 J Clin Aesthet Dermatol. 2018;11(9):37–41
J Clin Aesthet Dermatol. 2018;11(9):37–41
by Natalya Chalyk, MD; Viktor Klochkov, MD; Louise Sommereux; Tatiana Bandaletova, PhD; Nigel Kyle; and Ivan Petyaev, PhD
Dr. Petyaev and Mr. Kyle are with Lycotec Ltd. in Cambridge, United Kingdom. Ms. Sommmereux is with Cosmex Clinic in Cambridge, United Kingdom. Dr. Bandaletova is with DiagNodus Ltd. in Cambridge, United Kingdom. Drs. Chalyk and Klochkov are with the Research Institute of Cardiology at Saratov State Medical University in Saratov, Russia.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Background.Nutrition can influence skin health. Dark chocolate possesses health promoting properties, but its consumption can exacerbate acne vulgaris in young people.
Objective.We evaluated effects of continuous dark chocolate intake on morphological characteristics of the residual skin surface components (RSSCs) collected from the facial skin of young and middle-aged men.
Methods. RSSC samples were taken from 17 young and 16 middle-aged men before and after a four-week consumption period of dark chocolate (10g per day). Lipid droplet size, corneocyte desquamation, and microbial presence levels were measured in the collected RSSC. The project was registered as ISRCTN89815519 in the ISRCTN registry (https://www.isrctn.com/).
Results.Chocolate consumption caused a significant increase in corneocyte desquamation only in the group of young men, whereas Gram-positive microorganism presence significantly increased in both the young and middle-aged men, though this effect was noticeably stronger in the young men.
Conclusion. Dark chocolate consumption appears to affect the facial skin of young men by enhancing corneocyte desquamation and promoting bacterial colonization of the RSSC. These changes might potentially contribute to acne development.
Keywords: Acne vulgaris, corneocyte desquamation, dietary chocolate, facial epidermis, microbial presence
Introduction
Nutritional factors can strongly affect skin health.1,2 Acne vulgaris is one of the most common dermatological conditions worldwide,3 and it has been well established that diets with high glycemic load can exacerbate acne.4 Several recent studies suggest that chocolate consumption is linked with the development of acne vulgaris in young people.5–9 However, these findings might be regarded as controversial, since chocolate is also known as having a food-exerting, health-promoting action.
Chocolate is extremely rich in phenolic antioxidants, especially flavonoids, that have been repeatedly shown to be protective against cardiovascular diseases and diabetes.10–12 For this reason, it is important to understand the mechanisms of adverse effects of chocolate intake on the facial skin as well as discover possible means of eliminating these adverse effects without having to exclude chocolate from the diet.
The investigation of human facial skin is technically challenging, as invasive skin sampling cannot be used for cosmetic reasons. Nevertheless, it is now established that a thin layer of residual skin surface components, or RSSC,14 composed of lipids produced by the sebaceous glands and epidermal cells, desquamated corneocytes, and sweat overlay the surface of the facial epidermis and can be collected for investigation.
We recently developed a noninvasive method of sampling RSSC from the human facial skin surface,14 and we detected age-related changes in morphological characteristics of the collected material in healthy men and women.14,15
In this report, we describe the early results of our ongoing study assessing the effects of dark chocolate consumption on the composition of the RSSC in young and middle-aged male volunteers.
Materials and Methods
Study design and participants. This study was a fragment of a collaborative project undertaken by Lycotec Ltd. (Cambridge, United Kingdom) and the Research Institute of Cardiology of Saratov Medical University (Saratov, Russian Federation). The project is registered as ISRCTN89815519 in the ISRCTN registry. All study subjects were enrolled and provided written informed consent in Saratov, Russia. The study protocol was approved by the local ethics committee (FGBU SarNIIK 02/18/2014).
Only clinically healthy adult Caucasian men aged either between 20 and 30 years (young group) or 45 and 75 years (middle-aged group) were considered for recruitment on the conditions that they were nonsmokers and did not consume excessive amounts of alcohol (i.e., more than 35 UK units per week). The absence of visible manifestations of acne was another inclusion criterion.
The study included 33 eligible participants (17 young men and 16 middle-aged men). All of them had their body mass index (BMI) determined by measuring body mass and height in the morning and calculating the index in kg/m2. BMI values below 25kg/m2 were regarded as normal weight, those between 25kg/m2 and 30kg/m2 were classified as overweight, and values over 30kg/m2 were deemed to indicate obesity.
All study participants received a dietary intervention defined as the intake of 10g daily doses of dark (70% cocoa) chocolate (Green & Black’s, London, United Kingdom). The chocolate was consumed after lunch for four weeks. Study participants were also instructed not to consume any other cocoa-containing products during this period.
The study employed a longitudinal (before–after) design; therefore, there was no separate control (placebo) group. Measurements taken at the initial (preintervention) point constituted an internal control for measurements done at the end of the study.
Sampling. For RSSC sampling, study participants were requested to avoid facial hygienic manipulations for 24 hours before material collection, which was carried out in the morning. These samples were collected immediately before initiating chocolate consumption and at the end of the study (i.e., after 4 weeks of daily chocolate consumption). RSSC sampling and the preparation of smears on microscope slides were performed as described elsewhere.14 All collected samples (four slides per collected sample) were sent to the laboratory of Lycotec Ltd. for further processing and eventual microscopic examination.
RSSC sample analysis. For performing microscopic morphological analyses of the collected samples, one slide was stained with hematoxylin and eosin (H&E) to identify any cells or cell remnants. The second slide was stained using Oil Red O (Lipid Stain, ab150678; Abcam, Cambridge, United Kingdom) for lipid visualization and lipid droplet size evaluation. One more slide was stained with crystal violet solution (Gram staining) to assess the level of Gram-positive microorganism presence. The remaining slides were kept for possible future use.
All slides were examined microscopically using an Olympus BX41 laboratory microscope (Olympus, Tokyo, Japan) at a ×1,000 magnification by analyzing 40 fields of view within each smear. Photomicrographs were prepared using an Olympus DP71 camera.
RSSC analyses included lipid droplet size measurement, counting desquamated corneocytes, and bacterial presence evaluation. Briefly, lipid droplet size quantified using the Cell^B imaging software (Digital Imaging Solutions, Olympus, Japan) was measured as the diameter of round droplets or long axis of oval droplets. Measurement results were expressed as average droplet size for each subject per time point. Likewise, desquamated corneocyte counts (averages per microscope field) were recorded for each subject/time point. Microorganism presence per microscope field was assessed using our five-grade Bacterial Presence Assessment Scale (BPAS) as previously described.14 All samples used for microscopy were anonymized to guarantee blind analysis.
Statistical analysis. The IBM SPSS 19.0 statistical package (IBM Corp., Armonk, New York) was used for quantitative data handling and statistical analysis of the results. All quantitative results were calculated for the two age groups and the BMI-defined subgroups (i.e., normal weight and overweight/obese) within the young group.
Results of all quantitative measurements were compared between the initial and final time points of the study. Descriptive statistics were used. Means, standard deviations (SD), and standard errors of the means (SEM) were determined. A paired t-test (two-sided P-values calculated) was applied to determine statistical significance for the differences between time points within age-defined groups or BMI-defined subgroups of young subjects. A t-test for independent means was used for comparisons between groups or BMI-defined subgroups. Line charts were used for presenting result dynamics during the study.
Results
General characteristics of study participants. From 40 individuals who volunteered to take part in the study, 33 (17 young and 16 middle-aged men) were enrolled as eligible. Table 1 shows general characteristics of the study participants.
It should be noted that BMI distributions in the age-defined groups were different. Among the young subjects, there were eight individuals with normal weight and nine who were either overweight or obese. In contrast, 14 out of the 16 men in the middle-aged group were overweight or obese. For this reason, BMI influence on RSSC characteristics could be assessed only in young individuals.
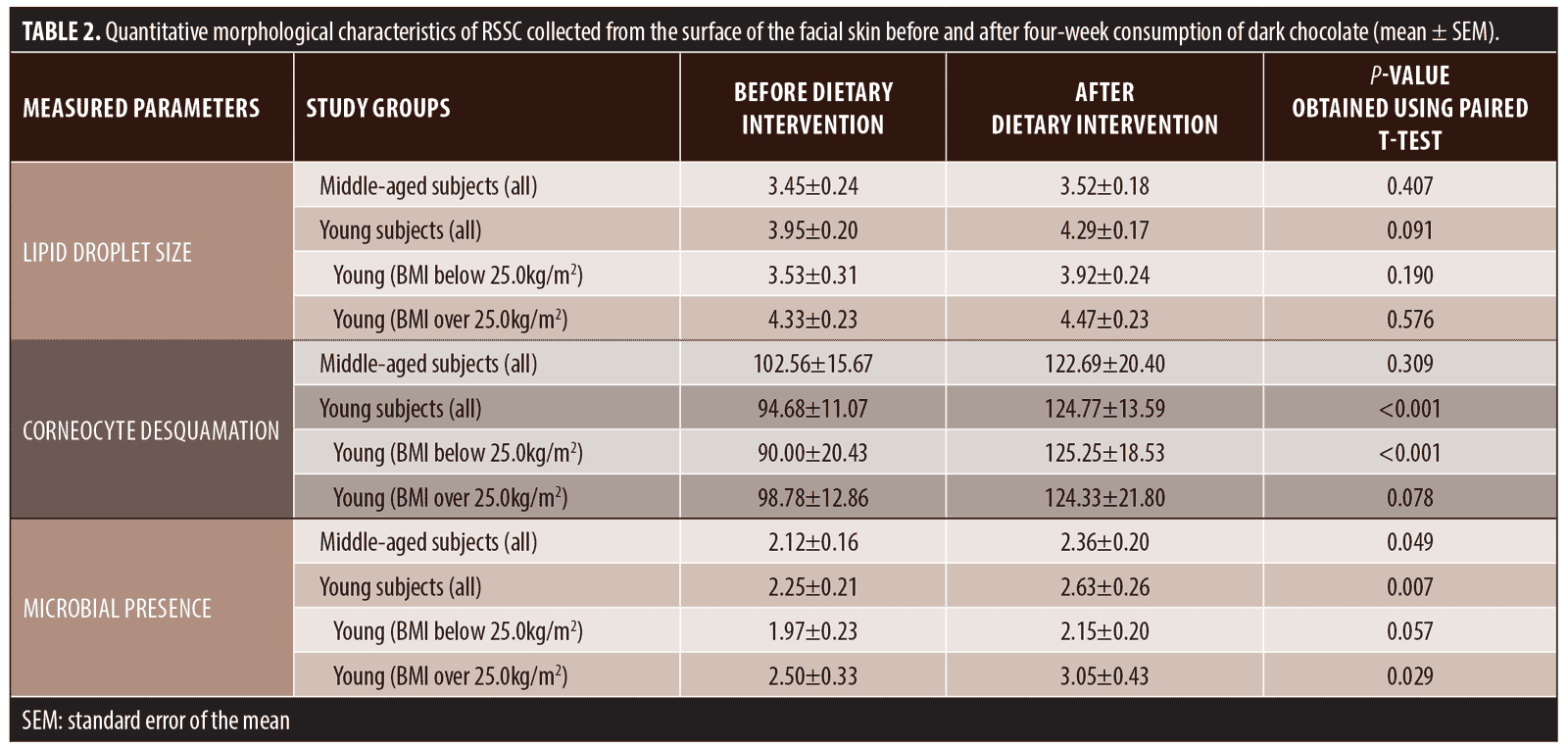
RSSC analysis. Lipid droplet size. Figure 1a and Table 2 present the outcome of lipid droplet size measurements before and after chocolate consumption. Lipid droplets tended to be larger in the young group; however, the difference between the groups was not statistically significant prior to the dietary intervention. Although chocolate consumption seemed to increase lipid droplet size in young men, the increase did not reach statistical significance (Table 2). Nevertheless, average droplet size in the young group became significantly higher as compared with in the middle-aged group (P=0.003). It is also noteworthy that, before chocolate consumption, lipid droplets were significantly larger in the young men with overweight or obesity than in those with normal weight (P=0.042).

Corneocyte desquamation. No significant difference in corneocyte desquamation was observed between the two age-defined study groups either before or after four weeks of chocolate consumption (Figure 1b and Table 2). Chocolate consumption appeared to increase corneocyte desquamation in both groups (Figure 1), but the increase was statistically significant only in the young men (P<0.001). When the BMI-defined subgroups within the young group were assessed separately, the outcome of the paired t-test showed that there was a highly significant (P<0.001) desquamation increase among individuals with normal weight, whereas such was marginally nonsignificant in the subjects with overweight or obesity (Table 2).
Microbial presence. Our previously described semiquantitative BPAS scale14 was employed for microbial presence analysis. The obtained results presented in Figure 1c and Table 2 show that chocolate consumption for four weeks resulted in significant increases of microbial presence in both the young and middle-aged men. No statistical differences in microbial presence levels could be detected between the age-defined groups, but BPAS grade increase appeared steeper in the young men (Figure 1c). With regard to BMI, microbial presence seemed to be higher in young subjects with overweight or obesity, but the difference between the BMI-defined subgroups did not reach statistical significance.
Discussion
Recent reports showing that chocolate consumption can worsen acne in young individuals have aroused interest in studying possible mechanisms behind this phenomenon.5–9 The manifestations of dietary influences on the surface of human facial skin are poorly investigated to date, and the present study, to our knoweldge, is the first attempt to assess the impact of continuous consumption of dark chocolate on morphological characteristics of the RSSC.
The sebum is the most abundant component of the RSSC; therefore, our results are likely to primarily reflect changes in sebum production and composition. This is important because excessive sebum production and hypercornification, as well as pilosebaceous duct colonization with Gram-positive Propionibacterium acnes (P. acnes) and Staphylococcus aureus (S. aureus), are known major factors in the pathogenesis of acne vulgaris.3,16,17
The results presented in this report reveal at least two interesting associations that seem to be more pronounced in young people. The latter observation is important since acne, which is very common among adolescents,3,16 now also seems definitively common in young adults, especially in those in their 20s.18,19
According to our results, dark chocolate appears to increase both epidermal corneocyte desquamation and Gram-positive microorganism presence on the surface of the facial skin (Figures 1b and 1c); however, the difference between the initial and concluding points of the study was convincing only in the young group (Table 2). Although the molecular mechanisms of these shifts remain obscure, cocoa components have been previously shown to increase production of interleukin 1-beta (IL1?), an inflammatory cytokine,20 and results of a recent study suggest that chocolate potentiates IL1? induction by P. acnes.21 It is interesting that IL1? expression was shown to precede keratinocyte cornification,22 thus being directly linked to eventual corneocyte desquamation, the enhancement of which might be interpreted as a proinflammatory phenomenon.
Our observation of a chocolate-induced increase in Gram-positive microorganism presence in the RSSC is also in line with published reports on the prompting of acne exacerbation by chocolate,5–9 but the mechanisms of this increase remain to be elucidated. Although Netea et al21 could not detect any effect of chocolate on the growth of P. acnes, it is well-established that chocolate flavonoids can modify the human gut microbiome;12 therefore, it is impossible to exclude the possibility that chocolate consumption can create favorable conditions for bacterial colonization of the skin surface. Unfortunately, we could not determine the types of bacteria present in RSSC samples, so further studies are needed for evaluating this assumption.
While no significant influence of chocolate intake on lipid droplet size could be detected, our study results corroborate our previous observations of the presence of larger lipid droplets in younger subjects.14 Indeed, younger men might have more active lipid production by sebocytes. Moreover, within the young group, individuals with overweight or obesity had significantly larger lipid droplets compared with men with normal BMI (Table 2). This might reflect obesity-related metabolic shifts in the epidermis. Individuals with higher BMI also had the highest level of microbial presence in the RSSC, which might explain the increased risk of acne development reported for young men with higher BMIs.23
Limitations. It should be noted that the present study had a few limitations. We did molecularly characterize skin microbiome changes. Furthermore, the small study size warrants the necessity of larger confirmatory studies that would include both men and women and permit a fuller assessment of the possible influences of BMI.
Nonetheless, the results obtained herein in young men look convincing, and it would be intriguing to explore whether new formulations of dark chocolate enriched with antioxidants could alleviate the observed undesirable effects. Our previous study showed that lycopene-containing dark chocolate exerted a systemic lipid-lowering effect,24 and continuation of this project will allow for the examination of possible beneficial influences of new dark chocolate formulations on skin.
Conclusion
Our results suggest that dark chocolate consumption can increase acne development risk in young individuals by stimulating hypercornification and promoting bacterial colonization of the surface of the facial skin; however, it remains unclear if these effects alone are sufficient to provoke acne development. Also, it should not be forgotten that chocolate generally acts as a highly beneficial nutrient.10–12 For this reason, efforts directed toward the reduction or elimination of acne-promoting properties of this valuable food by finely modifying its composition deserve to be seriously considered.
Acknowledgments
Volunteers who took part in the study are thanked for their cooperation. Dr. Alexandre Loktionov of DiagNodus Ltd. in Cambridge, United Kingdom, is thanked for his critical advice and help with manuscript preparation.
References
- McCusker M, Sidbury R. Nutrition and skin: kids are not just little people. Clin Dermatol. 2016;34(6):698–709.
- Pappas A, Liakou A, Zouboulis CC. Nutrition and skin. Rev Endocr Metab Disord. 2016;17(3): 443–448.
- Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, et al. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63(1):124–141.
- Block SG, Valins WE, Caperton CV, et al. Exacerbation of facial acne vulgaris after consuming pure chocolate. J Am Acad Dermatol. 2011;65(4):e114–e115.
- Caperton C, Block S, Viera M, et al. Double-blind, placebo-controlled study assessing the effect of chocolate consumption in subjects with a history of acne vulgaris. J Clin Aesthet Dermatol. 2014;7(5):19–23.
- Delost GR, Delost ME, Lloyd J. The impact of chocolate consumption on acne vulgaris in college students: a randomized crossover study. J Am Acad Dermatol. 2016;75(1):220–222.
- Vongraviopap S, Asawanonda P. Dark chocolate exacerbates acne. Int J Dermatol. 2016;55(5):587–591.
- Wolkenstein P, Machovcova A, Szepietowski JC, et al. Acne prevalence and associations with lifestyle: a cross-sectional online survey of adolescents/young adults in seven European countries. J Eur Acad Dermatol Venereol. 2018;32(2):298–306.
- Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15(10):2779–2811.
- Ellam S, Williamson G. Cocoa and human health. Annu Rev Nutr. 2013;33:105–128.
- Strat KM, Rowley TJ, Smithson AT, et al. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J Nutr Biochem. 2016;35:1–21.
- Shetage SS, Traynor MJ, Brown MB, et al. Effect of ethnicity, gender, and age on the amount and composition of residual skin surface components derived from sebum, sweat, and epidermal lipids. Skin Res Technol. 2014;20(1):97–107.
- Chalyk NE, Bandaletova TY, Kyle NH, et al. Age-related differences in morphological characteristics of residual skin surface components collected from the surface of facial skin of healthy male volunteers. Skin Res Technol. 2017;23(2):212–220.
- Chalyk NE, Bandaletova TY, Kyle NH, et al. Morphological characteristics of residual skin surface components collected from the surface of facial skin in women of different age. Ann Dermatol. 2017;29(4):454–461.
- Williams HC, Dallavalle RP, Garner S. Acne vulgaris. Lancet. 2012;79(9813):361–372.
- Gollnick HPM. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol. 2015;29(Suppl 5):1–7.
- Collier CN, Harper JC, Cantrell WC, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485.
- Mao T, Van de Water J, Keen CL, et al. Cocoa procyanidins and human cytokine transcription and secretion. J Nutr. 2000;130(Suppl 8):2093S–2099S.
- Netea SA, Janssen SA, Jaeger M, et al. Chocolate consumption modulates cytokine production in healthy individuals. Cytokine. 2013;62(1): 40–43.
- Lachner J, Mlitz V, Tschachler E, et al. Epidermal cornification is preceded by the expression of a keratinocyte-specific set of pyroptosis-related genes. Sci Rep. 2017;7(1):17446.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol. 2012;67(6):1129–1135.
- Petyaev IM, Dovgalevsky PY, Chalyk NE, et al. Reduction in blood pressure and serum lipids by lycosome formulation of dark chocolate and lycopene in prehypertension. Food Sci Nutr. 2014;2(6):744–750.

