J Clin Aesthet Dermatol. 2018;11(9):28–31
by James Del Rosso, DO; Neil Swanson, MD; Brian Berman, MD, PhD; George M. Martin, MD; Tina Lin, PharmD; and Ted Rosen, MD
Dr. Del Rosso is with JDR Dermatology Research/Thomas Dermatology in Las Vegas, Nevada. Dr. Swanson is with the Department of Dermatology at Oregon Health and Science University in Portland, Oregon. Dr. Berman is with the Department of Dermatology and Cutaneous Surgery at the Miller School of Medicine, University of Miami in Miami, Florida and the Center for Clinical and Cosmetic Research in Aventura, Florida. Dr. Martin is with the Dermatology and Laser Center of Maui in Maoi, Hawaii. Dr. Lin is with Valeant Pharmaceuticals in Bridgewater, New Jersey. Dr. Rosen is with the Department of Dermatology at Baylor College of Medicine in Houston, Texas.
Funding: The original studies were funded by Graceway Pharmaceuticals LLC. The meta-analysis was funded by Ortho Dermatologics (Raleigh, North Carolina), a division of Valeant Pharmaceuticals International Inc. The authors were not compensated for the preparation of this manuscript.
Disclosures: All authors are investigators, speakers, and/or consultants for Ortho Dermatologics.
Abstract: Background. Ad-hoc reports within clinical studies of imiquimod for the treatment of actinic keratosis (AK) have suggested the drug can improve both skin texture and overall signs of photodamage.
Objective.We sought to assess the efficacy and tolerability of imiquimod 3.75% and 2.5% cream for the treatment of photodamage in patients with AK of the full face or balding scalp.
Methods.A meta-analysis of four identical multicenter, randomized, double-blind, vehicle-controlled studies was conducted. The studies included a total of 969 adult subjects (aged 33–91 years) with 5 to 20 visible lesions or palpable AKs in an area exceeding 25cm2 on either the face or balding scalp. Patients were randomized to imiquimod 3.75%, imiquimod 2.5%, or vehicle cream (1:1:1). Up to two packets (250mg each) were applied per dose once daily for two two-week treatment cycles, separated by a two-week no-treatment interval. Photodamage improvement was assessed at study end based on subjects’ baseline assessments using a seven-point scale. Local skin reactions were recorded throughout the study.
Results.Combined Investigator’s Global Integrated Photodamage (IGIP) score was “significantly” or “much” improved in 57.6 percent (n=175) of patients treated with imiquimod 2.5% cream and in 69.6 percent (n=208) of patients treated with imiquimod 3.75% cream versus in 25.7 percent (n=76) of patients treated with the vehicle. Mean IGIP scores at end of study were 1.67, 1.98, and 0.73, respectively (both actives P<0.0001 versus vehicle).
Conclusion.Both imiquimod 2.5% and 3.75% creams showed a positive effect on photodamage when compared with the vehicle cream.
Keywords: Imiquimod, photodamage, meta-analysis, topical, actinic keratosis
Introduction
Photodamage of the skin occurs as a consequence of chronic or repeated exposure to sunlight. It is characterized by increased tactile roughness; dyspigmentation; sallowness; laxity; deep, coarse wrinkles; and telangiectasia.1 Histological changes include increased epidermal thickness or pronounced epidermal atrophy, increased melanogenesis, progressive degeneration and disorganization of dermal collagen and fibrillin, and the deposition of abnormal elastic tissue.2
Wrinkles and telangiectasia are associated with increased risks of actinic keratosis (AK) and nonmelanoma skin cancer. As a result, it is recommended that individuals exhibiting substantial photoaging should be examined periodically for AK and skin cancers.3
Topical imiquimod is well-established in many clinical trials as a therapy for the eradication of AKs.4–9 However, there exists only limited evidence demonstrating the use of topical imiquimod as an antiaging treatment because its impact on treating photodamage has not been formally studied.
An open-label study of daily application of 5% imiquimod for three months indicated its potential use as an antiaging treatment in chronically sun damaged skin associated with lentigo maligna in 26 patients.10 Following daily application for three months, there was a significant increase in dermal fibroplasia, an associated reduction in solar elastosis, and a restoration of normal epidermal thickness.
In addition, topical 5% imiquimod treatment of chronically sun-exposed and eventually photodamaged skin without overt clinical signs for AK has been shown to detect and potentially clear early (subclinical) lesions before they can be clinically diagnosed as AK.11 Another small, open-label study of 10 women with moderate signs of facial photodamage demonstrated that imiquimod 5% applied once daily for five days a week for five weeks completely corrected epidermal atrophic dysplasia, with an associated marked improvement in physical appearance.12
Lower concentrations of imiquimod have been developed to be used in short-term cycle therapy and expanded treatment areas. The objective of our study was to assess the efficacy and tolerability of imiquimod 3.75% and 2.5% cream in treating photodamage through a meta-analysis of four previously performed, large-scale studies of patients being treated for AK of the full face or balding scalp.
Methods
A meta-analysis was conducted of four identical multicenter, randomized, double-blind, vehicle-controlled studies. These studies involved a total of 969 adult subjects (aged 33–91 years) with 5 to 20 visible or palpable AKs with an area smaller than 25cm2 on either the face or balding scalp. Patients were randomized (1:1:1) to self-treatment with imiquimod 3.75%, imiquimod 2.5%, or vehicle creams once daily (i.e., two two-week treatment cycles separated by a two-week no-treatment interval), with a follow-up period of eight weeks.
For each study, the site investigators selected the treatment area (either the entire face or the entire balding scalp, but not both). Patients applied a thin layer of cream to the treatment area (up to two packets, or 500mg of product, per application), avoiding the periocular areas, lips, and nares. The study medication was applied prior to normal sleeping hours and removed approximately eight hours later with mild soap and water. The ears were excluded from both assessment and treatment. Rest periods from daily treatment were instituted by the investigator as needed to manage local skin reactions (LSRs) or application site reactions, with resumption of treatment upon adequate resolution. LSRs were a defined set of local adverse events (AEs), including erythema, edema, weeping/exudate, flaking/scaling/dryness, scabbing/crusting, and erosion/ulceration that were assessed independently of other AEs.
At the end-of-study (EOS) visit at Week 14, eight weeks after the final treatment cycle, the site investigator completed an overall assessment of each patient’s photodamage in the treatment area (including an integrated assessment of fine wrinkling, coarse wrinkling, mottled pigmentation, roughness, sallowness, skin laxity, and telangiectasias) by comparing the change in Investigator’s Global Integrated Photodamage (IGIP) score from baseline (i.e., the patient’s appearance at baseline visit) to that at Week 14 using a seven-point scale (where -3=significantly worse than baseline and +3=significantly improved from baseline). Mean scores were calculated and p-values assessed using the Mann–Whitney U test. As the IGIP score was recorded at EOS, patients lost to follow-up were not included. The IGIP score was a tertiary endpoint with no formal baseline assessment.
In addition to assessing impact of imiquimod on photodamage, investigators assessed LSRs at each visit, using a four-point scale (0=none and 3=severe).
Results
Overall, 969 patients were enrolled in the four chosen studies. Pooled and individual results from two of these studies have been previously published, primarily focusing on efficacy and tolerability in treating AK lesions.4–7
Patient demographics were very similar for all four studies across the three treatment regimens (Table 1). Patients (mean age: 63.0–66.4 years) were predominantly male (80.0%) and Caucasian (99.6%). The majority had Fitzpatrick Skin Types I (13.9%), II (41.4%), or III (31.3%). The face was treated in 73.2 percent (n=709) of patients, while the scalp was treated in 26.8 percent (n=260).
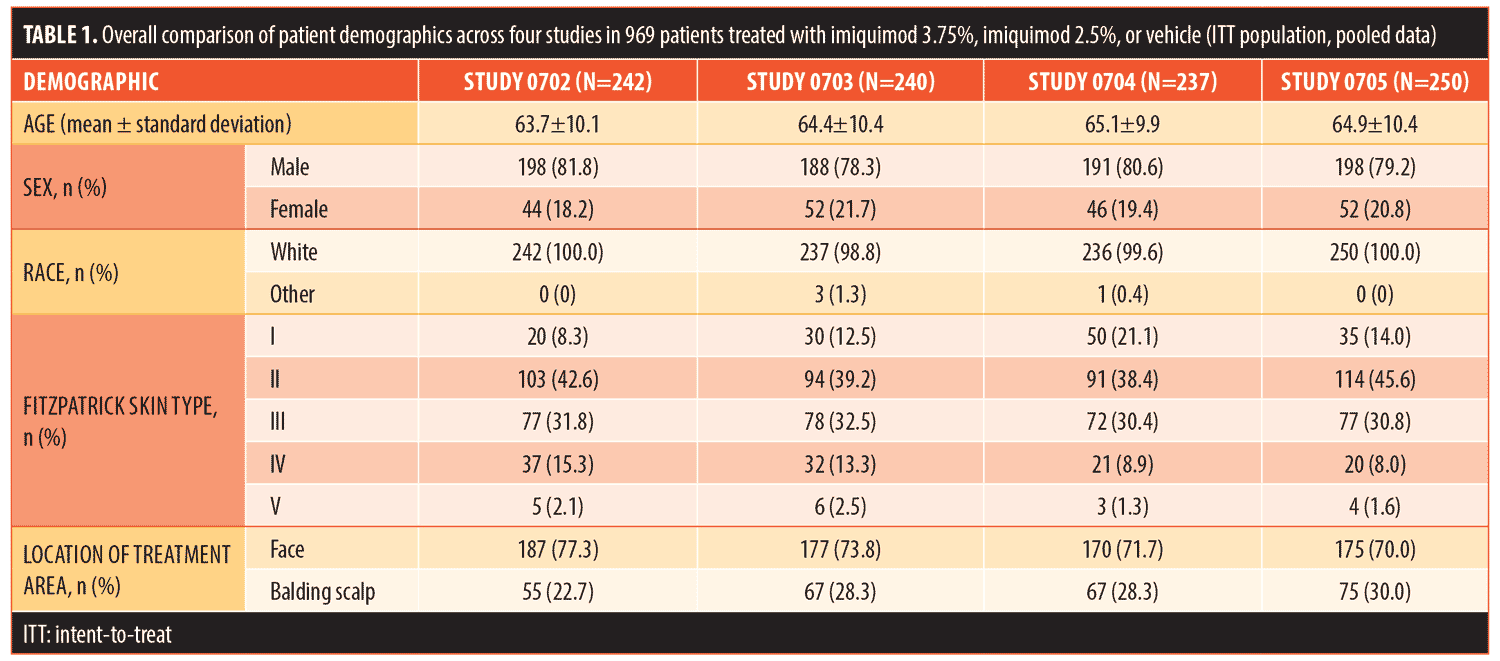
The mean IGIP scores were 1.67, 1.98, and 0.73 for imiquimod 2.5%, imiquimod 3.75%, and the vehicle, respectively (actives both P<0.0001 versus vehicle; Table 2). Of the total number of patients, 44.1 percent (n=132) treated with imiquimod 3.75% were significantly improved (score=3) as compared with 31.9 percent (n=97) treated with imiquimod 2.5% and only 9.5 percent (n=28) treated with the vehicle. Only five patients treated with imiquimod 3.75% (1.7%) were adjudged to have worse photodamage at EOS, versus six patients treated with imiquimod 2.5% (2.0%) and 21 treated with the vehicle (7.1%).
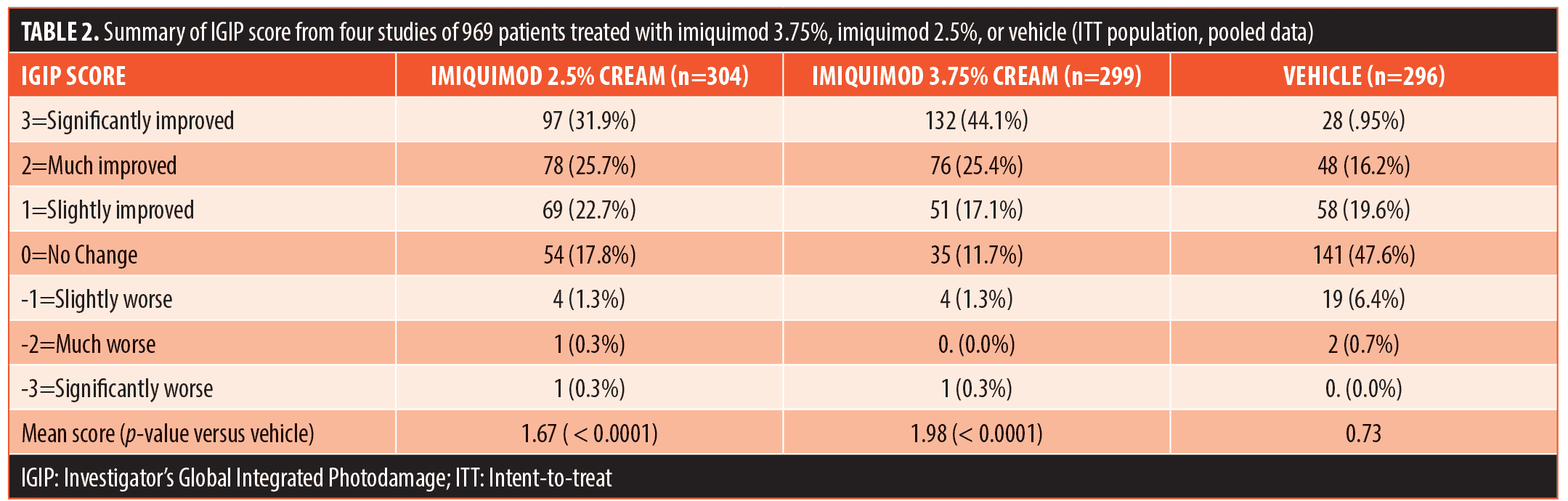
LSRs were observed in almost all of the patients during treatment and pooled results reported across the four studies (Table 3). There was a greater incidence of patients experiencing LSRs and in particular severe LSRs with increasing imiquimod concentration. Erythema was the LSR reported with the greatest frequency; specifically, it was noted in 97.2 percent and 97.5 percent of patients in the 2.5% imiquimod and 3.75% imiquimod treatment groups, respectively, compared with 85.0 percent of patients in the vehicle group. The incidence of severe erythema (intense redness) appeared to be dose-dependent, reported in 21.4, 35.0, and 0.0 percent of patients in the three groups, respectively. The mean erythema score increased during each treatment cycle and then decreased to below baseline during the follow-up period for both imiquimod groups. The LSRs largely did resolve, as shown by the mean LSR sum score, which returned to baseline levels by the end of the study.
Discussion
To date, there have been ad-hoc reports that imiquimod can improve skin texture and signs of photodamage, although published clinical data are limited to being sourced from several small, open-label studies. The effects of imiquimod appear to be confined to the epidermis, with improvements in epidermal dysplasia resulting in a marked improvement in the physical appearance of photodamaged skin due to increased hydration and removal of the dry, scaly skin surface.12 Imiquimod 5% cream has also been shown to reverse epidermal dysplasia in immunosuppressed renal transplant patients, with a corresponding improvement in skin quality.13
By pooling data from four identical double-blind, placebo-controlled studies that considered imiquimod 2.5% and 3.75%, we were also able to show a positive effect on photodamage. There was a statistically significant and dose-related effect on IGIP score as compared with using the vehicle, with 87 percent of patients demonstrating an overall improvement with the administration of imiquimod 3.75%. The incidence of LSRs was also dose-related, although treatment was well tolerated, even in patients with the most severe reactions, with local AEs resolving following treatment cessation.4–7,14,15 It was not clear as to whether LSRs were observed solely at the sites of AK or with similar frequency and severity in treated skin between visible sites of AK.
Importantly, the treatment of photodamage with imiquimod not only provides a cosmetic benefit but also represents a management strategy to prevent cutaneous nonmelanoma carcinoma. Imiquimod has been shown to detect and potentially clear subclinical lesions before they can be visually recognized as AK, which is an important step in initiating a program to prevent cutaneous tumors.11
Limitations. Notably, there are limitations in our investigation. Photodamage was a tertiary endpoint of the four studies, and investigator assessments were made at eight weeks posttreatment without obvious baseline comparison. In addition, the only assessment made (IGIP) is a composite of a number of aspects of photodamage, including fine wrinkling, coarse wrinkling, mottled pigmentation, roughness, sallowness, skin laxity, and telangiectasias. It is therefore not known which specific aspects of photodamage benefited the most from the use of imiquimod.
As far as we know, this is the first study to consider the use of lower concentrations of imiquimod (2.75% and 3.75%) in photodamage, with a dosing regimen primarily designed to treat AKs. It is possible that a longer treatment period (e.g., between 3 and 6 months) is required to elucidate optimal clinical and histologic benefits.
Conclusion
In a meta-analysis of four well-controlled Phase III studies, both imiquimod 2.5% and 3.75% creams showed a positive effect on photodamage when compared with a vehicle cream. This finding warrants further study to determine which aspects of photodamage benefit the most from administration and what the optimal dosing regimen(s) might be.
Acknowledgments
The authors acknowledge Brian Bulley, MSc, of Konic Limited for medical writing support. Valeant Pharmaceuticals North America LLC funded Konic’s activities pertaining to this manuscript.
References
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Investig Dermatol Symp Proc. 2002;7(10): 51–58.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405.
- Stern RS. Treatment of photoaging. N Eng J Med. 2004;350(15):1526–1534.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicentre, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13(2):166–169.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicentre, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2013;12(11): 1278–1282.
- Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62(4):582–590.
- Hanke CW, Beer KR, Stockfelth E, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 3-week cycles. J Am Acad Dermatol. 2010;62(4):573–581.
- Lebwohl M, Dinehart S, Whiting D, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol. 2004;50(5):714–721.
- Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57(2):265–268.
- Metcalf S, Crowson AN, Naylor M, et al. Imiquimod as an antiaging agent. J Am Acad Dermatol. 2007;56(3):422–425.
- Kopera D, Kerl H. Visualization and treatment of subclinical actinic keratosis with topical imiquimod 5% cream: an observational study. Biomed Res Int. 2014:135916.
- Kligman AM, Zhen Y, Sadiq I, et al. Imiquimod 5% cream reverses histologic changes and improves appearance of photoaged facial skin. Cos Dermatol. 2006;19(11):704–711.
- Brown VL, Atkins CL, Ghali L, et al. Safety and efficacy of 5% imiquimod cream for the treatment of skin dysplasia in high-risk renal transplant recipients: randomized, double-blind, placebo-controlled trial. Arch Dermatol. 2005;141(8):985–993.
- Korman N, Moy R, Ling M, et al. Dosing with 5% imiquimod cream 3 times per week for the treatment of actinic keratosis: results of two phase 3, randomized, double-blind, parallel-group, vehicle-controlled trials. Arch Dermatol. 2005;141(4): 467–473.
- Szeimies RM, Gerritsen MJ, Gupta G, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J Am Acad Dermatol. 2004;51(4):547–555.

