASI score was 13.142±10.7. Seventeen (34%) patients had a positive family history of vitiligo. The most common type of vitiligo among the studied patients was vitiligo vulgaris (n=24, 48%), followed by focal vitiligo (n=16, 32%), and acrofacial vitiligo (n=10, 20%).
Laboratory investigations. The mean serum level of zinc was significantly lower (P<0.001) and the serum levels of IL-4, IL-6, and IL-17 were significantly higher in the vitiligo group compared to the controls (P<0.001) (Table 1).
Serum markers and the study variables. The serum zinc levels showed significant negative correlation with serum IL-4, IL-6, and IL-17 levels (P< 0.05). Serum IL-6 levels showed significant positive correlation with serum IL-4 and IL-17 levels (P< 0.001). Moreover, there was a significant positive correlation between serum IL-4 and IL-17 levels (P< 0.001) (Figure 1).

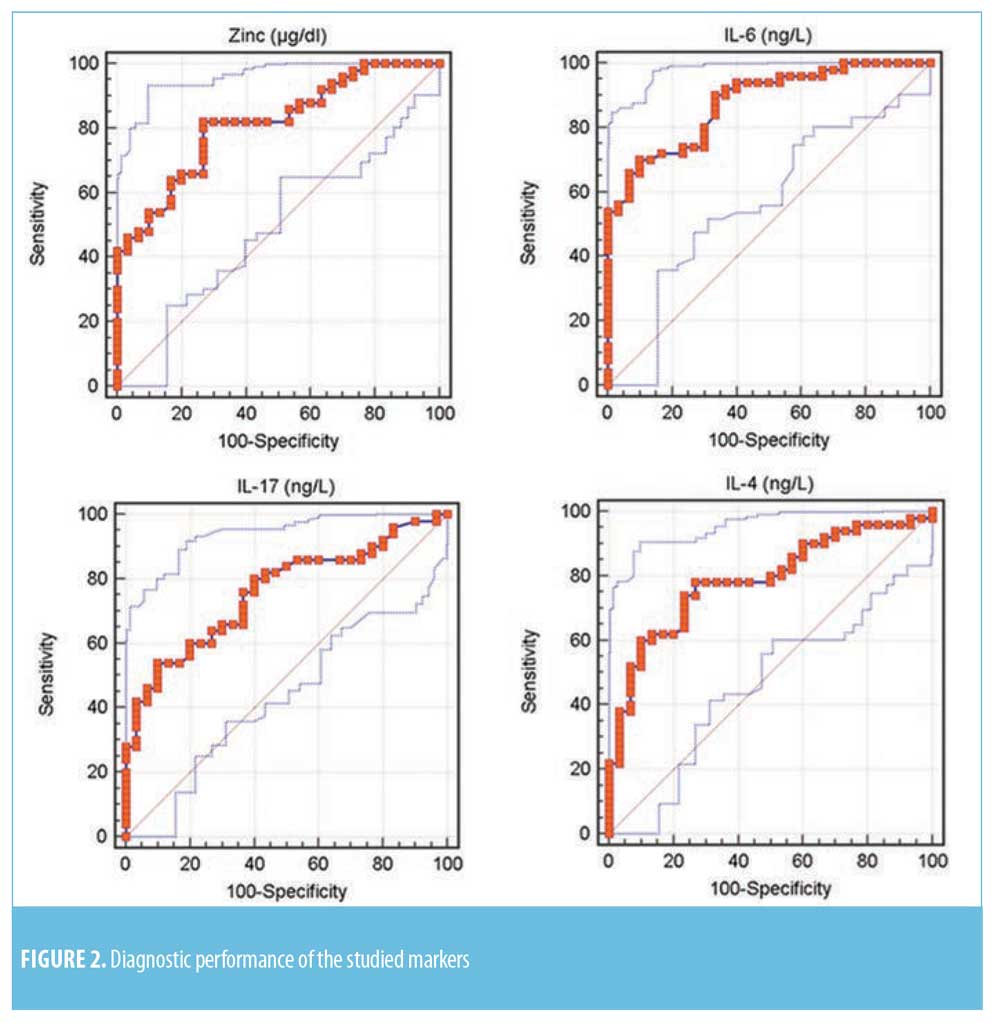
Diagnostic performance of the studied serum markers. ROC curve for the studied markers showed that the most sensitive serum levels for diagnosis was zinc (82%), while serum levels of IL-17 and IL-6 were the most specific (90%) (Table 2 and Figure 2).
Discussion
Zinc and other nutrients play vital roles in melanin synthesis, specifically by catalyzing the terminal stages of eumelanin formation; thus, zinc dysregulation might contribute to the pathogenesis of vitiligo.15 In this study, zinc serum levels were significantly lower in patients than in controls. These results were consistent with the results reported by Shameer et al,7 Mogaddam et al,16 and Salem et al.17Arora et al18 also reported lower zinc levels in patients with vitiligo; however, the difference was insignificant, which might be related to their small sample size (N=15 patients).
IL-17 is an active participant in melanocyte destruction. It is responsible for recruiting neutrophils and macrophages to the site of antigen, activating keratinocytes in local melanocyte units, and increasibng production of IL-1beta and TNFalpha.19 The exact role of this particular cytokine and its receptors in vitiligo is still not fully understood; however, it could potentially be a target for therapeutic interventions.20
In the current study, the mean serum levels of IL-17 were significantly higher in patients than in controls. These results were consistent with the results reported by Bassiouny and Shaker,21 Khan et al,9 Elela et al,19 Tembhre et al,22 and Zhou et al.23 However, Jandus et al24 and Esmaeili et al25 did not observe a significant difference in IL-17 serum levels or mRNA expression in lymphocytes between patients with vitiligo and healthy subjects. This might also be attributed to their small sample size (patients with vitiligo n=15, controls n=5). Osman et al26 also did not find a significant difference in IL-17 serum levels of Sudanese patients with vitiligo compared to control subjects. This lack of signficance can possibly be explained by the inclusion of patients with vitiligo under treatment, which could cause modulation of the serum levels of the inflammatory cytokines.
IL-4 has been shown to stimulate B-cell proliferation, to regulate immunoglobulin class switching, and to promote T-cell development. Alterations in IL-4 levels can disturb immune functioning, with subsequent development of several autoimmune diseases.27 IL-4 Inhibits the melanogenesis of normal human melanocytes through the JAK2–STAT6 signaling pathway, and it decreases the gene transcription of melanogenesis-associated proteins in melanocytes.28
In this study, IL-4 serum levels in patients were significantly higher than those of the control subjects. These results were consistent with the results reported by Imran et al27 and Khan et al9.
IL-6 could possibly be involved in the pathogenesis of vitiligo by enhancing the expression of intercellular adhesion molecule 1(ICAM-1) and inducing the polyclonal activation of B lymphocytes, which increases activation of autoantibodies and the subsequent destruction of active melanocytes. Moreover, IL-6 production is considerably increased by the presence of IL-17A, a leading interleukin related to the mechanism of melanocyte destruction.29
In the current study, IL-6 serum levels were significantly higher in patients with vitiligo compared to control subjects. These results confirm findings reported by Yu et al,3, Singh et al,10 Sushama et al,31 and Yang et al.32
A relationship between serum levels of inflammatory cytokines and zinc in different inflammatory conditions has been reported.33 In this study, there was a significant negative correlation between zinc serum levels and the serum levels of the measured cytokines IL-4, IL-6, and IL-17. Zinc metabolism and serum levels are somehow related to these interleukins either directly or indirectly.
Zinc deficiency has been reported in different inflammatory conditions. The proposed mechanism of this deficiency is the inflammatory cytokines’ mediated increase in the expression of zinc transporters that internalize zinc in the cells leading to hypozincemia and aggravation of the inflammatory process. IL-6 and IL-17 enhance the expression of the zinc transporters ZIP14 and ZIP8, respectively.34,35
Th17 cell development requires activation of STAT3 by IL-6. Zinc deficiency enhances STAT3 phosphorylation and activation,13 with subsequent increase in IL-6 responses and IL-6 gene expression.36 Zinc supplements disrupt the IL-6-related signals that activate STAT3. This STAT3 attenuation impairs TH17 development and proliferation.37 On the other hand, the relationship between zinc and IL-4 is more complex. Zinc deficiency attenuates STAT6, a molecule involved in IL-4 signaling.13 In-vitro zinc treatment of T cells in the presence of activated monocytes inhibited IFN-gamma– and IL-17-producing cells, but not IL-4-producing cells.38
Currently, zinc is considered to be an adjuvant antioxidant treatment for vitiligo management, and it is not always prescribed to patients. In this study, the significant relation between this trace element and a group of key players in vitiligo pathogenesis led us to theorize that zinc supplements could be an effective treatment for this disfiguring cutaneous disorder. The question remains, however, as to how much zinc is required per dose. It seems that degree to which serum concentrations of inflammatory cytokines are effected by supplemental zince is related to the administered dosage. Daily administration of 45mg of zinc seems to reduce IL-6 serum levels, while lower doses (10mg zinc/day) can aggravate the inflammatory response.33 The lack of data regarding the appropriate dosage required to elicit beneficial effects may explain why some patients improve on zinc supplements and others do not (or even deteriorate). Adjusting the dose of zinc may allow this trace element to induce major effects.
References
- Picardo M, Dell’Anna ML, Ezzedine K et al. Vitiligo. Nat Rev Dis Primers. 2015;1:15011.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206–1212.
- Halder RM, Chappell JL. Vitiligo update. Semin Cutan Med Surg. 2009;28(2):86–92.
- Kamer B, Wasowicz W, Pyziak K, et al. Role of selenium and zinc in the pathogenesis of food allergy in infants and young children. Arch Med Sci. 2012;8(6):1083–1088.
- Institute of Medicine. United States Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc . Washington, DC: National Academy Press; 2001.
- Hotz C, Brown H. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25(1):191–203.
- Shameer P, Prasad PV, Kaviarasan PK. Serum zinc level in vitiligo: a case control study. Indian J Dermatol Veneol Leprol. 2005;71:206–207.
- Bagherani N, Yaghoobi R, Omidian M. Hypothesis: Zinc can be effective in treatment of vitiligo. Indian J Dermatol. 2011 Sep–Oct;56(5):480-484.
- Singh S, Singh U, Pandey SS. Serum concentration of IL-6, IL-2, TNF-alpha, and IFNgamma in vitiligo patients. Indian J Dermatol. 2012;57(1):12–14.
- Khan R, Gupta S, Sharma A. Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J Am Acad Dermatol. 2012;66(3):510–511.
- Fu C, Chen J, Lu J, et al. Roles of inflammation factors in melanogenesis (Review). Mol Med Rep. 2020 Mar;21(3):1421–1430.
- Gruber K, Maywald M, Rosenkranz E, et al. Zinc deficiency adversely influences interleukin-4 and interleukin-6 signaling. J Biol Regul Homeost Agents.
2013;27(3):661–671. - Kitabayashi C, Fukada T, Kanamoto M, et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol. 2010;22(5):375–386.
- Hamzavi I, Jain H, McLean D, et al. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: The vitiligo area scoring index. Arch Dermatol. 2004;140(6):677–683.
- Cohen BE, Elbuluk N, Mu EW, Orlow SJ. Alternative systemic treatments for vitiligo: a review. Am J Clin Dermatol. 2015;16(6):463–474.
- Mogaddam MR, Ardabili NS, Maleki N, Chinifroush MM, Fard EM. Evaluation of the serum zinc level in patients with vitiligo. Adv Dermatol Allergol. 2017;XXXIV(2):116–119.
- Salem MA, Abd El-Raheem TA, boraia NM. Serum copper and zinc levels in vitiligo Patients. Egypt J Intern Med. 2018; 70(2):273–281.
- Arora PN, Dhillon KS, Rajan SR, Sayal SK, Das AL. Serum zinc level in cutaneous disorders. Med J Armed Forces India. 2002;58(4):304–306.
- Elela MA, Hegazy RA, Fawzy MM, Rashed LA, Rasheed H. Interleukin 17, interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: a case-controlled study on eighty-four patients. Eur J Dermatol. 2013;23(3):350–355.
- Lukinovic-Skudar S. Autoimmune factors in the ethiopathogenesis of vitiligo. MOJ Anat Physiol. 2015;1(3):60–62.
- Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol.
2011;36(3):292–297. - Tembhre MK, Sharma VK, Sharma A, Chattopadhyay P, Gupta S. T helper and regulatory T cell cytokine profile in active, stable and narrow band ultraviolet B treated generalized vitiligo. Clin Chim Acta. 2013;424:27–32.
- Zhou L, Shi Y-L, Li K, et al. Increased circulating Th17 cells and elevated serum levels of TGF-beta and IL-21 are correlated with human non-segmental vitiligo development. Pigment Cell Melanoma Res.
2015;28(3):324–329. - Jandus C, Bioley G, Rivals JP, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58(8):2307–2317.
- Esmaeili B, Rezaee SA, Layegh P, et al. Expression of IL-17 and COX2 gene in peripheral blood leukocytes of vitiligo patients. Iran J Allergy Asthma Immunol. 2011;10(2):81–89.
- Osman AM, Mukhtar MM, Bakheit KH, Hamdan HZ. Plasma levels of interleukin 17, interleukin 23, and transforming growth factor beta in sudanese patients with vitiligo: a case control study. Indian J Dermatol. 2015;60(6):635.
- Imran M, Laddha N, Dwivedi M, et al. Interleukin-4 genetic variants correlate with its transcript and protein levels in patients with vitiligo. Br J Dermatol. 2012;167(2):314–323.
- Choi H, Choi H, Han J, et al. IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J Invest Dermatol.
2013;133(2):528–536. - Kotobuki Y, Tanemura A, Yang L, et al. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. 2012;25(2):219–230.
- Yu HS, Chang KL, Yu CL, et al. Alterations in IL-6, IL-8, GM-CSF TNF-alpha, and IFN-gamma release by peripheral mononuclear cells in patients with active vitiligo. J Invest Dermatol. 1997;108(4): 527–529.
- Sushama S, Dixit N, Gautam RK, et al. Cytokine profile (IL-2, IL-6, IL-17, IL-22, and TNF-alpha) in vitiligo—new insight into pathogenesis of disease. J Cosmet Dermatol.
2018;18(1):337–341. - Yang X, Yan L, Ha D, et al. Changes in sICAM-1 and GM-CSF levels in skin tissue fluid and expression of IL-6, IL-17 and TNF-alpha in blood of patients with vitiligo. Exp Ther Med. 2019;17(1):408–412.
- Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676–694.
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci. 2005;102(19):6843–6848.
- Bonaventura P, Lamboux A, Albarède F, Miossec P. A feedback loop between inflammation and Zn uptake. PLoS One. 2016 Feb 4;11(2):e0147146.
- Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res. 2015;59(5):991–999.
- Kitabayashi C, Fukada T, Kanamoto M, et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol. 2010;22(5):375–386.
- Lee W-W, Lee H, Yoon B, Hwang Y. Inhibition of IL-6 signaling by zinc leads to repression of the Th17 response (IRM11P.763). J Immunol. 2014;192(1 Supplement):130.5.
