J Clin Aesthet Dermatol. 2020;13(12):E56–E83
J Clin Aesthet Dermatol. 2020;13(12):E56–E83
by Mark Nestor, MD, PhD; Joel L. Cohen, MD; Marina Landau, MD; Said Hilton, MD; Andreas Nikolis, MD; Syed Haq, MBBS, PhD; Maurizio Viel, MD; Bill Andriopoulos, PhD; Inna Prygova, MD; Keith Foster, PhD; Alessio Redaelli, MD; and Philippe Picaut, PharmD, PhD
Dr. Nestor is with the Miller School of Medicine at the University of Miami in Miami, Florida, and the Center for Cosmetic Enhancement and Center for Clinical and Cosmetic Research in Aventura, Florida. Dr. Cohen is with AboutSkin Dermatology and DermSurgery in Greenwood Village and Lone Tree, Colorado and the University of California, Irvine, in Irvine, California. Dr. Landau is with Wolfson Medical Center in Holon, Israel. Dr. Hilton is with Dr. Hilton and Partner in Düsseldorf, Germany. Dr. Nikolis is with the University of Montreal in Montreal, Québec, Canada. Dr. Haq is with Invictus Humanus in London, United Kingdom. Dr. Viel is with London Center for Aesthetic Surgery in London, United Kingdom. Dr. Andriopoulos is with Galderma Aesthetics in Uppsala, Sweden. Drs. Prygova, Foster, and Picaut are with Ipsen Pharma in Boulogne-Billancourt, France. Dr. Redaelli is with Visconti di Modrone Medical Center in Milan, Italy.
FUNDING: This study was sponsored by Ipsen.
DISCLOSURES: Mark Nestor has served as investigator for and received research grants from Galderma, Allergan, and Evolus and received consultancy fees from Ipsen; Joel L. Cohen has received consultancy fees from Galderma, Allergan, Merz, Evolus, Revance, and CROMA; Marina Landau has received consultancy fees from Ipsen; Said Hilton has served as an investigator in clinical trials from Allergan, Ipsen, and Evolus; Andreas Nikolis has received consultancy fees and research grants from Galderma, Allergan, and Merz; Syed Haq is Director of Invictus Humanus Ltd, Chief Scientific & Medical Officer of Tarian Group Holdings Ltd, and has received consultancy fees from Galderma; Maurizio Viel has received consultancy fees from Ipsen; Bill Andriopoulos is an employee of Galderma; Inna Prygova is employed by Ipsen Pharma; Keith Foster and Philippe Picaut were employees of Ipsen at the time this research was conducted; Alessio Redaelli received consultancy fees from Ipsen and FillMed and is editor of Officina Editoriale Oltrarno Firenze.
ABSTRACT: Objective:We sought to analyze the current literature regarding time to onset and duration of effect of abobotulinumtoxinA (aboBoNT-A, Dysport®/Azzalure®) for upper facial aesthetic indications.
Methods: We conducted a systematic review of literature databases (PubMed/MEDLINE, Embase, Cochrane Library, and Google Scholar) to identify English-language publications relevant to: population (patients with aesthetic indications [including glabellar lines and wrinkles]); interventions (aboBoNT-A); comparators (no restrictions); outcomes (efficacy, including onset of action and duration of effect); and settings (clinical). A manual search of review paper bibliographies was performed. Structured data extraction was used to enable interstudy analysis.
Results: Overall, 42 original research papers relevant to aboBoNT-A onset and/or duration were identified. All 24 studies assessing efficacy within one week post-injection demonstrated some response at the first time point assessed, and all 37 studies assessing duration showed some response after 12 weeks. Although methodologies for assessing onset and duration differed, when outcomes were refined by reported mean/median, at least 50 percent of patients responding to treatment, or significance versus placebo or baseline at a given time point, onset was most often reported within 2 to 3 days (7 studies), and as early as 24 hours (2 studies). Duration was most often reported as four months (18 studies), although four studies provided evidence that aboBoNT-A efficacy was maintained at five months and three studies at or after six months post-injection.
Conclusion: This review indicates that aboBoNT-A has a median onset of efficacy of 2 to 3 days and a longer duration of action (3–6 months across studies) than the current labelled minimum treatment interval (12 weeks).
KEYWORDS: abobotulinumtoxinA, upper face, duration, onset
AbobotulinumtoxinA (aboBoNT-A; Dysport®/Azzalure®) has been approved for glabellar lines treatment in adult patients under 65 years in many countries worldwide, including European countries and the United States, since 2009.1,2 AboBoNT-A approval in European and Asian countries, among others, includes treatment of lateral canthal lines (i.e., crow’s feet),1,3 and numerous studies have assessed aboBoNT-A efficacy in other areas of the upper face, such as the frontalis muscles (i.e., forehead region).4
Other botulinum neurotoxin type A (BoNT-A) products approved for aesthetic indications include onabotulinumtoxinA (onaBoNT-A; Botox®) and incobotulinumtoxinA (incoBoNT-A; Xeomin®), among other less widely used toxins, such as prabotulinumtoxinA (Jeuveau®). Although all BoNT-As are derived from a 150kDa neurotoxin, dosing units are non-interchangeable and formulations differ notably in excipients, such as albumin content; thus, products might differ in terms of time of onset and duration of clinical effect.5 Moreover, total 150kDa neurotoxin content varies among the three main commercially available toxins (aboBoNT-A, onaBoNT-A, and incoBoNT-A).6 It is thought that these products each have a different molecular potency, which might impact their onset of action and duration of effect.7 Furthermore, recent in-vitro studies revealed that the amount of active neurotoxin available in Food and Drug Administration (FDA)-approved doses for each clinical indication differed between these three BoNT-A products.8
Current dosing guidelines recommend a minimum treatment interval of 12 weeks;1,2 however, aboBoNT-A efficacy in glabellar lines at the recommended dose (50U) has been shown to extend to 4 to 5 months in several double-blind, randomized, placebo?controlled studies.9 In an open-label, repeated-cycle study with aboBoNT-A (minimum 85 days between cycles), the duration of response was up to six months for a small number of patients.10 Moreover, aboBoNT-A has a reported median time to onset of action of 2 to 3 days,9,10 with some patients responding within one day.10,11
Here, we report results from a systematic literature review on time to onset of action and duration of effect of aboBoNT-A for glabellar lines, and other areas of the upper face, including lateral canthal lines and forehead regions, as reported up to September 2018.
Methods
Systematic literature search and study selection. This systematic literature review was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 Searches were conducted in PubMed, EMBASE, the Cochrane Library, and Google Scholar between September 11, 2018, and September 12, 2018. Search terms were developed by reviewing the background literature for terms related to the research question, in line with the objective outlined above. The full search strategy is shown in Supplementary Table 1. Searches were limited to English-language manuscripts with no date restrictions. Abstracts from conferences and meetings were excluded. Review articles were also excluded from the search, although their bibliographies were manually searched to identify further articles that met the inclusion criteria. Citations were downloaded into the reference management software EndNote X7 to check for duplicates; deduplication was then manually replicated. Citations and abstracts were screened for inclusion by two reviewers using the eligibility criteria defined in terms of population, intervention, comparator, outcomes, and setting (PICOS, Supplementary Table 2). The patient population was defined as patients with aesthetic indications, such as glabellar lines, and the intervention as aboBoNT-A. No restrictions were placed on comparators. Outcomes were defined as onset of action or duration of effect. Study type was any clinical setting, excluding case reports.

Full manuscripts were obtained for eligible abstracts. For each manuscript, the relevance was assessed by two reviewers; one reviewer checked all manuscripts for inclusion and consistency of data extraction forms.
Data extraction and assessment. Study design, population, and outcome results data were extracted by each reviewer using a standardized data extraction form. An assessment of bias was made using the Cochrane Collaboration’s tool for assessing risk of bias for randomized controlled trials.13 A similar approach was used to assess risk of bias for non-randomized studies using the methodological index for non-randomized studies (MINORS) checklist.14 As a statistical meta-analysis was not conducted, an overall assessment of quality across studies, such as heterogeneity or publication bias, was not performed. The primary objective was to assess the onset of action and duration of effect of aboBoNT-A for aesthetic use in the upper face. Results are presented in tables and figures and narratively described.
Results
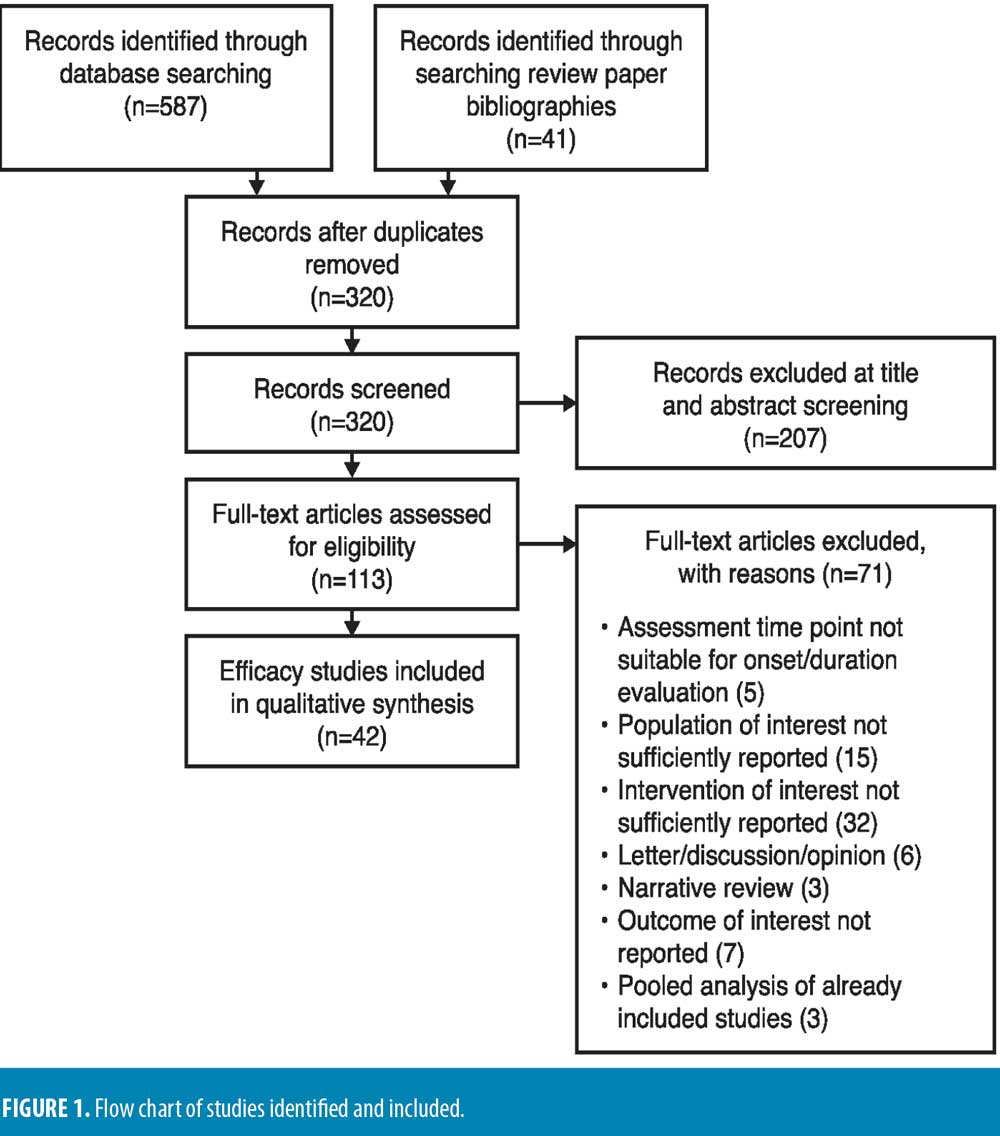
Literature search and selected studies. In total, 320 studies were identified from medical literature databases and manual selection from review article bibliographies. Of these, 42 publications met the criteria for primary analysis (defined in methods section). Figure 1 presents the PRISMA diagram of all publications evaluated for inclusion and reasons for exclusion.
Assessment of onset and duration. In most studies, onset of effect was assessed by either the patient or an objective assessor via in-person or photographic assessment. Onset was most commonly assessed using a diary-based patient self-assessment during the first week post-injection, defined as the first day on which the patient reported a response, often in answer to the question, “since being injected, have you noticed an effect on the appearance of your glabellar lines?”10,15–20 Another commonly used measure of onset was a four-point severity scale (where 0=none and 3=severe, although exact wording differed between studies), with onset defined as the first day improvement was observed.21–25 For most studies, response was defined as at least a one-grade improvement or a post-injection score of 0 or 1. In some studies, the scale used had an additional grade (4=very severe).17,26–29 One study assessed onset by frontalis activity measurement, defined as percentage change in frontalis muscle activity (i.e., difference between frontalis height at maximum elevation and at rest).11 A modified seven-point Fitzpatrick wrinkle grading scale was employed in one study (0.5 grade intervals; 0=absence of wrinkles, 3=a deep furrow of >3mm in depth).30 In two studies, onset was patient-reported at follow-up visits or by telephone.31,32 A few studies included other measures for assessing onset, such as change in compound muscle action potential (CMAP),17 electromyography assessment of the injected muscles,33 or using computerized software for the measurement of wrinkle lines,30,34 muscle strain,35 or mobility.36
For many studies, duration of action was assessed using the same
four-point10,15,16,18,20–25,32,34,37–49 and five-point severity scales,17,46,50,51 and modified Fitzpatrick scale,30 as defined for onset. One study used a four-point qualitative scale to assess improvement in wrinkle severity, where 1=unaltered and 4=very reduced.52 Although these measures are designed to assess efficacy, duration is indicated by patients exhibiting a response at later study time points. Similarly to onset, one study quantified duration of altered frontalis muscle activity.45 Duration was also reported as time until observed improvements in wrinkles regressed to their baseline appearance, 28 and video assessment of time to return to baseline muscle activity.26 Additional patient-reported methods for assessing duration included a four-point scale from “ineffective” to “very effective,”53 as well as an eight-point43 or nine-point50 self-assessment scales for wrinkle severity, rated from “marked/very strong worsening” to “complete/very strong improvement,” or response to questionnaires completed at clinic visits or by telephone follow up.31 A few studies included other measures for assessing duration, such as change in CMAP17 or electromyography assessment33 of injected muscles, or using computerized software for the measurement of lines,30,34 muscle strain,35 as well as blinded investigator assessment based on imaging software.54
Further details of the outcomes used to assess onset and duration of treatment are described in Tables 1 to 5.
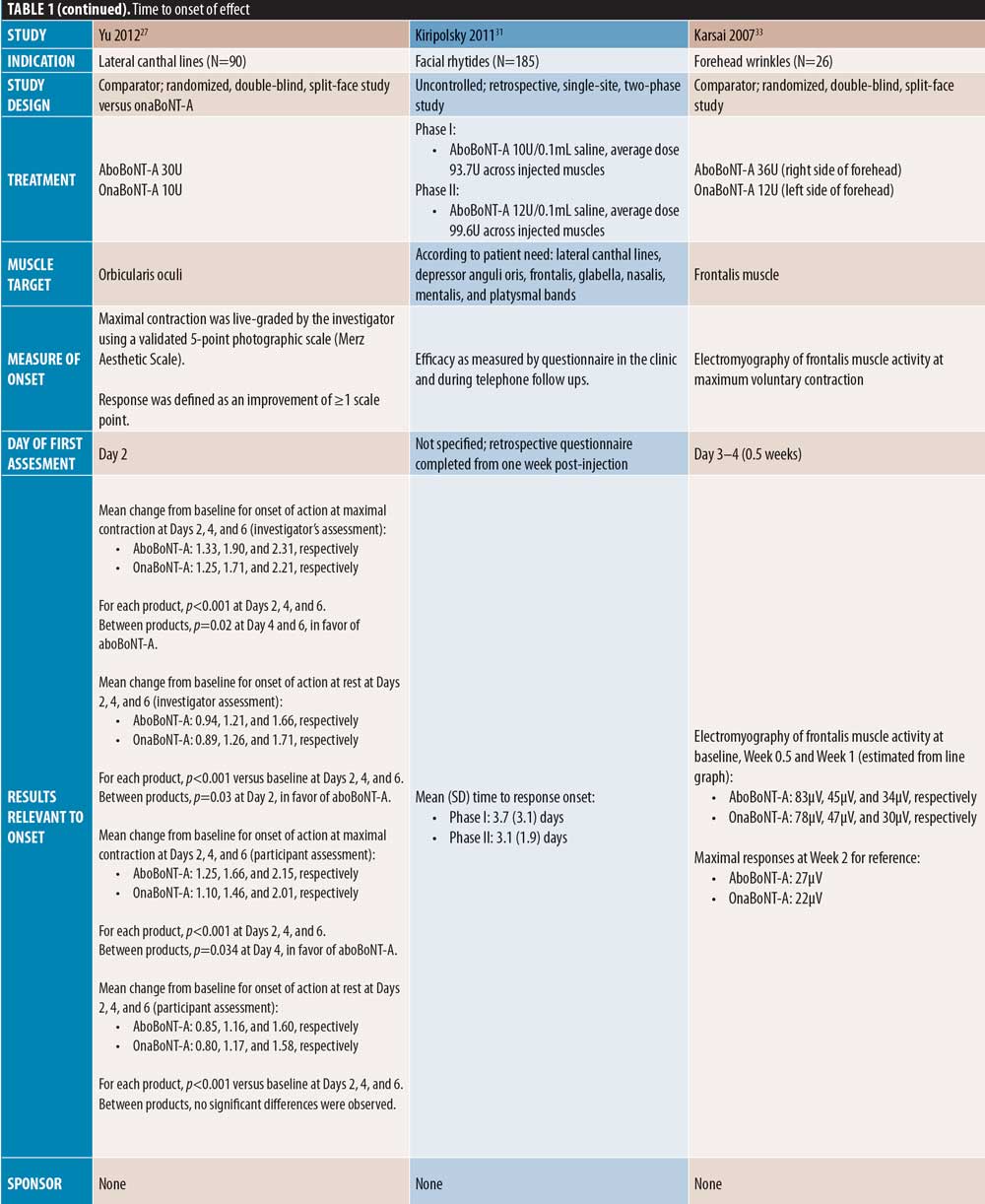
Onset of effect of aboBoNT-A. Of the 42 publications identified, 24 evaluated the efficacy of aboBoNT-A during the first week following treatment. Of these publications, six were placebo-controlled, nine investigated aboBoNT-A alongside a comparator (one study was both placebo- and comparator-controlled), and nine were uncontrolled studies. Unless stated otherwise, dose ratios for aboBoNT-A compared with onaBoNT-A or incoBoNT-A were 2.5:1U. It should be noted that study methodologies and methods of determining onset of action differed, and that some studies were conducted at doses higher than those recommended in the product label.
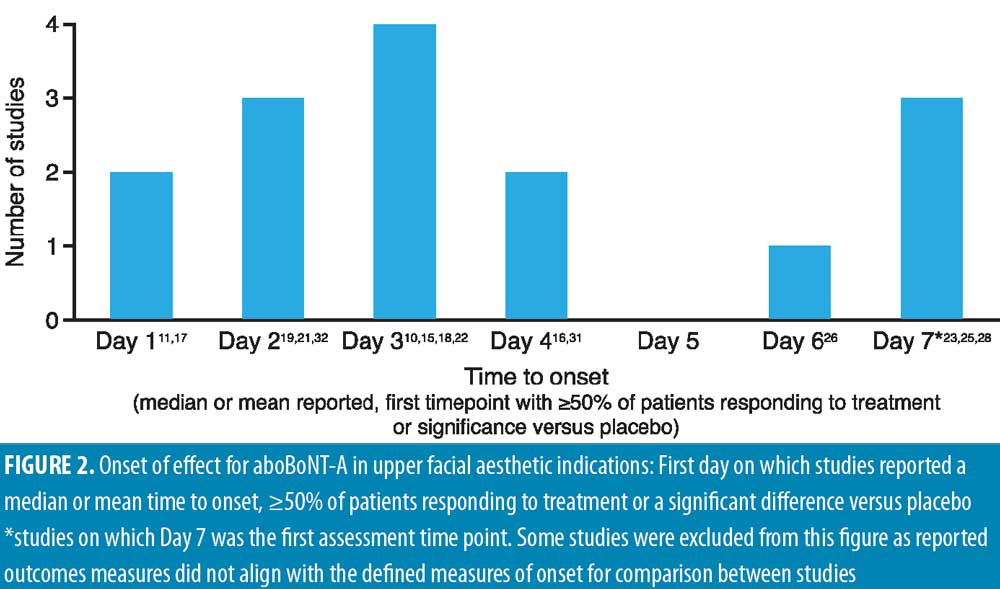
The first day of post-injection assessment was post-injection Day 1 in nine studies, Day 2 in four studies, Days 3 to 4 in two studies, Day 5 in one study, and Day 7 in six studies (two not specified), and all 24 studies demonstrated some response to aboBoNT-A at these first assessment time points (Table 1). As shown in Figure 2, although methods of assessment differed across studies, most reported a median time to onset, 50 percent or more of patients responding to treatment, or first significant difference versus baseline or placebo on Day 2 (observed in 3 studies) or Day 3 (observed in 4 studies).
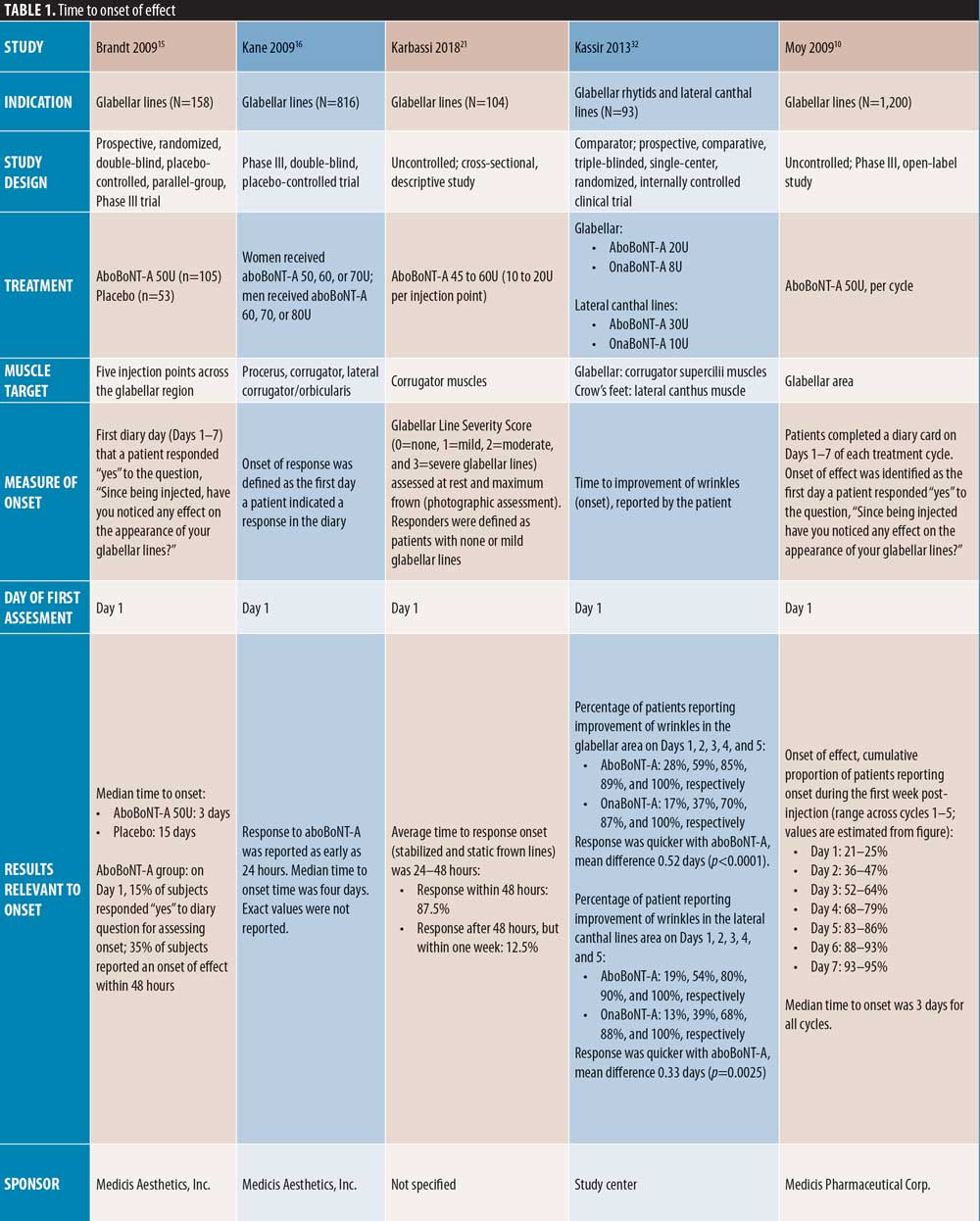
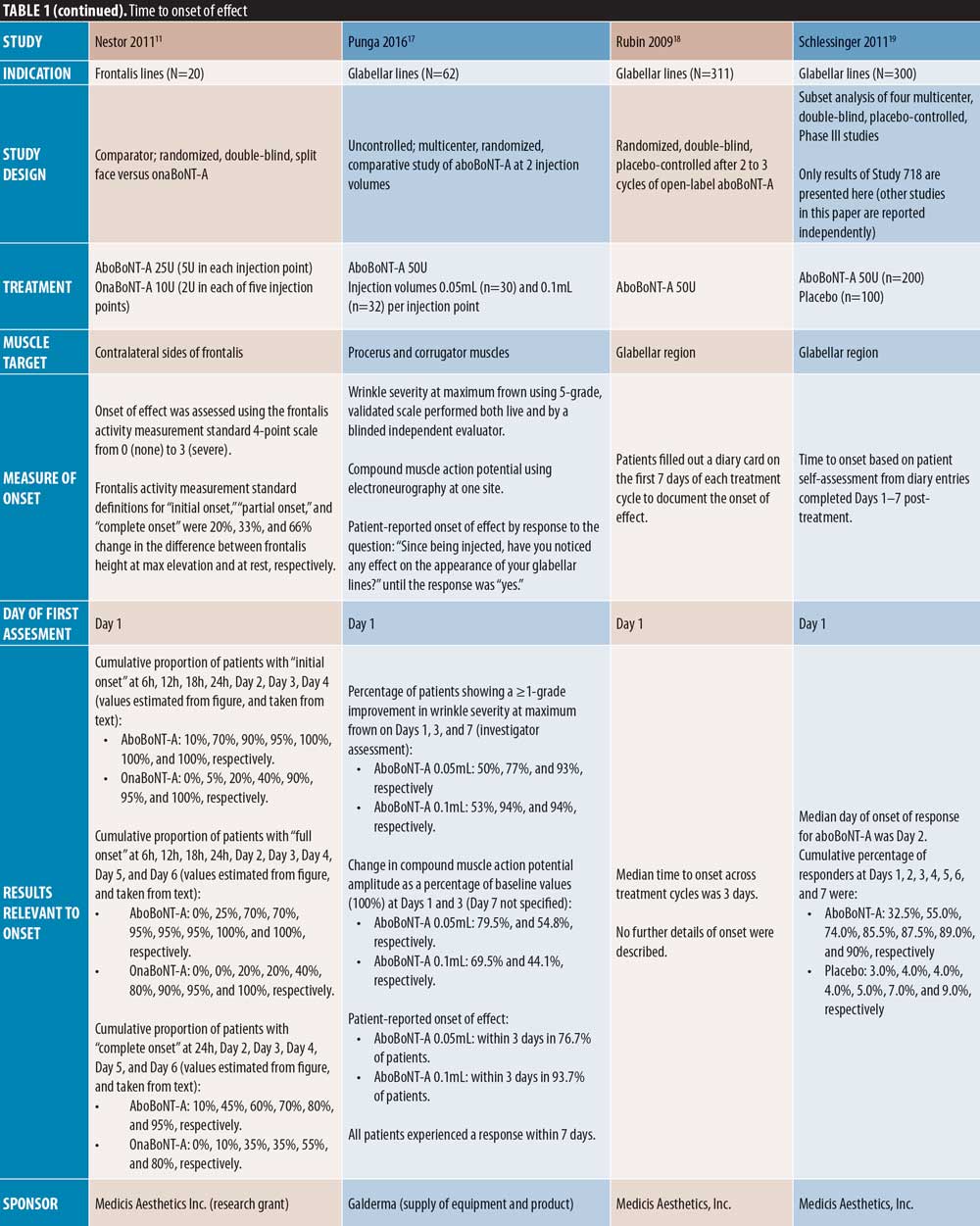
Glabellar lines. Early onset of action of aboBoNT-A in glabellar lines is demonstrated in a number of placebo-controlled studies (Table 1). Of those with an assessment on post-injection Day 1, Schlessinger et al19 reported the median day of onset as Day 2, with 55 percent of patients achieving onset by this time point, compared to four percent in the placebo group, and Brandt et al15 reported a median time to onset of three days compared to 15 days for placebo, with 35 percent of aboBoNT-A-treated patients reporting onset within 48 hours. Similarly, across multiple treatment cycles, Rubin et al18 reported a median time to onset of three days (onset not described for placebo). In another study with a time point at Day 1, Kane et al16 reported a median time to onset of four days, with response observed at 24 hours in some patients.
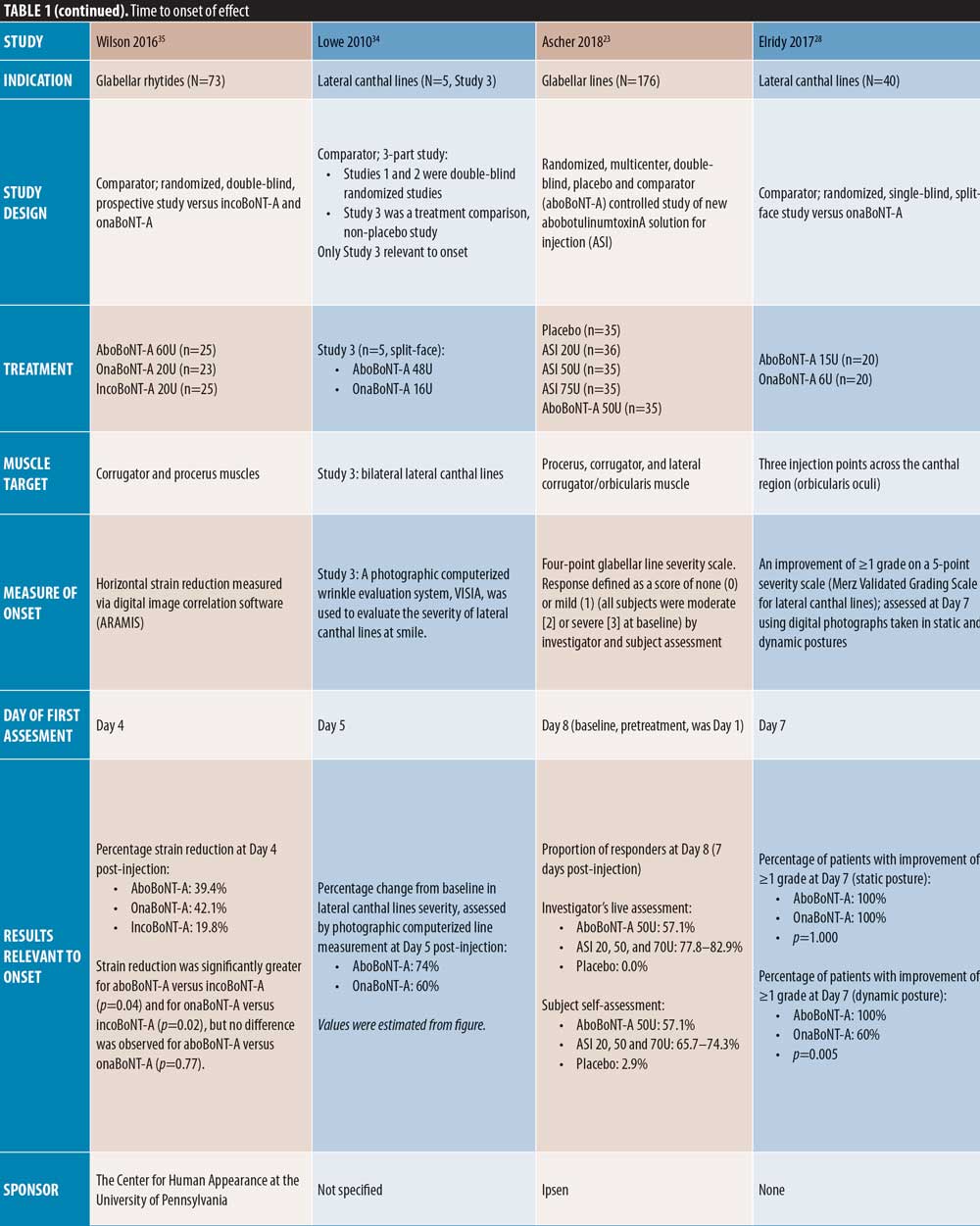
In the remaining placebo-controlled studies of patients with glabellar lines, the first day of assessment was Day 7; Monheit et al25 reported significant changes from baseline at all aboBoNT-A doses investigated (all p<0.001 vs. placebo). Ascher et al23 reported that 57 percent of aboBoNT-A-treated patients were responders by both investigator and patient assessment, compared with zero percent and three percent in the respective placebo groups (significance not reported). Ascher et al23 also investigated the efficacy of a new liquid formulation of aboBoNT-A (as opposed to a powder that requires reconstitution prior to injection) at varying doses for the treatment of glabellar lines, achieving a response rate of 78 to 83 percent and 66 to 74 percent at Day 7 by investigator and patient assessment, respectively.
Of the comparator-controlled studies, only Kassir et al32 assessed efficacy from Day 1. In this study, a higher proportion of patients reported onset with aboBoNT-A compared to onaBoNT-A at each time point up to Day 5. At this time point, both products achieved onset in 100 percent of patients, with a mean difference in time to onset of 0.52 days (p<0.0001). Rappl et al26 (dose ratio: 3:1:1) assessed onset from Day 2 and reported a quicker median onset of effect with incoBoNT-A (3.4 days in men and 3.0 days in women) compared to onaBoNT-A and aboBoNT-A (both 5.9 days in men and 5.3 days in women); with treatment identity (p<0.0001) and sex (p=0.02) identified as significant predictors of time to onset. Furthermore, Wilson et al35 (dose ratio: 3:1:1), after first assessing on Day 4 post-injection, reported significant differences in strain reduction in the glabellar region between BoNT-A products using digital imaging software: aboBoNT-A and onaBoNT-A both showed significantly greater strain reduction compared with incoBoNT-A (p=0.04 and p=0.02, respectively), although no difference was observed between aboBoNT-A and onaBoNT-A (p=0.77).
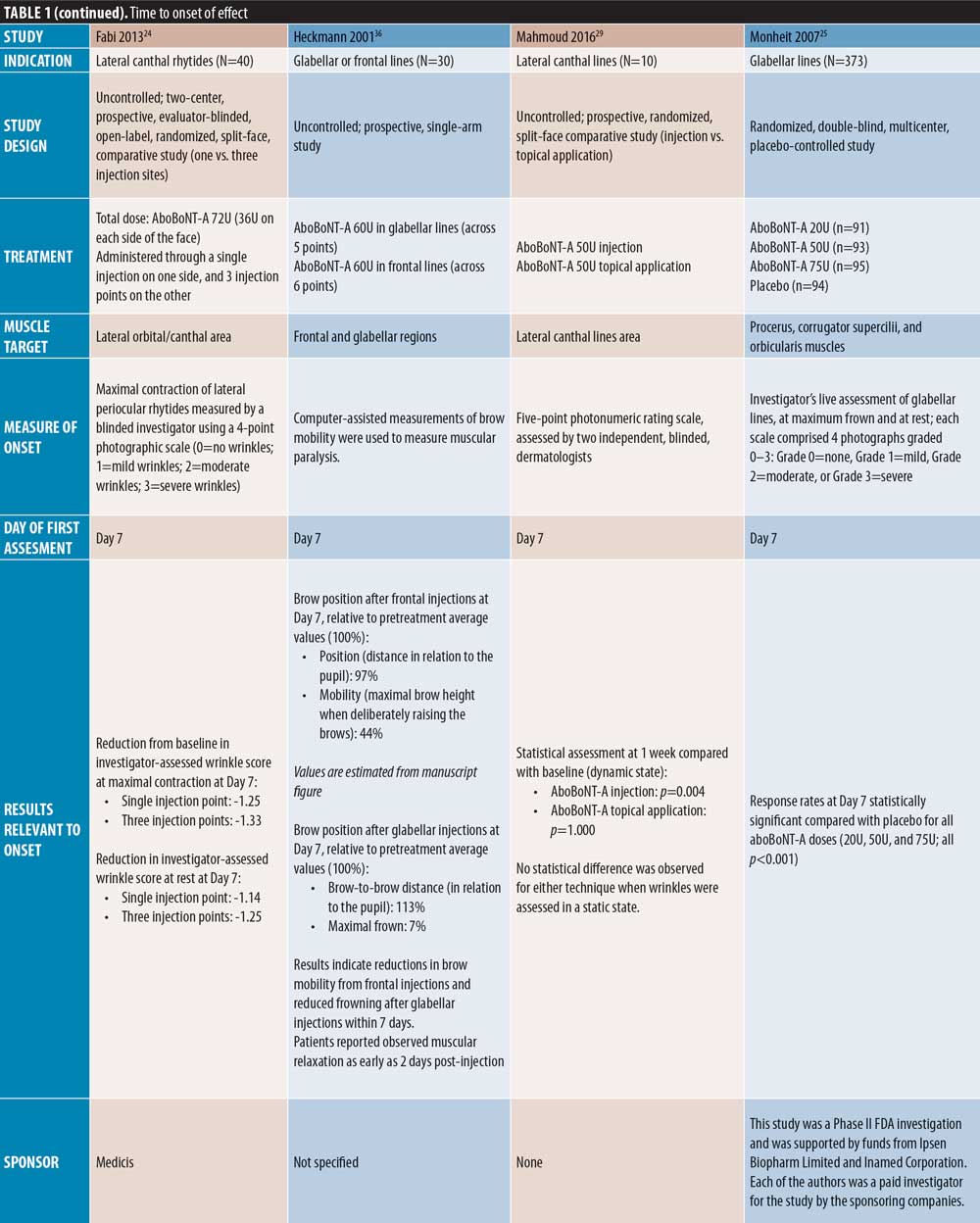
Of the uncontrolled studies, three assessed onset from Day 1, one did not specify, and one assessed onset at Day 7. Karbassi et al21 reported an average time to response of 24 to 48 hours, with 87.5 percent of patients achieving onset within 48 hours. Across five treatment cycles, Moy et al10 reported a median time to onset of three days, with an estimated 21 to 25 percent of patients reporting onset on Day 1 and 36 to 47 percent by Day 2. Punga et al17 reported onset in 50 percent and 53 percent of patients on Day 1 at 0.05mL and 0.1mL dilutions, respectively, and within three days in 77 percent and 94 percent of patients, respectively. Across multiple injection cycles, Schlessinger et al20 reported a median time to onset of 2 to 4 days in women and 2 to 5 days in men, and Heckmann et al36 demonstrated a reduction in frowning within seven days post-injection.
Lateral canthal lines. Several comparator-controlled studies of aboBoNT-A for the treatment of lateral canthal lines were identified. Kassir et al32 assessed efficacy from Day 1 and reported a quicker time to onset with aboBoNT-A compared to onaBoNT-A, with a mean difference of 0.33 days (p<0.0025). In a split-face study by Yu et al27 comparing aboBoNT-A and onaBoNT-A, a response was observed from Day 2 by both investigator and patient assessment at maximum contraction with both products. However, a significant difference was observed in mean change from baseline in favor of aboBoNT-A on Days 4 and 6 by investigator assessment (p=0.02 on both days vs. onaBoNT-A) and on Days 4 by patient assessment (p=0.03 vs. onaBoNT-A). Lowe et al34 observed a greater change from baseline in lateral canthal line severity with aboBoNT-A (74%) compared with onaBoNT-A (60%) at Day 5 post-injection, although significance was not assessed. In these three studies, the dose ratio of aboBoNT-A to onaBoNT-A was 3:1. At Day 7 post-injection, Elridy et al28 reported 100 percent of patients achieving onset of effect following aboBoNT-A injection, when assessed in a dynamic posture, compared with 60 percent of patients treated with onaBoNT-A (p=0.005).
Two uncontrolled studies reported on aboBoNT-A for the treatment of lateral canthal lines, both with first assessment on Day 7. At this time point, Mahmoud et al29 reported significant reductions from baseline in lateral canthal line severity in a dynamic state (p=0.004) and Fabi et al24 reported a one-grade or greater improvement on a four-point severity scale for lateral canthal lines at maximal contraction and at rest.
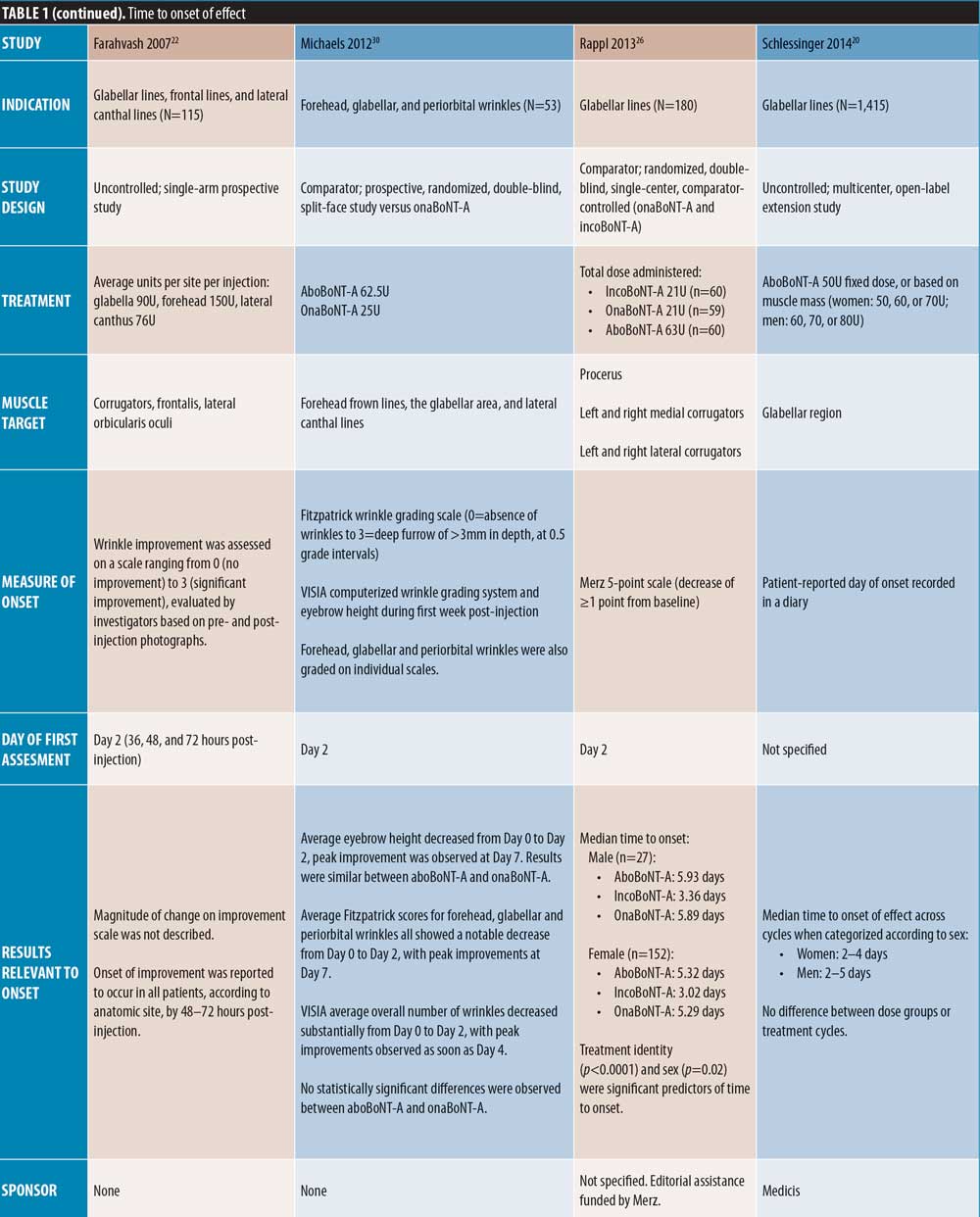
Forehead lines. Of the comparator-controlled studies of aboBoNT-A in forehead lines, one study assessed onset from Day 1 post-injection. Nestor et al11 described onset of effect in patients treated with aboBoNT-A and onaBoNT-A in frontalis lines in terms of initial, full and complete onset (?20%, ?33%, and ?66% change in frontalis height, respectively, assessed at maximum elevation and at rest). Initial onset was achieved by 100 percent of patients by Day 2 for aboBoNT-A and Day 4 for onaBoNT-A, and full onset was achieved by all patients on Day 5 for aboBoNT-A and Day 6 for onaBoNT-A. By Day 6, complete onset had been achieved in 95 percent and 80 percent of patients receiving aboBoNT-A and onaBoNT-A, respectively. At the 24-hour assessment time point, initial, full, and complete onset were achieved in 95 percent, 70 percent, and 10 percent of patients receiving aboBoNT-A compared to 40 percent, 20 percent, and zero percent receiving onaBoNT-A, respectively, although statistical significance was not assessed. In another comparator-controlled study, Karsai et al33 (dose ratio of 3:1) showed similar reductions in frontalis muscle activity compared to baseline for both aboBoNT-A and onaBoNT-A at 3 to 4 days post-injection, with further reductions at Day 7. Furthermore, an uncontrolled study by Heckmann et al36 with first assessment on Day 7 demonstrated a reduction in brow mobility following frontal line injections with aboBoNT-A.
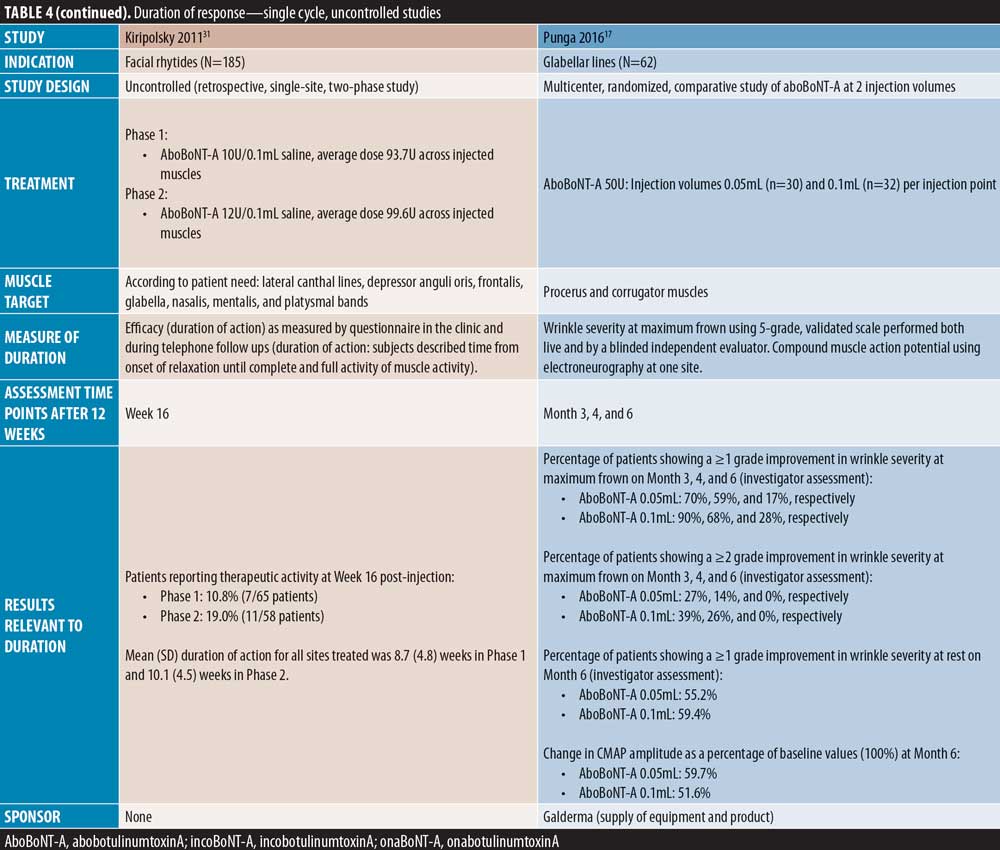
Full-face studies. Three studies were identified that assessed onset of effect of aboBoNT-A globally following injections across multiple injections sites in the upper face. A comparator-controlled split-face study by Michaels et al30 reported a notable decrease in wrinkles in the forehead, glabellar, and periorbital regions by Day 2 post-injection, with peak improvements at Day 7, although no significant difference was observed between aboBoNT-A and onaBoNT-A. In an uncontrolled study by Farahvash et al22 in which patients received injections for glabellar, frontal, and lateral canthal line injections, all patients achieved onset of effect within 48 to 72 hours. In a further uncontrolled study by Kiripolsky et al31, mean time to onset was 3.1 to 3.7 days across different dilutions of aboBoNT-A.
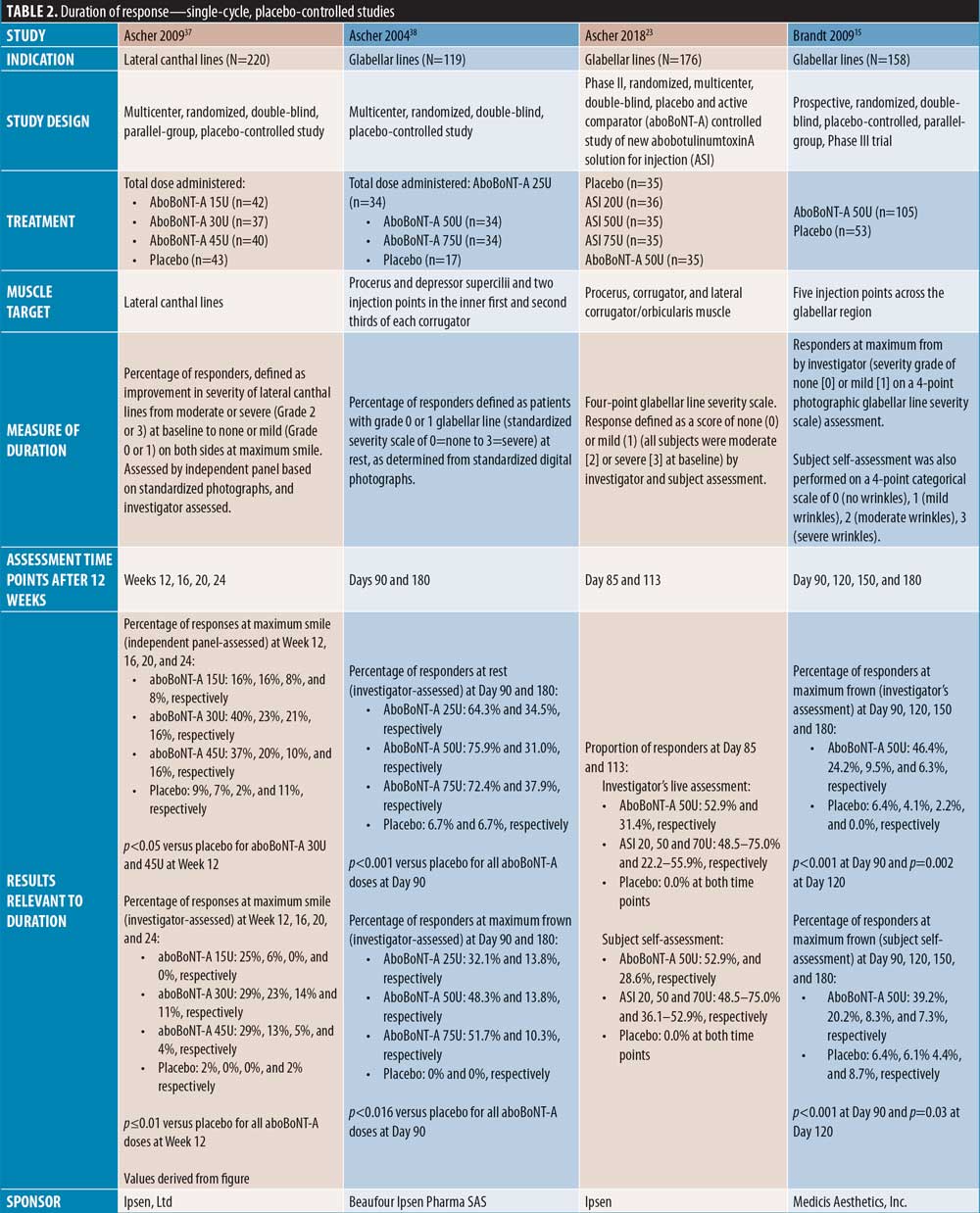
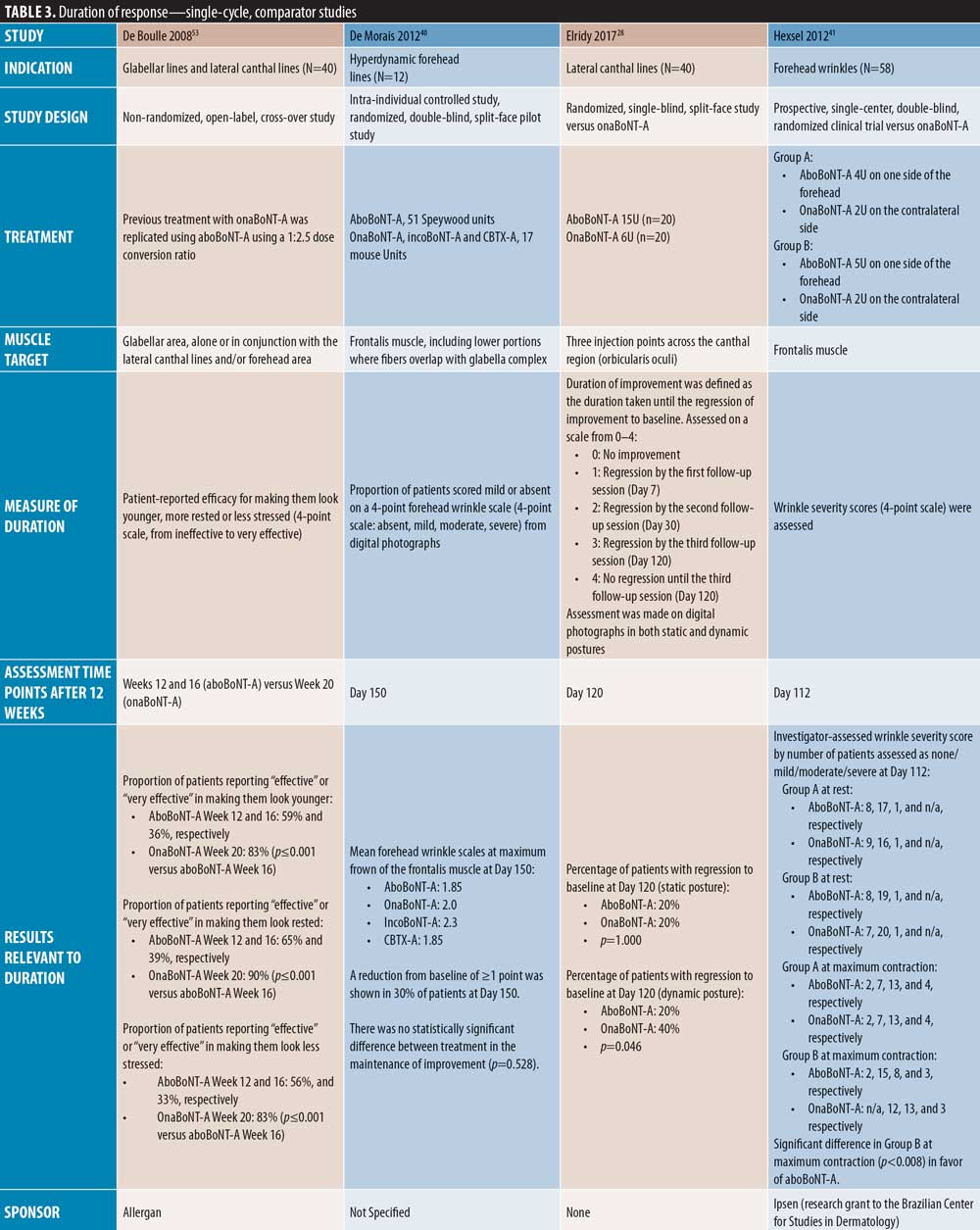
Duration of action of aboBoNT-A. Of the 42 publications identified, 37 assessed the efficacy of aboBoNT-A at time points after the current minimum treatment interval of 12 weeks. Of these publications, seven were single-cycle, placebo-controlled studies (Table 2); 17 were single-cycle comparator-controlled studies (Table 3); nine were single-cycle uncontrolled studies (Table 4); and four were repeat-cycle studies (Table 5). Unless stated otherwise, dose ratios for aboBoNT-A compared to onaBoNT-A or incoBoNT-A were 2.5:1U. It should be noted that study methodologies and methods of determining onset of action differed, and that some studies were conducted at doses higher than those recommended in the product label.
All studies demonstrated efficacy with aboBoNT-A after 12 weeks. As shown in Figure 3, although methods of assessment differed across studies, most studies reported a median duration of effect, or a last time point with 50 percent or more of patients responding to treatment or showing a significant difference versus baseline or placebo at Month 4 in 18 studies, Month 5 in four studies, and Month 6 in three studies. Further studies, detailed below, reported maintained efficacy in some patients at or after Month 6, although criteria defined for inclusion in Figure 3 were not met.
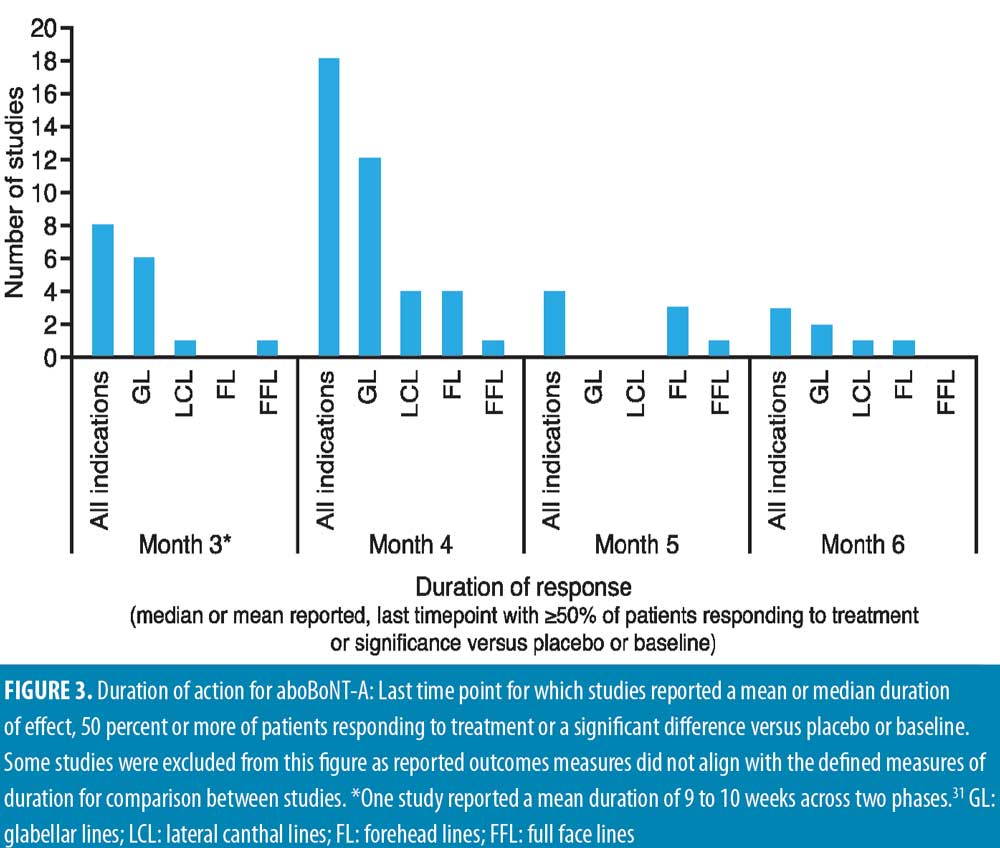
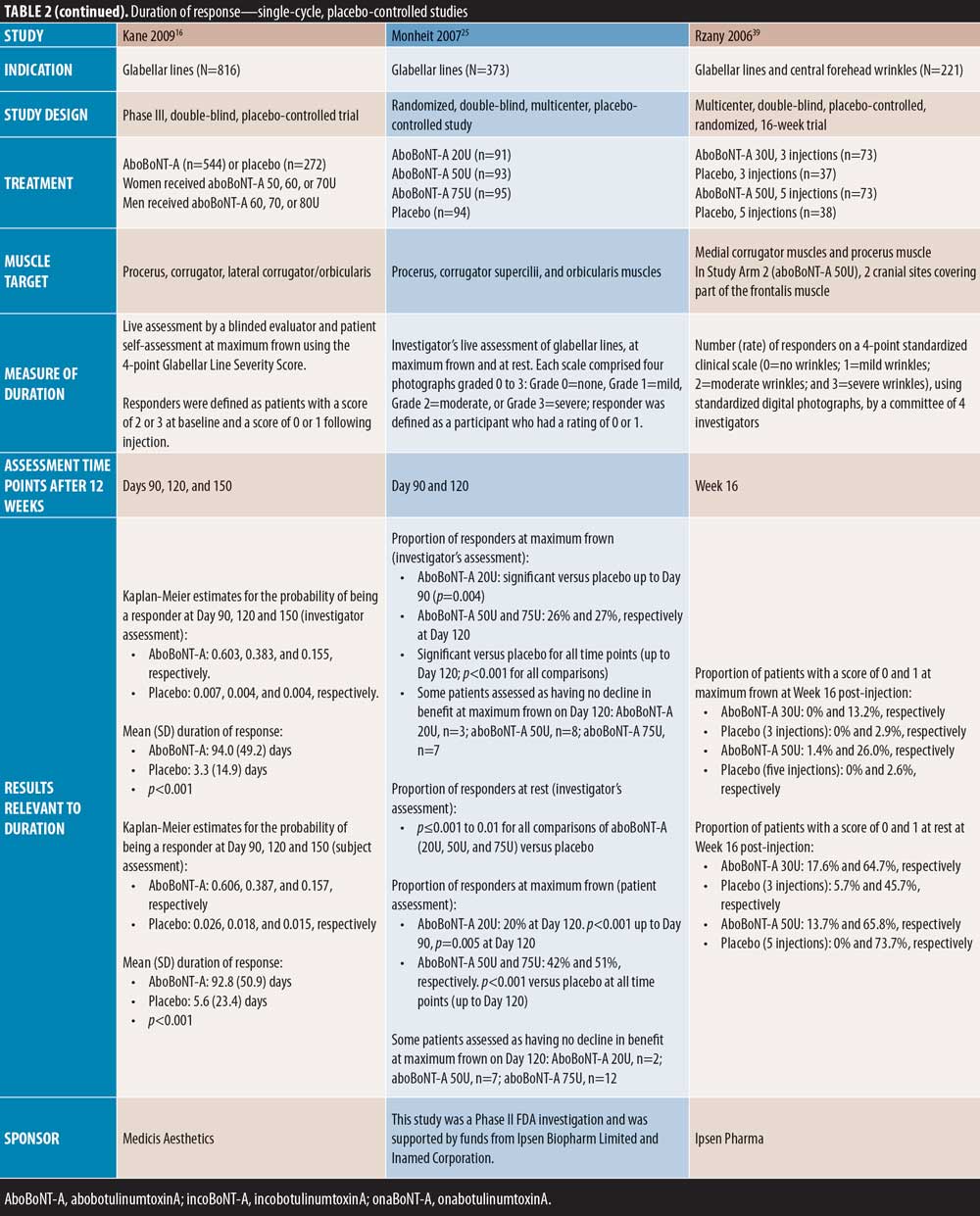
Glabellar lines. A number of single-cycle placebo-controlled studies assessed aboBoNT-A efficacy in glabellar lines at time points after Week 12, thus providing data for duration of action.
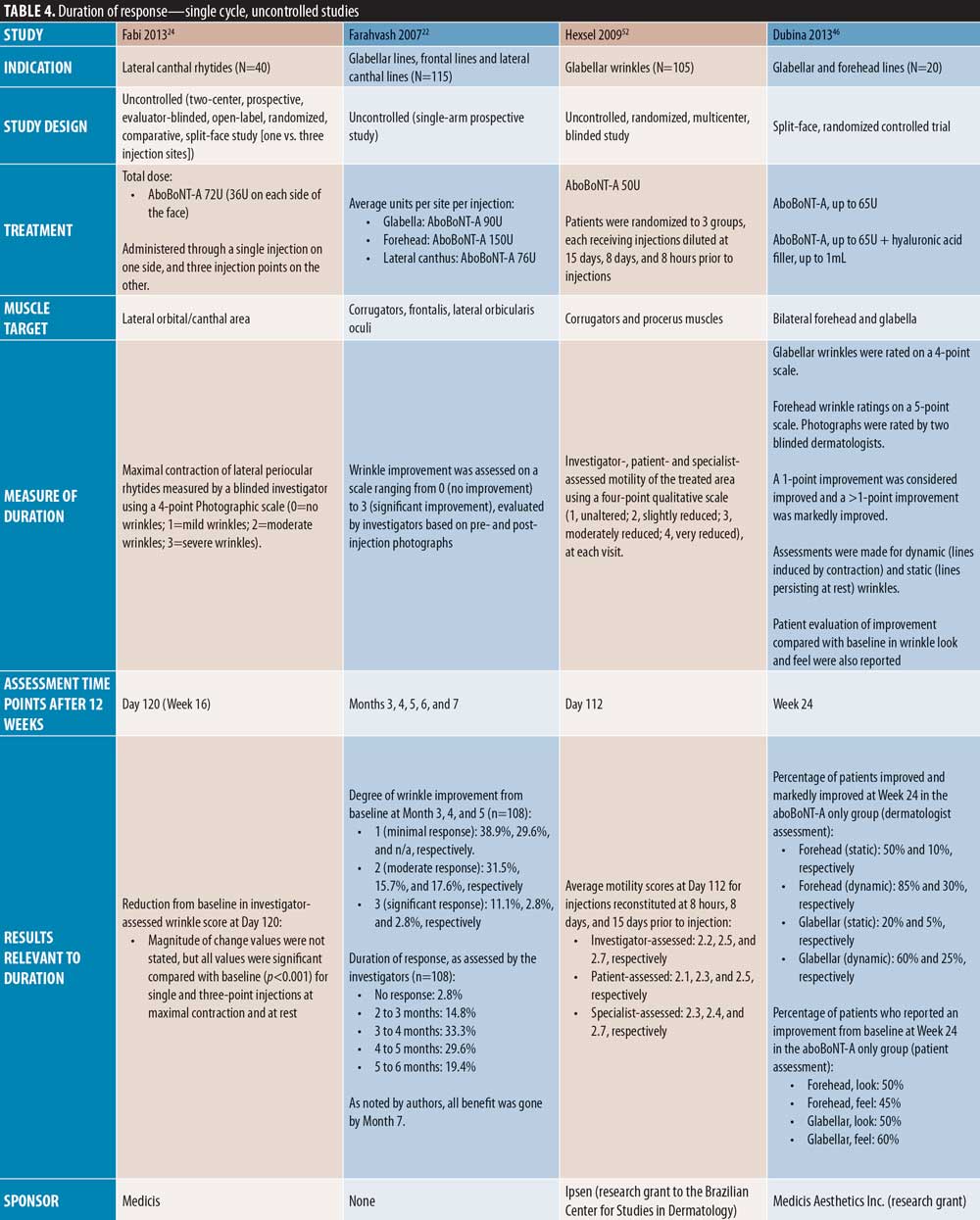
Three placebo-controlled studies had assessments up to around four months and one study up to five months. Ascher et al23 reported maintained efficacy of aboBoNT-A at Day 113 following treatment in 31 percent of patients compared to zero percent in the placebo group; no significance was assessed. The new liquid formulation of aboBoNT-A investigated by Ascher et al 23 showed maintained efficacy at Day 113 in 22 to 56 percent of patients across assessed doses, as described in Table 2. Similarly, Monheit et al25 reported response at maximum frown on Day 120 in 26 percent and 27 percent of patients receiving aboBoNT-A 50U and 75U, respectively (p<0.001 vs. placebo, all time points). A small number of patients in this study (n=3/91, n=8/93, and n=7/95 for 20U, 50U, and 75U, respectively) were assessed to have no decline in benefit (i.e., peak improvement was maintained) at Day 120. Rzany et al39 assessed the efficacy at Week 16 post-injection, and reported a score of no or mild wrinkles at maximum frown in 13 to 26 percent of patients compared to three percent in placebo groups, although no statistical significance was assessed. Furthermore, Kane et al16 reported a significant improvement from baseline with aboBoNT-A compared to placebo up to Day 150 post-injection by both investigator and patient assessment (p=0.004 to 0.0015); the mean duration of response for aboBoNT-A was 94 days and 93 days by investigator and patient assessment, respectively.
Further, in two placebo-controlled studies, aboBoNT-A efficacy was assessed up to approximately six months. Ascher et al38 reported prolonged efficacy up to Day 180, with 31 to 38 percent of patients remaining responders to treatment at rest and 10 to 14 percent at maximum frown, compared with seven percent and zero percent in the respective placebo groups, although differences were no longer significant. Brandt et al15 reported significant differences in the proportion of responders to treatment with aboBoNT-A compared with placebo at Day 120 following treatment, by investigator (p=0.002) and patient (p=0.03) assessment; at Day 180, six percent and seven percent of patients were considered responders by investigator and patient assessment, respectively, although this was not significant versus placebo.
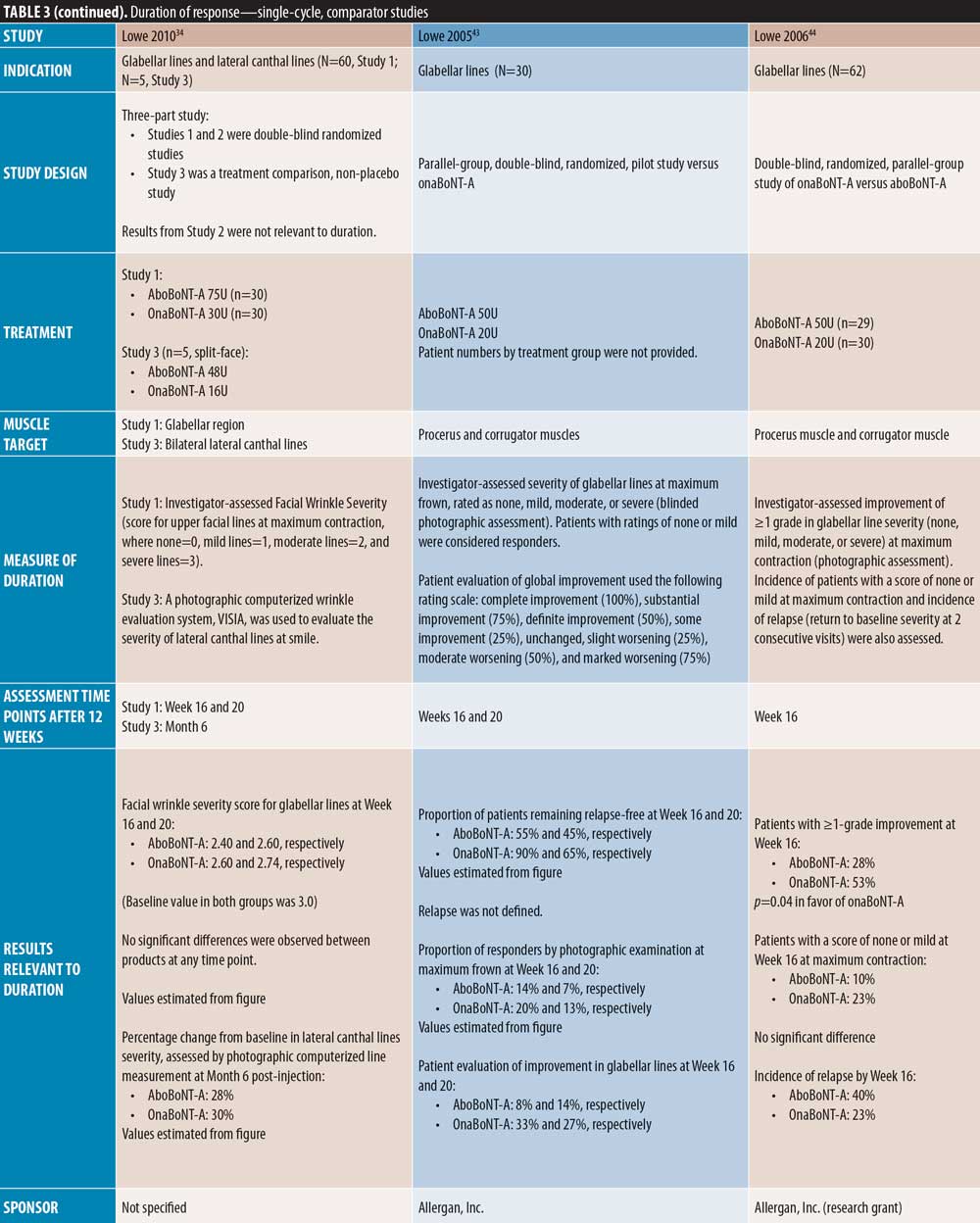
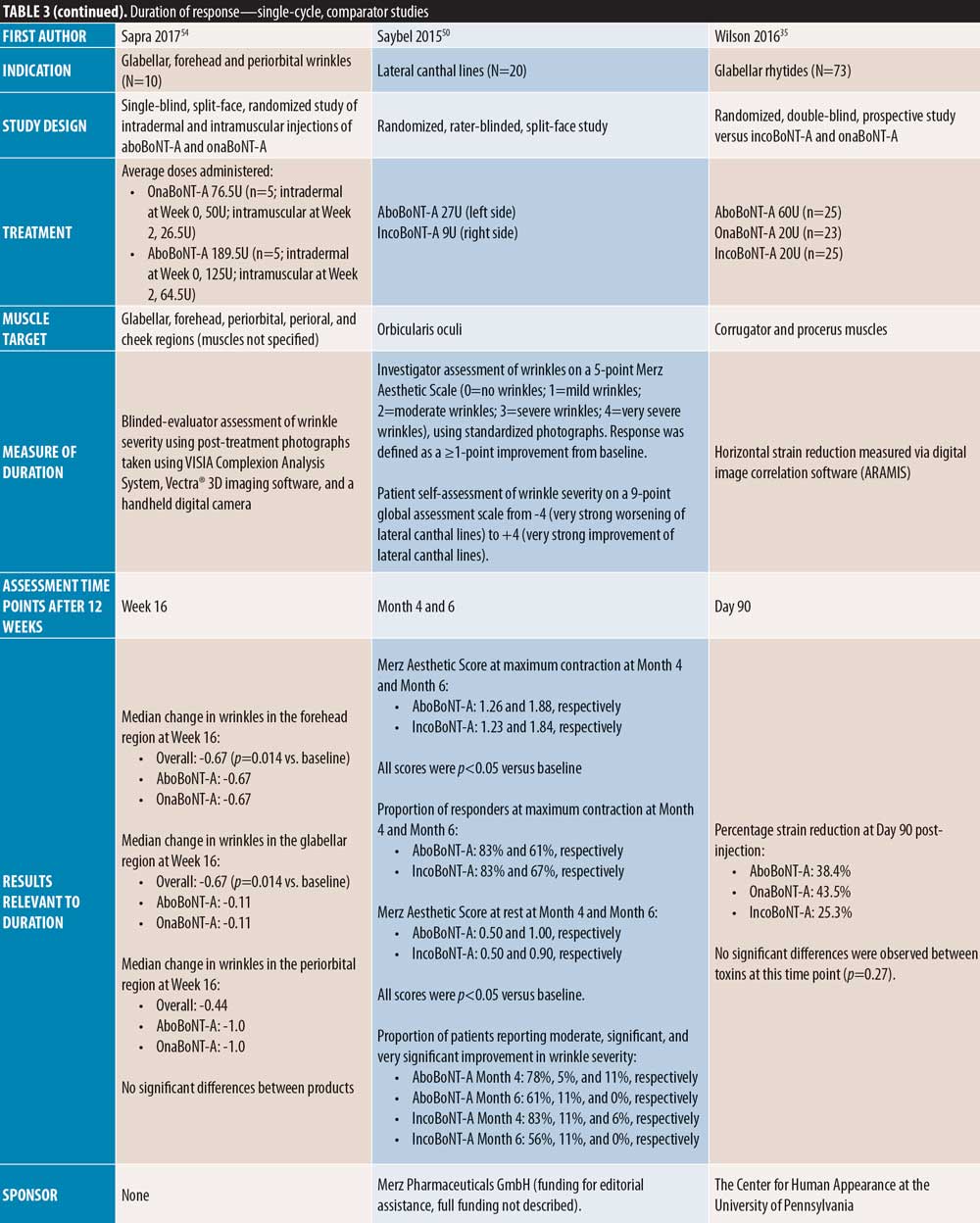
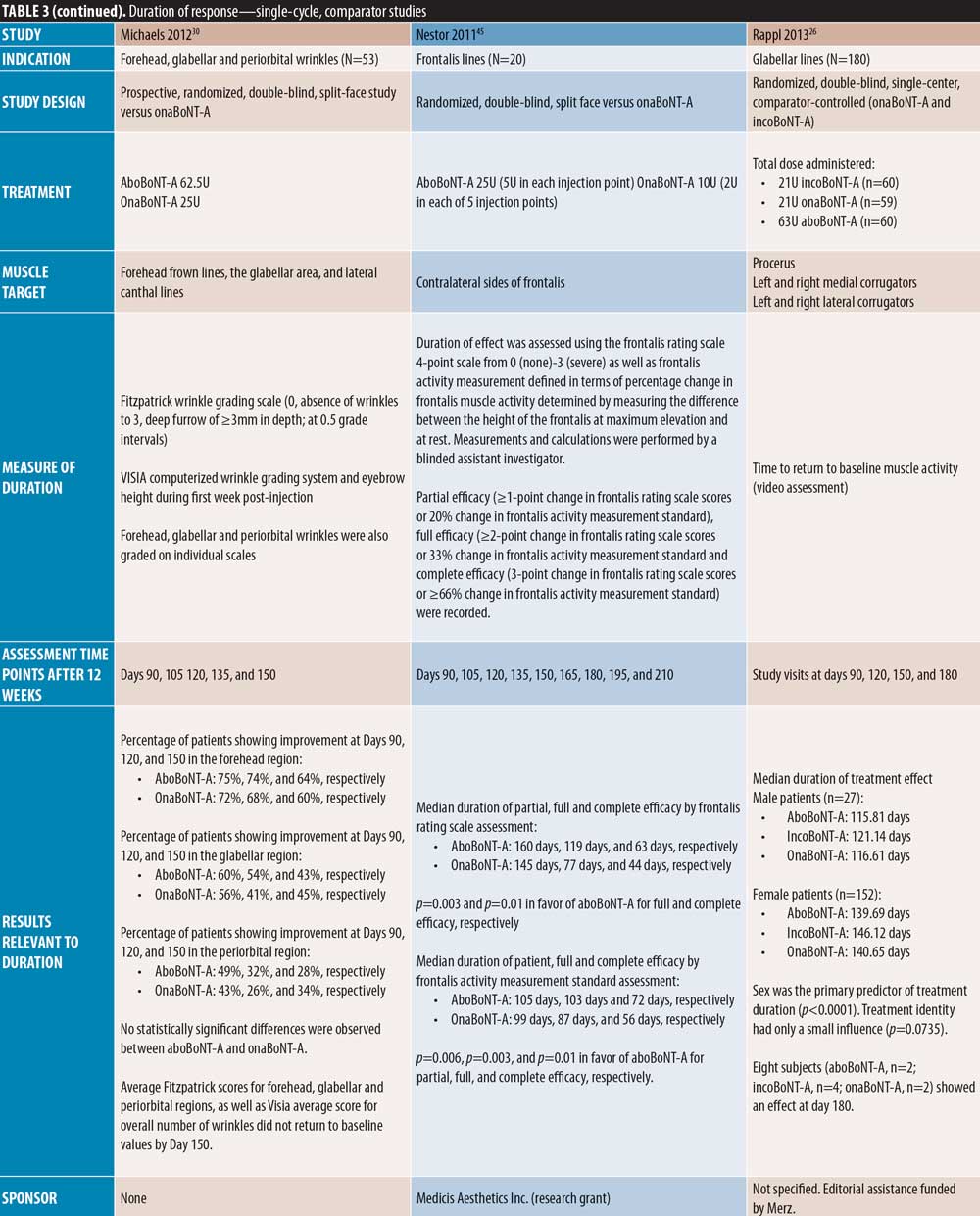
Single-cycle comparator-controlled studies for aboBoNT-A in glabellar lines were identified with assessments up to six months in some studies. Wilson et al35 (dose ratio 3:1:1) reported no significant difference in treatment effect between products at Day 90 following treatment with aboBoNT-A, onaBoNT-A, and incoBoNT-A (p=0.27). Lowe et al34 reported maintained reductions from baseline in severity scores following treatment with aboBoNT-A and onaBoNT-A at Week 16 and Week 20 post-injection, with no significant differences between products at any time point. A study by Lowe et al43 reported sustained efficacy of aboBoNT-A and onaBoNT-A in 55 percent and 90 percent of patients, respectively, at Week 16 following treatment, and in 45 percent and 65 percent of patients, respectively, at Week 20, by investigator-assessment, although significance was not reported. Sapra et al54 reported maintained reductions in wrinkle severity compared to baseline values at Week 16 following treatment with aboBoNT-A and onaBoNT-A (p=0.014 vs. baseline), with no significant difference between products, although patient numbers were low (n=10). Similarly, Michaels et al30 showed maintained improvements in wrinkle severity scales for both aboBoNT-A and onaBoNT-A at Day 150 post-injection (43% and 45% of patients, respectively). Rappl et al26 (dose ratio 3:1:1) showed a long duration of treatment effect with incoBoNT-A (121 and 146 days in men and women, respectively), aboBoNT-A and onaBoNT-A (116 to 117 days and 140 to 141 days in men and women, respectively), although no significance was determined between treatment groups.
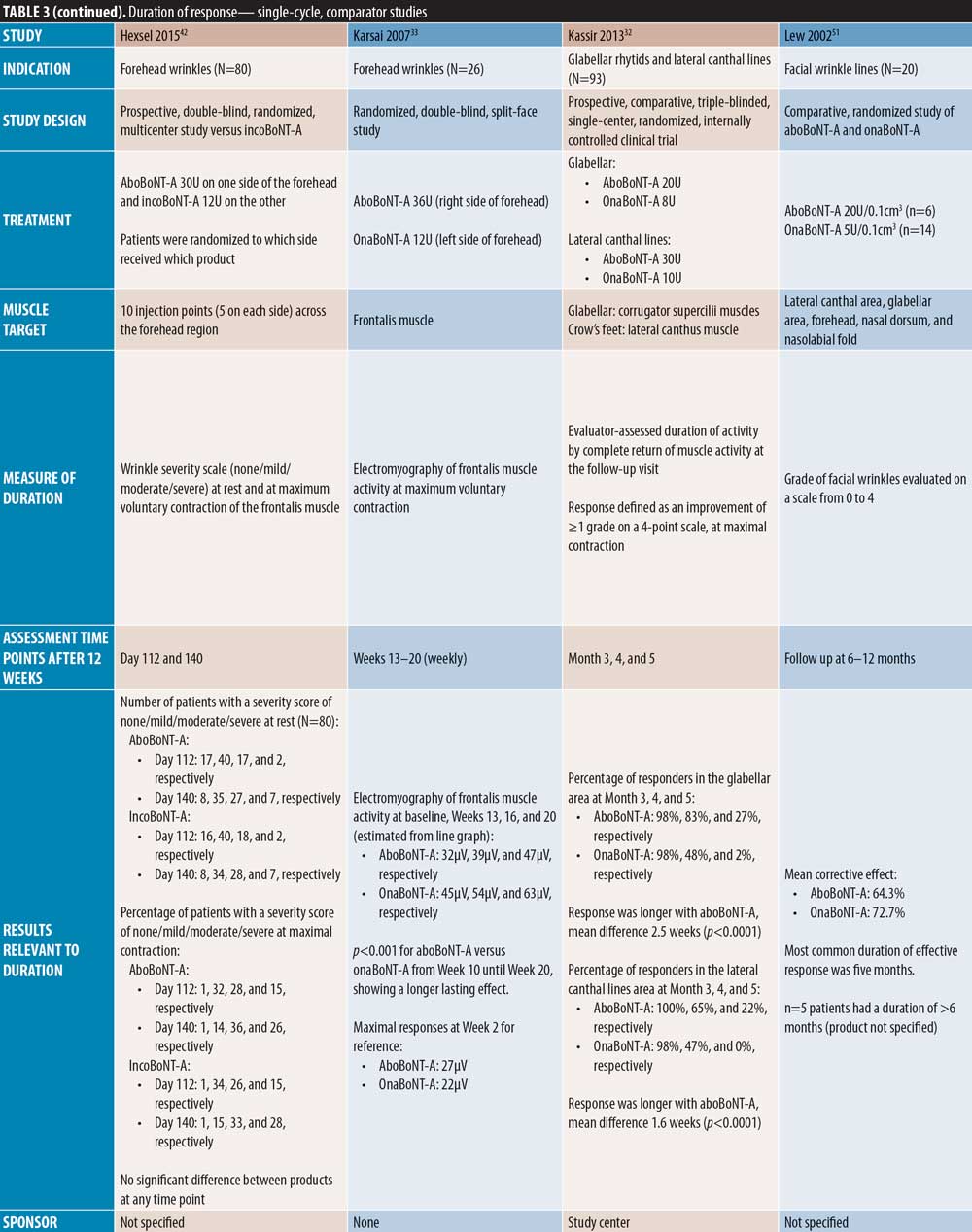
In two single-cycle, comparator-controlled studies, significant differences were observed between BoNT-A products. Lowe et al44 reported a higher proportion of responders to treatment with onaBoNT-A at Week 16 post-injection compared to aboBoNT-A (p=0.04). Additionally, Kassir et al32 demonstrated a significantly longer duration of effect with aboBoNT-A compared with onaBoNT-A (p<0.0001), with a mean difference in duration of response of 2.5 weeks and 27 percent of patients in the aboBoNT-A group remaining responders to treatment at Month 5 post-injection. Furthermore, a non-randomized crossover study by De Boulle,53 in which previous treatment with onaBoNT-A was replicated using aboBoNT-A (dose converted), patients self-reported how effective aboBoNT-A was at making them look younger. Overall, 59 percent of patients reported that aboBoNT-A was “effective” or “very effective” at Week 12 post-injection and 36 percent reported effectiveness at Week 16. These results were compared to onaBoNT-A treatment at Week 20, at which 83 percent of patient-reported treatment was “effective” or “very effective” at making them look younger (p<0.001 vs. aboBoNT-A at Week 16).
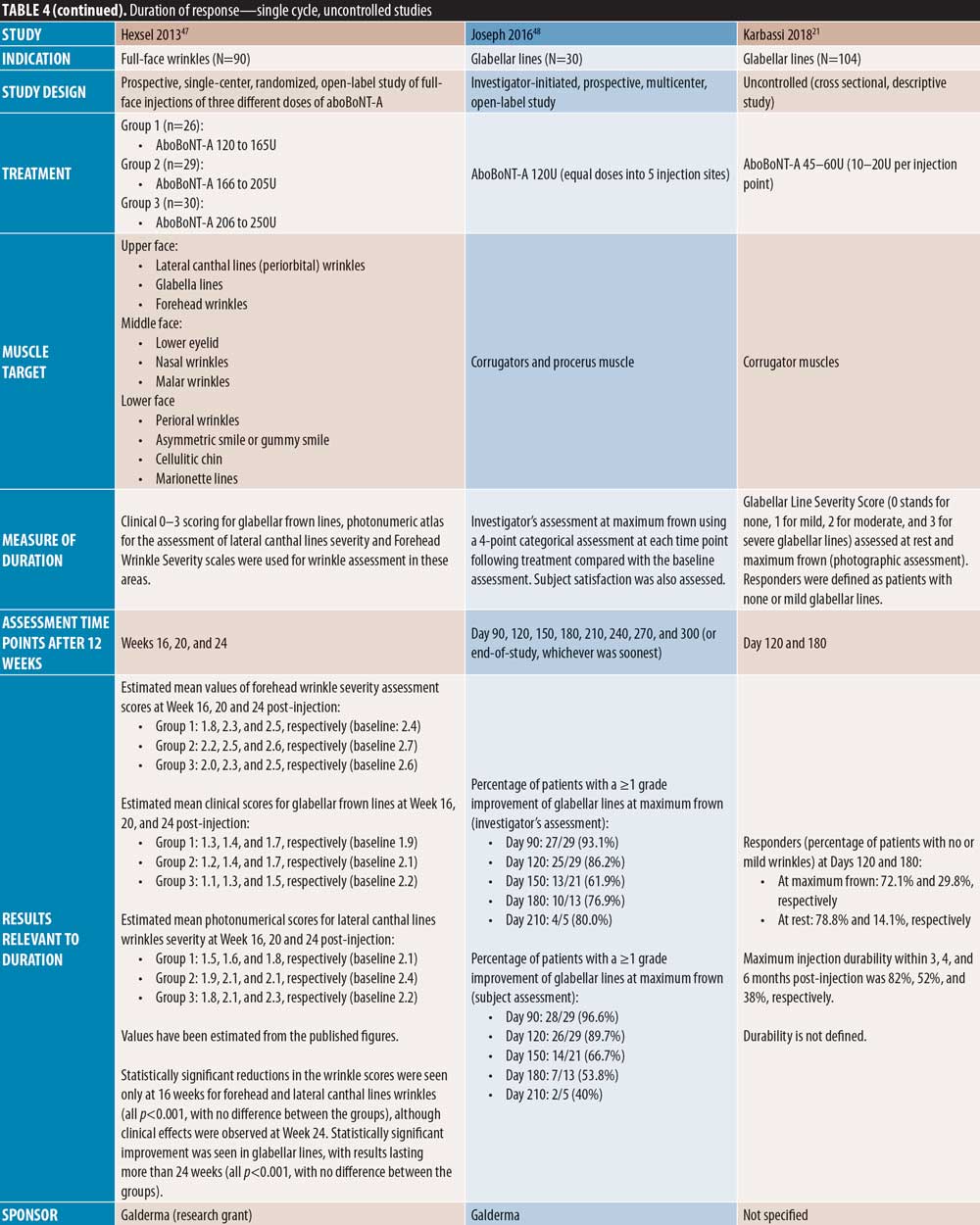
Five single-cycle uncontrolled studies of aboBoNT-A showed efficacy at assessment time points between four and seven months. Hexsel et al52 report scores for slightly to moderately reduced motility in the glabellar region following assessments on Day 112 post-treatment. In a study by Karbassi et al21, the percentage of patients with no or mild glabellar lines at maximum frown was 72 percent at Day 120 and 30 percent at Day 180, as assessed by the investigators. At rest, the proportion of responders was 79 percent at Day 120 and 14 percent at Day 180. Similarly, Punga et al17 reported that 17 to 28 percent of patients were considered responders by investigator assessment at maximum frown at Month 6, and 55 to 59 percent of patients were considered responders at rest. Dubina et al46 reported maintained improvements in wrinkle severity at Week 24 post-injection in 50 percent of patients in a static state and 85 percent in a dynamic state, and another study by Hexsel et al47 also showed significant reductions from baseline in wrinkle severity to Week 24 (p<0.001). Furthermore, Joseph et al48 reported response to treatment with a high dose (120U) of aboBoNT-A in some patients up to Day 210 by both investigator and patient assessment at maximum frown; of the 30 patients assessed, 10 were responders at Day 180 and four at Day 210 by investigator assessment.
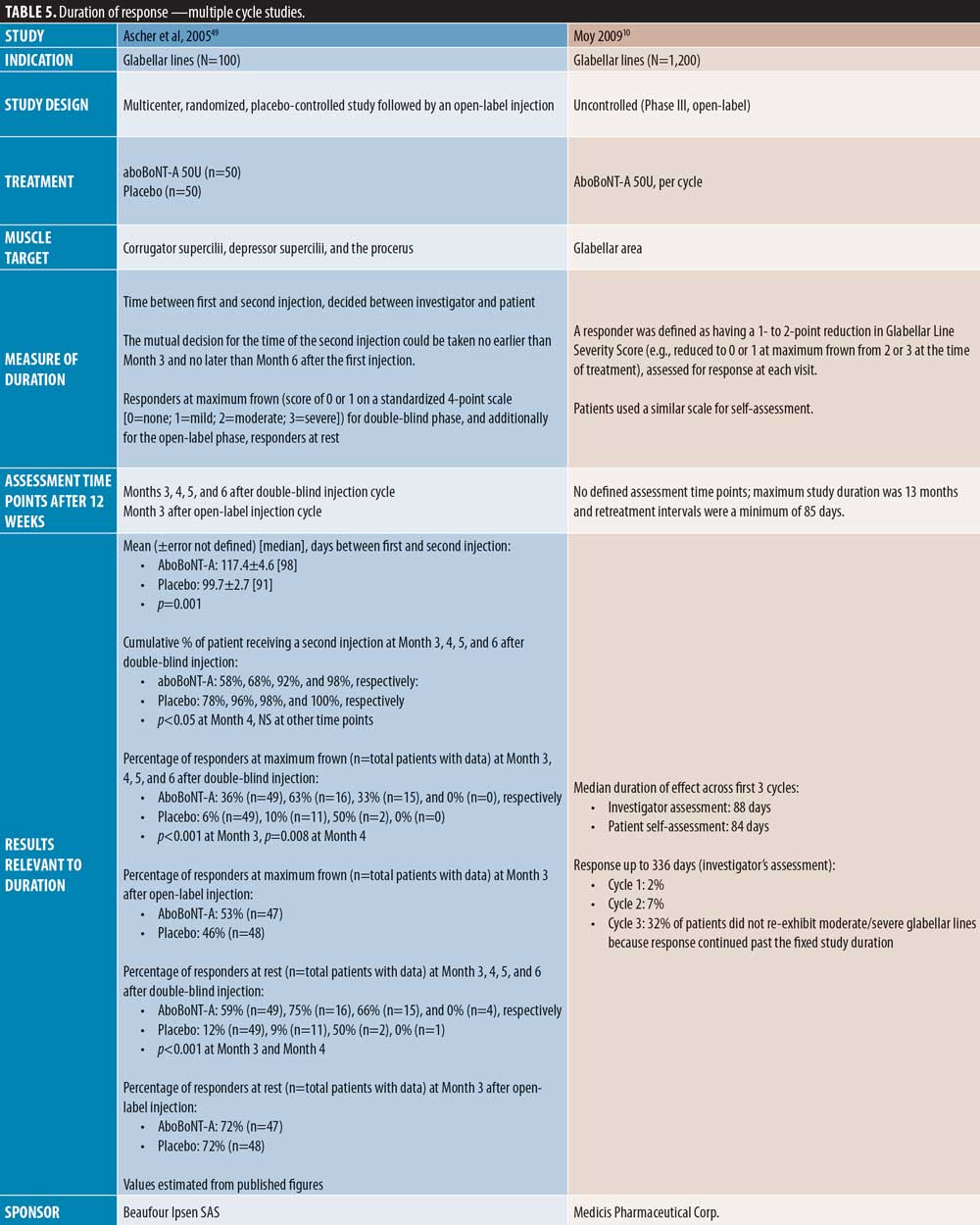
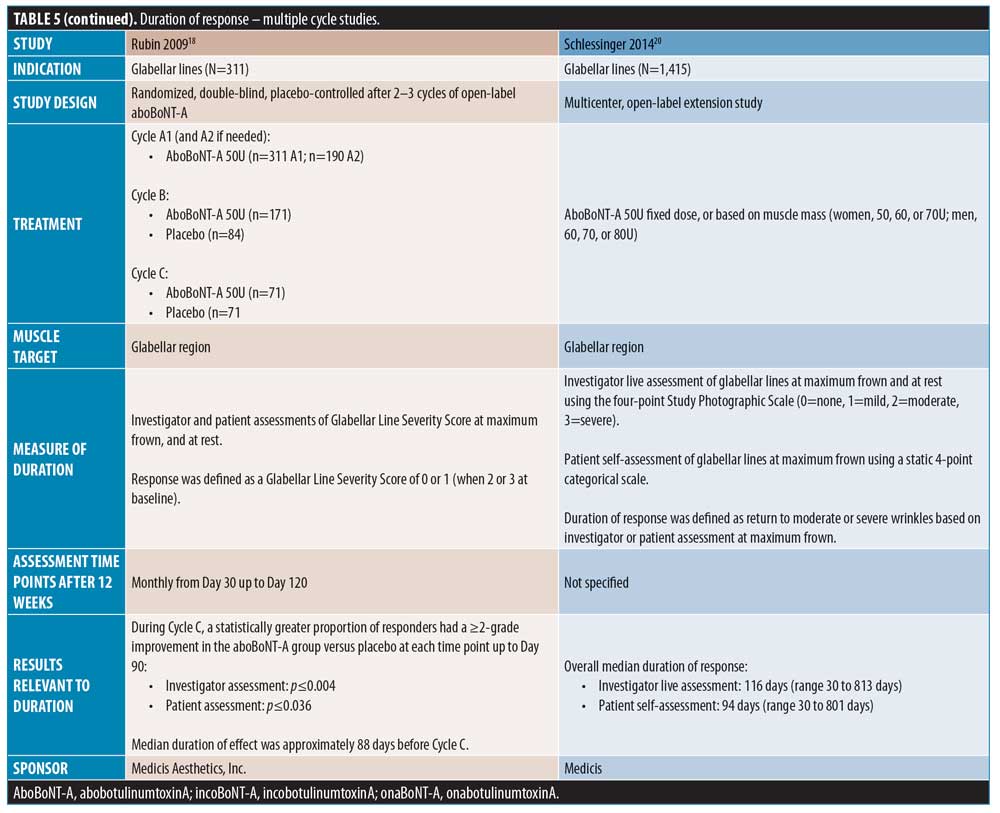
Four studies were identified in which patients received multiple aboBoNT-A injection cycles with efficacy assessments up to six months. As reported by Rubin et al,18 across three open-label injections cycles, the median duration of effect was 88 days and a significantly greater proportion of patients had a two-grade improvement from baseline in the aboBoNT-A group compared to placebo (p?0.004 and p?0.036 by patient and investigator assessment, respectively). Similarly, in a study by Moy et al10, the median duration of effect across the first three treatment cycles was 88 days by investigator assessment and 84 days by patient assessment. However, in Cycles 1 and 2, two percent and seven percent of patients, respectively, were considered by the investigators to show response to treatment up to 336 days. Schlessinger et al20 reported an overall median duration of effect of aboBoNT-A of 116 days by investigator assessment and 94 days by patient self-assessment. Furthermore, in a two-cycle study by Ascher et al,49 the mean time between first and second injection was significantly longer for aboBoNT-A (117 days) compared to placebo (100 days; p=0.001), and 15 of the 98 patients injected during the first injection cycle were considered responders at maximum frown in the aboBoNT-A group at Month 5.
Lateral canthal lines. A single placebo-controlled study of aboBoNT-A for the treatment of lateral canthal lines was identified in which efficacy was assessed up to six months post-injection. Ascher et al37 reported that 16 to 40 percent of patients remained responders to treatment at maximum contraction at Week 12 post-injection, compared with nine percent of patients in the placebo group (p<0.05 for 30U and 45U aboBoNT-A groups). At Week 24 post-injection, 8 to 16 percent of patients were responders to aboBoNT-A treatment, compared to 11 percent of patients in the placebo group (not significant).
Four comparator-controlled studies of aboBoNT-A in lateral canthal lines were identified. In a split-face study by Elridy et al28, 20 percent of patients in both the aboBoNT-A and onaBoNT-A treatment groups showed regression in wrinkle severity to baseline appearance at Day 120 post-injection when assessed in a static posture. However, when assessed in a dynamic posture, significantly fewer patients receiving aboBoNT-A (20%) compared to onaBoNT-A (40 percent) showed regression to baseline wrinkle severity (p=0.046). Kassir et al32 (dose ratio 3:1) demonstrated a significantly longer duration of effect with aboBoNT-A compared to onaBoNT-A (p<0.0001), with a mean difference in duration of response of 1.6 weeks and 22 percent of patients in the aboBoNT-A group remaining responders to treatment at Month 5 post-injection. Lowe et al34 (dose ratio 3:1) described maintained reductions from baseline values in severity scores at Month 6 post-injection in 28 percent and 30 percent of patients receiving aboBoNT-A and onaBoNT-A, respectively, with no significant differences between products due to small sample size (both N=2). Finally, Saybel et al50 (dose ratio 3:1) reported statistically significant differences from baseline up to Month 6 post-injection (p<0.05) in patients receiving aboBoNT-A or incoBoNT-A, assessed at maximum contraction. At Month 6, a high proportion of patients were considered responders to treatment (61% and 67% for aboBoNT-A and incoBoNT-A, respectively).
Additionally, in one uncontrolled study by Fabi et al,24 efficacy was demonstrated at Week 16 compared with baseline (p<0.001) at maximal contraction and at rest, and in another uncontrolled study by Hexsel et al,47 significant reductions from baseline in wrinkle severity were observed up to Week 16 in lateral canthal lines regions, although treatment effects were observed throughout the 24-week study duration.
Forehead lines. A number of comparator-controlled studies were identified that assessed aboBoNT-A for the treatment of forehead lines at time points up to six months. In four studies, no significant difference was observed between comparators. A study by Hexsel et al42 reported a comparable decrease in wrinkle severity at Days 112 and 140 following injection in the forehead region with aboBoNT-A and incoBoNT-A, and Sapra et al54 reported maintained reductions in wrinkle severity compared to baseline values at Week 16 following treatment with aboBoNT-A and onaBoNT-A (p=0.014 vs. baseline, no significant difference between products). At Day 150 post-injection, Michaels et al30 also showed maintained improvements in wrinkle severity scales for both aboBoNT-A and onaBoNT-A (64% and 60% of patients, respectively). Additionally, De Morais et al40 (dose ratio 3:1:1) reported a maintained reduction from baseline of one point or more on a four-point forehead wrinkle scale in 30 percent of patients at Day 150 post-injection in those receiving aboBoNT-A, onaBoNT-A, incoBoNT-A, and Chinese type A botulinum toxin, with mean wrinkle scores of 1.9, 2.0, 2.3, and 1.9, respectively, which were not considered significantly different (p=0.0528).
By contrast, three comparator-controlled studies demonstrated a significantly longer duration with aboBoNT-A compared with onaBoNT-A. A split-face study by Hexsel et al41 that assessed efficacy up to Day 112 showed that significantly more patients had a wrinkle severity score of “none” or “mild” in the forehead region at maximum contraction with aboBoNT-A compared to onaBoNT-A (p<0.008). Karsai et al33 demonstrated a significantly longer-lasting effect with aboBoNT-A compared to onaBoNT-A by electromyography assessment of frontalis muscles activity following treatment at each time point assessed after Week 10 until Week 20 (all p<0.001). Furthermore, Nestor et al45 showed significantly longer median duration of partial, full, and complete efficacy (?20%, ?33%, and ?66% change in the difference between frontalis height at max elevation and at rest, respectively) for aboBoNT-A (160 days, 119 days, and 63 days, respectively) compared to onaBoNT-A (145 days, 77 days, and 44 days, respectively; p=0.003 and 0.01 for full and complete efficacy, respectively).
In one uncontrolled study, Hexsel et al47 reported significant reductions from baseline in wrinkle severity up to Week 16 in forehead lines, although treatment effects were observed throughout the study duration (24 weeks). In another uncontrolled study, Dubina et al46 showed a high proportion of patients with improvement at Week 24 following treatment, as wrinkle severity was improved in 20 percent of patients in a static state and 60 percent and in a dynamic state.
Full-face lines. One comparator-controlled study and two uncontrolled studies reported overall efficacy assessments for aboBoNT-A treatment across multiple injections sites in the upper face. Lew et al51 (dose ratio 4:1) summarized that most patients experienced a duration of effect of five months following treatment with aboBoNT-A or onaBoNT-A, although exact duration was not described by product in order to provide a comparison. Kiripolsky et al31 reported a mean duration of effect of aboBoNT-A of 9 to 10 weeks in patients injected in multiple facial injection sites, as determined by the investigator according to patient need, while 11 to 19 percent of patients remained responders to treatment at Week 16 post-injection. Furthermore, Farahvash et al22 reported that the duration of response of aboBoNT-A across glabellar lines, frontal lines, and lateral canthal lines was assessed by investigators to be 5 to 6 months in 19 percent of patients, and greater than three months in 82 percent of patients.
Discussion
Many studies in this review were designed to gain approval from relevant regulatory agencies for aboBoNT-A use in aesthetic indications, therefore, their primary objective was not focused on demonstrating fast onset or duration of action. Nonetheless, this systematic literature review indicates that aboBoNT-A has a median onset of efficacy of 2 to 3 days, with efficacy observed within 24 hours in some studies, as primarily recorded in patient diaries post-injection, and a longer duration of action (3–6 months across studies) than the current labeled minimum treatment interval (12 weeks).
This review presents evidence that aboBoNT-A efficacy was maintained at or after six months post-injection in some patients.15,17,21,34,37,38,46–48,50 Of these studies, three reported 50 percent or more of patients showing efficacy or statistical significance versus baseline at six months post-injection;46,47,50 in particular, at six months, Saybel et al50 reported 61 percent of patients were responders to treatment (p<0.05 vs. baseline). However, of the placebo-controlled studies showing efficacy at six months, differences versus placebo were not significant at this time point.15,37,38 A Phase III study of a new ready-to-use liquid formulation of aboBoNT-A reported onset within three days in 60 percent of patients and a duration of up to six months in five percent (vs. placebo) by investigator assessment (p=0.0441) and 27 percent (p=0.0036) by patient self-assessment.55 Furthermore, a recent real-world study of satisfaction with twice-yearly aboBoNT-A injections reported that 75 percent of patients were satisfied with aesthetic outcome at six months post-injection and 37 percent had a one-grade improvement from baseline in investigator-assessed glabellar line severity at this time point.56
Onset and duration of action of BoNT-A products can depend on individual patient factors, such as muscles targeted, injection technique, reconstitution method and storage following reconstitution, post-injection procedure, and inter-patient variation in muscle mass, which is influenced by factors such as age and sex.5 As patients age and their skin laxity increases, the interplay between superficial fibers of the corrugator and their insertion into overlying skin might be affected, leading to changes in efficacy in older patients over time.57,58
Field et al8 hypothesized the rationale for prolonged duration of effect with aboBoNT-A. Their study assessed the quantity and light chain activity of BoNT-A in the three main commercially available BoNT-A products: aboBoNT-A, onaBoNT-A, and incoBoNT-A. The mean (±standard deviation) 150kDa BoNT-A content per vial measured by enzyme-linked immunosorbent assay was 2.69ng (±0.03)/500U vial aboBoNT-A, 0.90ng (±0.03)/100U vial onaBoNT-A, and 0.40ng (±0.01)/100U vial incoBoNT-A. For clinical relevance, investigators quantified the amount of 150kDa BoNT-A in FDA-approved glabellar lines doses: 0.27ng aboBoNT-A (50U), 0.18ng onaBoNT-A (20U), and 0.08ng incoBoNT-A (20U); these data were also calculated for therapeutic indications.8 Field et al also assessed enzymatic light chain activity of the three products using an EndoPep assay. This was reported as light chain activity per ng of neurotoxin, and results showed no significant difference between products, meaning that the activity of 150kDa neurotoxin molecules were consistent across products. Together, the results of the Field et al study revealed that a greater amount of 150kDa neurotoxin, and thus a higher concentration of active light chain, are delivered with the FDA-approved dose of aboBoNT-A compared to other currently approved BoNT-A products.
The results of the study by Field et al are reflected in the clinical postulates set out by Nestor et al in their 2017 paper.7 Nestor et al noted firstly that all BoNT-A products act identically, with the same mechanism of action, reflected in the similar light chain activity demonstrated by Field et al.7,8 The second postulate explained that clinical effects of BoNT-A products are dependent on the kinetic relationship between active neurotoxin and receptors at the neuromuscular junction, where molecular potency is defined as the number of active 150kDa molecules available for binding.7 This, in turn, determines the clinical effect of BoNT-A as increased molecular potency, in other words, a greater concentration of active neurotoxin being delivered to target muscles, will allow for a greater amount of bound receptors at the neuromuscular junction between motor neurons and the given muscle.7 This high rate of saturation, as noted in the third postulate by Nestor et al,7 might determine efficacy, rate of onset, and duration of action of the BoNT-A product and could therefore explain the fast onset of response and long duration of effect of aboBoNT-A observed in the clinical studies discussed in this review.
Despite the high quantity of active BoNT-A in aboBoNT-A, it has a safety profile similar to that of other BoNT-A products in terms of incidence rate and type and severity of adverse events.60 Several studies over the past decade have demonstrated the safety and tolerability of aboBoNT-A in aesthetic indications. A systematic review of safety of aboBoNT-A for aesthetic use reported that across the identified Phase III and extension studies in glabellar lines, no serious treatment-emergent adverse events had occurred that were considered treatment-related.61 In particular, Cohen et al62 assessed the long-term safety of repeat treatments with aboBoNT-A in both fixed-unit and variable-dosing settings in an open-label study of 1,415 patients over 24 months. In this study, no new safety issues were identified with repeat cycles of aboBoNT-A; in fact, the incidence of treatment-emergent adverse events remained constant or decreased across repeat treatment cycles, with most adverse events being mild or moderate in severity.62
In clinical practice, retreatment intervals with aboBoNT-A should be considered on an individual patient basis, and, based on the studies discussed in this systematic literature review, as few as two injections per year could be optimal in a number of patients, benefiting both the patient and their physician.
Acknowledgments
The authors thank Watermeadow Medical, an Ashfield Company, for their support in developing the systematic literature review.
The authors thank Jacqueline Harte BSc (Hons), of Watermeadow Medical, an Ashfield Company, for providing medical writing support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines.
References
- Electronic Medicines Compendium site. Azzalure Summary of Product Characteristics. 2019. https://www.medicines.org.uk/emc/product/6584/smpc. Accessed 06 Nov 2020.
- Ipsen Biopharm Ltd site. Dysport Full Prescribing Information. 2017. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2020/01/09195739/S115_2019_09_25_sBLA_Approval_PMR_Fulfilled_PI_MG_Sept-2019.pdf. Accessed 06 Nov 2020.
- Sundaram H, Huang P-H, Hsu N-J, et al. Aesthetic applications of botulinum toxin A in Asians: an international, multidisciplinary, pan-Asian consensus. Plastic and reconstructive surgery Global open. 2016;4(12):e872–e872.
- Maas C, Kane MA, Bucay VW, et al. Current aesthetic use of abobotulinumtoxinA in clinical practice: An evidence-based consensus review. Aesthet Surg J. 2012;32(1 Suppl):8S–29S.
- Nestor M, Ablon G, Pickett A. Key parameters for the use of abobotulinumtoxinA in aesthetics: onset and duration. Aesthet Surg J. 2017;37(1 Suppl):S20–S31.
- Frevert J. Content of botulinum neurotoxin in botox(R)/vistabel(R), dysport(R)/azzalure(R), and xeomin(R)/bocouture(R). Drugs R D. 2010;10(2):67–73.
- Nestor MS, Kleinfelder RE, Pickett A. The use of botulinum neurotoxin Type A in aesthetics: Key clinical postulates. Dermatologic Surgery. 2017;43(&NA;):S344–S362.
- Field M, Splevins A, Picaut P, et al. AbobotulinumtoxinA (dysport((R))), onabotulinumtoxinA (botox((R))), and incobotulinumtoxinA (xeomin((R))) neurotoxin content and potential implications for duration of response in patients. Toxins (Basel). 2018;10(12).
- Rzany B, Ascher B, Monheit G. Treatment of glabellar lines with botulinum toxin type A (Speywood unit): a clinical overview. J Eur Acad Dermatol Venereol. 2010;24 (Suppl 1):1–14.
- Moy R, Maas C, Monheit G, Huber MB, Reloxin investigational G. Long-term safety and efficacy of a new botulinum toxin type A in treating glabellar lines. Arch Facial Plast Surg. 2009;11(2):77–83.
- Nestor MS, Ablon GR. Comparing the clinical attributes of abobotulinumtoxinA and onabotulinumtoxinA utilizing a novel contralateral Frontalis model and the Frontalis Activity Measurement Standard. J Drugs Dermatol. 2011;10(10):1148–1157.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893–1901.
- Kane MA, Brandt F, Rohrich RJ, et al. Evaluation of variable-dose treatment with a new U.S. Botulinum Toxin Type A (Dysport) for correction of moderate to severe glabellar lines: Results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619–1629.
- Punga AR, Alimohammadi M, Fagrell D, et al. A randomized, comparative study to evaluate efficacy and safety of two injection volumes of abobotulinumtoxinA in treatment of glabellar lines. Dermatol Surg. 2016;42(8):967–976.
- Rubin MG, Dover J, Glogau RG, et al. The efficacy and safety of a new U.S. Botulinum toxin type A in the retreatment of glabellar lines following open-label treatment. J Drugs Dermatol. 2009;8(5):439–444.
- Schlessinger J, Monheit G, Kane MA, Mendelsohn N. Time to onset of response of abobotulinumtoxina in the treatment of glabellar lines: a subset analysis of phase 3 clinical trials of a new botulinum toxin type A. Dermatol Surg. 2011;37(10):1434–1442.
- Schlessinger J, Dover JS, Joseph J, et al. Long-term safety of abobotulinumtoxinA for the treatment of glabellar lines: results from a 36-month, multicenter, open-label extension study. Dermatol Surg. 2014;40(2):176–183.
- Karbassi E, Nakhaee N, Zamanian M. The efficacy and complications of a new technique of abobotulinum-toxin A (Dysport) injection in patients with glabellar lines. J Cosmet Dermatol. 2019 Feb;18(1):55–58.
- Farahvash MR, Arad S. Clostridium botulinum type A toxin for the treatment of upper face animation lines: an Iranian experience. J Cosmet Dermatol. 2007;6(3):152–158.
- Ascher B, Kestemont P, Boineau D, et al. Liquid formulation of abobotulinumtoxinA exhibits a favorable efficacy and safety profile in moderate to severe glabellar lines: a randomized, double-blind, placebo- and active comparator-controlled trial. Aesthet Surg J. 2018;38(2):183–191.
- Fabi SG, Sundaram H, Guiha I, Goldman MP. A two-center, open-label, randomized, split-face study to assess the efficacy and safety of one versus three intradermal injection sites of abobotulinumtoxinA in the treatment of lateral periocular rhytides. J Drugs Dermatol. 2013;12(8):932–937.
- Monheit G, Carruthers A, Brandt F, Rand R. A randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal dose. Dermatol Surg. 2007;33(s1): S51–S59.
- Rappl T, Parvizi D, Friedl H, et al. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double-blind study. Clin Cosmet Investig Dermatol. 2013;6:211–219.
- Yu KC, Nettar KD, Bapna S, Boscardin WJ, Maas CS. Split-face double-blind study comparing the onset of action of onabotulinumtoxinA and abobotulinumtoxinA. Arch Facial Plast Surg. 2012;14(3):198–204.
- Elridy AS, Zaki RGE, Elshinawy RF. Comparison of the clinical efficacy of abobotulinumtoxinA (ABO) and onabotulinumtoxin A (ONA) in the treatment of crow’s feet wrinkles: a split-face study. Semin Ophthalmol. 2017:1–9.
- Mahmoud BH, Ozog D, Burnett C, Cohen JL. Prospective randomized split-face comparative study between topical botulinum toxin a surface application and local injection for crow’s feet. Dermatologic Surgery. 2016;42(4):554–556.
- Michaels BM, Csank GA, Ryb GE, Eko FN, Rubin A. Prospective randomized comparison of onabotulinumtoxinA (Botox) and abobotulinumtoxinA (dysport) in the treatment of forehead, glabellar, and periorbital wrinkles. Aesthet Surg J. 2012;32(1):96–102.
- Kiripolsky MG, Peterson JD, Guiha I, Goldman MP. A two-phase, retrospective analysis evaluating efficacy of and patient satisfaction with abobotulinumtoxina used to treat dynamic facial rhytides. Dermatol Surg. 2011;37(10): 1443–1447.
- Kassir R, Kolluru A, Kassir M. Triple-blind, prospective, internally controlled comparative study between abobotulinumtoxinA and onabotulinumtoxinA for the treatment of facial rhytids. Dermatol Ther (Heidelb). 2013;3(2): 179–189.
- Karsai S, Adrian R, Hammes S, Thimm J, Raulin C. A randomized double-blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic activity. Arch Dermatol. 2007;143(11):1447–1449.
- Lowe NJ, Shah A, Lowe PL, Patnaik R. Dosing, efficacy and safety plus the use of computerized photography for botulinum toxins type A for upper facial lines. J Cosmet Laser Ther. 2010;12(2):106–111.
- Wilson AJ, Chang B, Taglienti AJ, et al. A quantitative analysis of onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA: A randomized, double-blind, prospective clinical trial of comparative dynamic strain reduction. Plast Reconstr Surg. 2016;137(5):1424–1433.
- Heckmann M, Schön-Hupka G. Quantification of the efficacy of botulinum toxin type A by digital image analysis. J Am Acad Dermatol. 2001;45(4):508–514.
- Ascher B, Rzany BJ, Grover R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow’s feet: double-blind, placebo-controlled, dose-ranging study. Dermatol Surg. 2009;35(10):1478–1486.
- Ascher B, Zakine B, Kestemont P, et al. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol. 2004;51(2):223–233.
- Rzany B, Ascher B, Fratila A, et al. Efficacy and safety of 3-and 5-injection patterns (30 and 50 U) of botulinum toxin A (dysport) for the treatment of wrinkles in the glabella and the central forehead region. Arch Dermatol. 2006;142(3):320–326.
- Oliveira de Morais O, Matos Reis-Filho E, Vilela Pereira L, Martins Gomes C, Alves G. Comparison of four botulinum neurotoxin type A preparations in the treatment of hyperdynamic forehead lines in men: a pilot study. J Drugs Dermatol. 2012;11(2):216–219.
- Hexsel D, Brum C, do Prado DZ, et al. Field effect of two commercial preparations of botulinum toxin type A: a prospective, double-blind, randomized clinical trial. J Am Acad Dermatol. 2012;67(2):226–232.
- Hexsel D, Soirefmann M, Porto MD, et al. Fields of muscular and anhidrotic effects of 2 botulinum toxin-A commercial preparations: a prospective, double-blind, randomized, multicenter study. Dermatol Surg. 2015;41 Suppl 1:S110–118.
- Lowe PL, Patnaik R, Lowe NJ. A comparison of two botulinum type a toxin preparations for the treatment of glabellar lines: double-blind, randomized, pilot study. Dermatol Surg. 2005;31(12):1651–1654.
- Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized study. J Am Acad Dermatol. 2006;55(6):975–980.
- Nestor MS, Ablon GR. Duration of action of abobotulinumtoxina and onabotulinumtoxina: a randomized, double-blind study using a contralateral frontalis model. J Clin Aesthet Dermatol. 2011;4(9):43–49.
- Dubina M, Tung R, Bolotin D, et al. Treatment of forehead/glabellar rhytide complex with combination botulinum toxin A and hyaluronic acid versus botulinum toxin a injection alone: a split-face, rater-blinded, randomized control trial. J Cosmet Dermatol. 2013;12(4):261–266.
- Hexsel D, Brum C, Porto MD, et al. Full-face injections of variable total doses of abobotulinum toxin type A: a randomized, phase IV clinical trial of safety and efficacy. J Drugs Dermatol. 2013;12(12):1356–1362.
- Joseph JH, Eaton LL, Robinson J, Pontius A, Williams EF, III. Does increasing the dose of abobotulinumtoxinA impact the duration of effectiveness for the treatment of moderate to severe glabellar lines? J Drugs Dermatol. 2016;15(12):1544–1549.
- Ascher B, Zakine B, Kestemont P, et al. Botulinum toxin A in the treatment of glabellar lines: Scheduling the next injection. Aesthet Surg J. 2005;25(4):365–375.
- Saybel A, Artemenko A, Nikitin S, Kurenkov A. A prospective, neurophysiologic comparative study to assess the efficacy and duration of effect of incobotulinumtoxinA and abobotulinumtoxinA in the treatment of crow’s feet. J Drugs Dermatol. 2015;14(11):1291–1296.
- Lew H, Yun Ys Fau – Lee SY, Lee Sy Fau – Kim SJ, Kim SJ. Effect of botulinum toxin A on facial wrinkle lines in Koreans. Opthalmologica. 2002;216(1):50–54.
- Hexsel D, Rutowitsch MS, De Castro LCM, Do Prado DZ, Lima MM. Blind multicenter study of the efficacy and safety of injections of a commercial preparation of botulinum toxin type a reconstituted up to 15 days before injection. Dermatologic Surgery. 2009;35(6):933–939.
- De Boulle K. Patient satisfaction with different botulinum toxin type A formulations in the treatment of moderate to severe upper facial rhytids. J Cosmet Laser Ther. 2008;10(2):87–92.
- Sapra P, Demay S, Sapra S, et al. A single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin A formulas to reduce signs of facial aging. J Clin Aesthet Dermatol. 2017;10(2):34–44.
- Ascher B, Rzany B, Kestemont P, et al. Liquid formulation of abobotulinumtoxinA: A 6-month, phase 3, double-blind, randomized, placebo-controlled study of a single treatment, ready-to-use toxin for moderate-to-severe glabellar lines. Aesthet Surg J. 2020;40(1):93–104.
- Shamban A, Cohen J, Schlessinger J, et al. Subject satisfaction with a twice-yearly retreatment schedule for abobotulinumtoxinA-interim results from a multi-center study. Presented at the 22nd International Master Course on Aging Science World Congress. 2020 Jan 30–Feb 1;Abstract 99884; available from: https://www.imcas.com/en/attend/imcas-world-congress-2020/program/session/52324. Accessed 5 Dec 2020.
- Khavkin J, Ellis DAF. Aging skin: histology, physiology, and pathology. Facial Plast Surg Clin North Am. 2011;19(2):229–234.
- Cheng CM. Cosmetic use of botulinum toxin type A in the elderly. Clin Interv Aging. 2007;2(1): 81–83.
- Bertucci V, Solish N, Kaufman-Janette J, et al. DaxibotulinumtoxinA for injection has a prolonged duration of response in the treatment of glabellar lines: pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). J Am Acad Dermatol. 2020;82(4): 838–845.
- Cavallini M, Cirillo P, Fundaro SP, et al. Safety of botulinum toxin A in aesthetic treatments: a systematic review of clinical studies. Dermatol Surg. 2014;40(5):525–536.
- Cohen JL, Scuderi N. Safety and patient satisfaction of abobotulinumtoxinA for aesthetic use: A systematic review. Aesthet Surg J. 2017;37(Suppl 1):S32–S44.
- Cohen JL, Schlessinger J, Cox SE, Lin X, Reloxin Investigational G. An analysis of the long-term safety data of repeat administrations of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6 Suppl):S43–S49.