 J Clin Aesthet Dermatol. 2018;11(9):42–46
J Clin Aesthet Dermatol. 2018;11(9):42–46
by Amrit Kaur, MBBS, MD; Monika Kucheria, MD; Rekha Gupta, MD; Gurvinder Pal Thami, MD; and Reetu Kundu, MD
Drs. Kaur, Kucheria, and Thami are with the Department of Dermatology, Venereology, and Leprosy and the Government Medical College and Hospital in Chandigarh, India. Dr. Gupta is with the Department of Radiodiagnosis at the Government Medical College and Hospital in Chandigarh, India. Dr. Kundu is with the Department of Pathology at the Government Medical College and Hospital in Chandigarh, India.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Extrapulmonary tuberculosis is uncommon and has an insidious onset with slow evolution and a paucibacillary nature. Here, we present a case of disseminated tuberculosis in an adult immunocompetent man presenting with morphologically different types of cutaneous lesions (i.e., multiple subcutaneous abcesses and multiple noduloulcerative lesions with discharging sinuses with seropurulent fluid). Extensive screening in the form of routine blood investigations, serologies, skin biopsy, Montoux test, sputum examination, chest and skull roentgenogram, noncontrast computed tomography chest and abdomen, contrast-enhanced computed tomography of the skull, and magnetic resonance imaging of lumbosacral spine with screening of the whole spine revealed extensive involvement of the skin, subcutaneous tissue, lungs, lymph nodes, skull bone, mandible, ribs, scapula, pelvis and Pott’s spine, and thyroid.
Keywords: Disseminated tuberculosis, immunocompetent, thyroid tuberculosis
Introduction
Tuberculosis (TB) is the most common cause of chronic infectious diseases, afflicting up to one-third of the world’s population and most commonly impacting the pulmonary system. Extrapulmonary TB is defined as the occurrence of TB at sites other than the lungs, such as the lymph nodes (19%), pleura (7%), gastrointestinal tract (4%), bone and joints (6%), meninges and central nervous system (3%), genitourinary system (1%), cutaneous (1%), and endocrine glands (<1%).1 Disseminated TB is defined as a TB infection involving the bloodstream, bone marrow, liver, or two or more noncontiguous sites or systems and includes miliary TB involving the lungs. Disseminated TB has been observed for centuries, but its exact incidence is not known. Moreover, the symptoms and signs are nonspecific and mimic a variety of diseases, requiring a high index of suspicion for early diagnosis. Disseminated TB is often missed antemortem and has been confirmed in 33 to 80 percent of autopsies.2 The disseminated forms of TB can arise in contiguous, lymphogenous, or hematogenous conditions, which can manifest as a single or multiorgan form of TB.3 The risk factors include the following: high bacillary-load immunosuppressive condition (e.g., human immunodeficiency virus [HIV]), diabetes, smoking, alcohol abuse, young age, malnutrition, exposure to indoor air pollution, connective tissue disorders, pregnancy (peri- or postpartum), underlying malignancy, use of immunosuppressive drugs like corticosteroids and biologicals,4 or exposure to an infectious person. In an immunocompetent adult, miliary TB constitutes less than two percent of all TB cases and up to 20 percent of all extrapulmonary TB cases.5 We present a case of an apparently immunocompetent adult man with disseminated TB with multisystem involvement. Treatment and etiological factors are described and discussed.
Case Presentation
A 24-year-old North Indian man who was referred to our tertiary care centre in Chandigarh, India, presented with multiple subcutaneous abscesses, noduloulcerative lesions, and discharging sinuses over the trunk, neck, scalp, and face, as well as stiffness of the neck and back for one year. He had an evening rise of fever along with occasional chills, sweats, fatigue, and significant weight loss. There was no history of cough, hemoptysis, dyspnea, urinary or gastrointestinal symptoms, or sensory or motor weakness. He had received multiple courses of antibiotics and had gone through multiple incisions and drainages of the abscesses without resolution. He had received a bacillus Calmette-Guerin vaccination in childhood. There was no past or family history of TB at the time of presentation; however, during the patient’s follow-up period, his father was diagnosed with spinal TB.
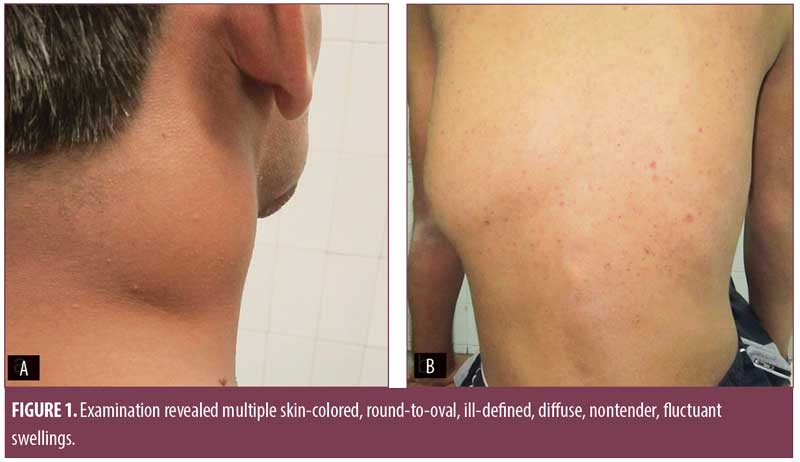
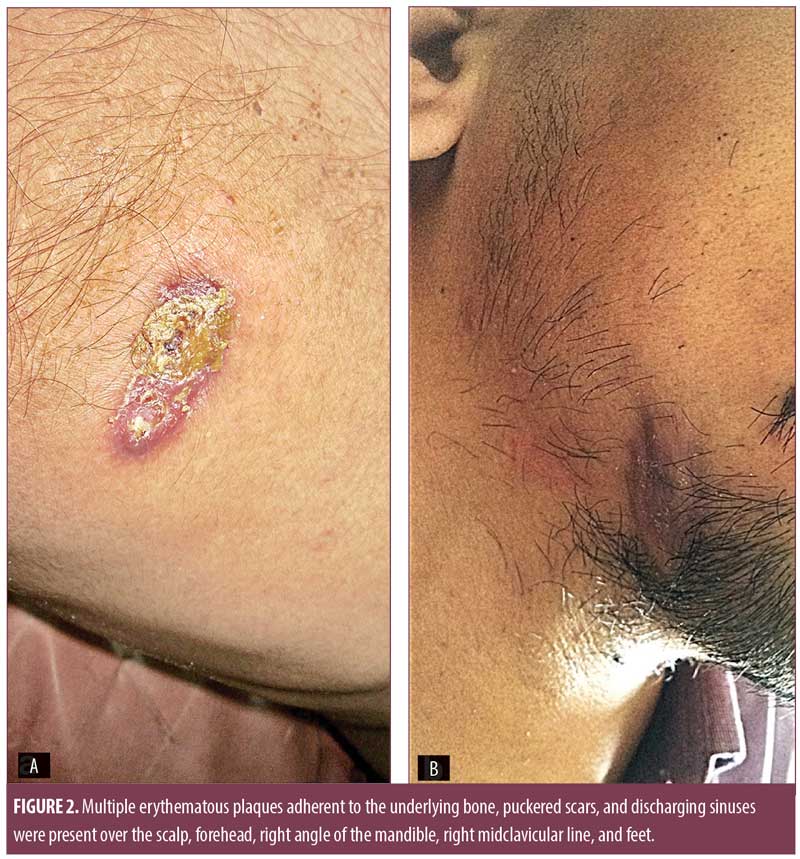
Examination revealed multiple skin-colored, round-to-oval, ill-defined, diffuse, nontender, fluctuant swellings over the right side of the neck along the sternocleidomastoid muscle (7cm×4cm), midline of the neck (4cm×4cm), right axilla (4cm×4cm), and left paraspinal area (12cm×10cm), with restriction of movement (Figure 1). Multiple erythematous plaques adherent to the underlying bone, puckered scars, and discharging sinuses were present over the scalp, forehead, right angle of the mandible, right midclavicular line, and feet (Figure 2). Multiple enlarged, nontender, firm, matted cervical and axillary lymph nodes were present bilaterally.
Pus smears were positive for acid-fast bacilli and negative for gram-stain. However, aerobic, anaerobic, mycobacterial, and fungal cultures were negative. Mantoux test was positive (12mm×14mm). Repeated sputum examination for acid-fast bacilli was negative on three consecutive days. Enzyme-linked immunosorbent assay for HIV and venereal disease research laboratory (VDRL) test was nonreactive. The patient had hemoglobin of 8.5g/L, a leukocyte count 9.8×103/µL, a platelet count of 425×103/µL, an erythrocyte sedimentation rate of 50mm/hr, and a C-reactive protein of 7mg/dL. Thyroid profile showed raised thyroid stimulating hormone (10.02mIU/mL), decreased T3 (2.01pg/mL), and normal T4 (0.97ng/dL). Renal and liver function tests were normal. Chest x-ray showed mild pleural effusion on the right side, and x-ray of the skull and right mandible revealed multiple osteolytic lesions in the frontal and parietal bones and over the body of the mandible in relation to the root of the molars (Figure 3a).
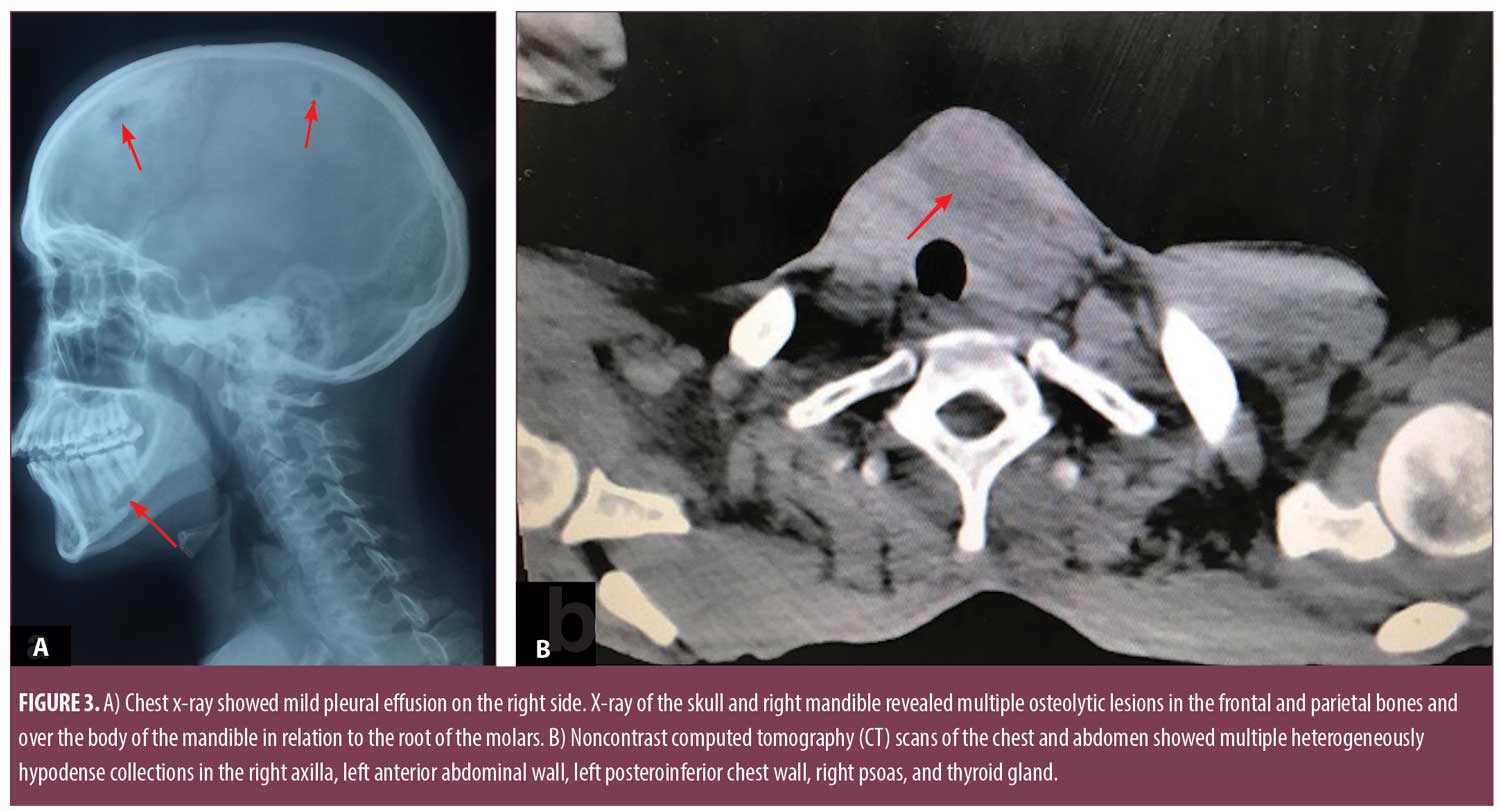
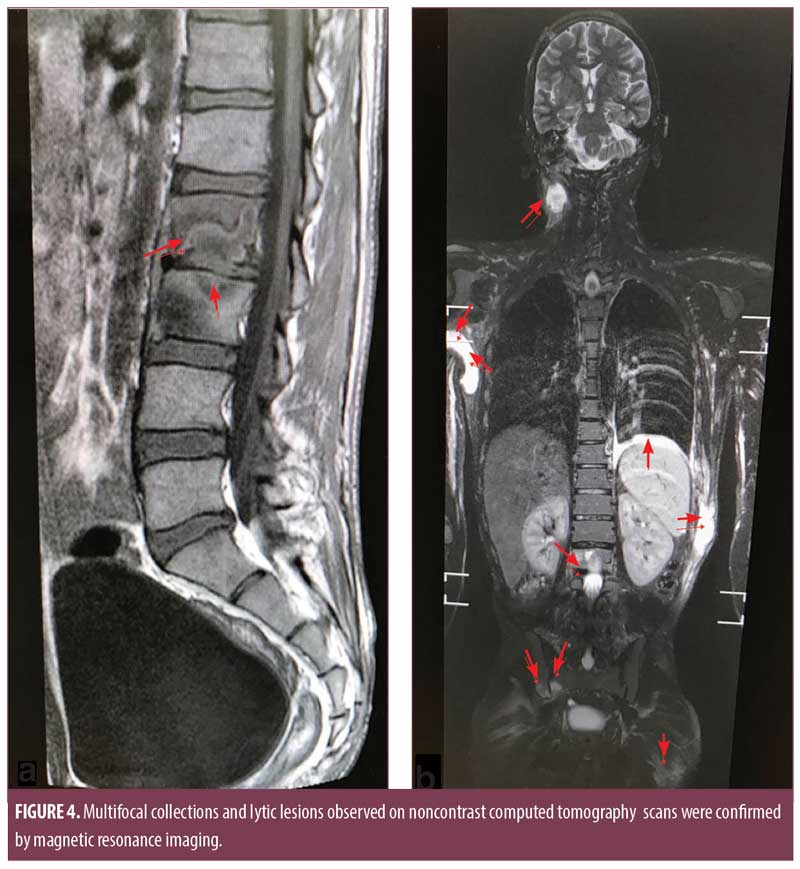
Noncontrast computed tomography (CT) scans of the chest and abdomen showed multiple heterogeneously hypodense collections in the right axilla, left anterior abdominal wall, left posteroinferior chest wall, right psoas, and thyroid gland (Figure 3b). There were osteolytic lesions at the right scapula; lumbar vertebrae (L2, L3); left eighth, ninth, and 10th ribs; bilateral iliac bones; posterior wall of the left acetabulum; bilateral femoral intertrochanteric regions; and right-sided sacral ala. Lungs showed consolidation and pleural effusion of the left lower lobe along with a fibroatelectatic right upper lobe. Contrast-enhanced CT scans of the head showed well defined osteolytic defects with a thin rim of enhancing soft tissue in the extradural space. The underlying brain tissue was normal. Magnetic resonance imaging (MRI) of the lumbosacral spine showed Pott’s spine with spondylodiscitis at the L2 to L3 level with associated prevertebral soft tissue component and right paravertebral collection extending along the right psoas muscle to the medial surface of the right iliac bone and obturator internus. Multifocal collections and lytic lesions observed on noncontrast CT scans were confirmed by MRI (Figure 4).
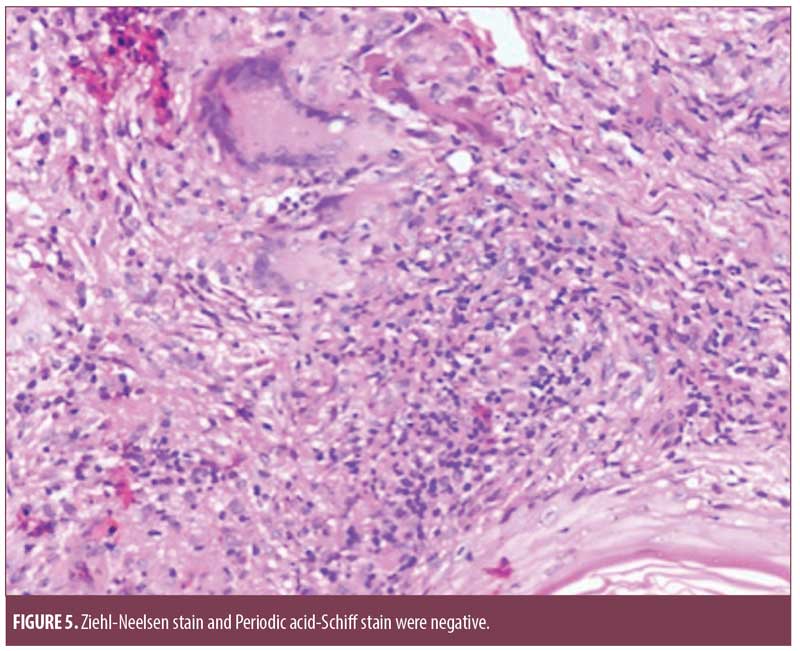
Histopathology from the chest lesion showed a focally thinned out epidermis with multiple epitheloid cell granulomas with occasional Langerhans giant cells in the dermis and the presence of perivascular lymphomononuclear cell infiltrate. Ziehl-Neelsen stain and Periodic acid-Schiff stain were negative (Figure 5).
In light of the aforementioned findings, a diagnosis of disseminated TB was made, with involvement of the skin, lungs, bone, joints, spine, subcutaneous tissue, and thyroid, along with primary hypothyroidism. Antitubercular treatment consisting of isoniazid, rifampin, pyrazinamide, and ethambutol was initiated and the abscesses were aspirated. In addition, levothyroxine 50?g was initiated for hypothyroidism.
After three months of antitubercular treatment, the fever resolved, the cutaneous lesions began disappearing, the abscesses healed, and the patient gained 10kg of weight. After six months, the skin lesions were completely resolved. Follow-up imaging showed resolution of all of lesions previously mentioned.
Discussion
The pathogenesis of TB is significantly intriguing and unclear. Modern immunology and imaging diagnostic techniques have given opportunity to understand the condition in a better way. TB behaves differently in immunocompromised versus immunocompetent patients, as indicated by its resurgence in association with HIV. In an immunocompromised patient, disseminated extrapulmonary TB is more common, has no site restriction, is multibacillary, and is often resistant to first-line treatment, whereas in an immunocompetent patient, it tends to be anatomically compartmentalized, paucibacillary, and rarely fatal.3,4 The concept of an immunological spectrum of TB akin to leprosy is evident in the case of cutaneous TB versus other forms of TB. An analogy can be drawn between the spectrum of clinical disease in leprosy, with its tuberculoid to lepromatous forms, and TB, with its localized to miliary forms. However, as emphasized by Nash and Douglass,6 anergy is also seen in patients with localized (i.e., pulmonary) TB. The spectrum of cutaneous TB comprises, at one end, the anergic, multibacillary forms (e.g., miliary, metastatic cutaneous abscess, periorificial) and at the other end, hyperergic, paucibacillary forms (e.g., lupus vulgaris and verrucous TB, tuberculoid [lichen scrofulosorum, papulonecrotic tuberculide, erythema induratum of Bazin]). There is also tentative evidence of a link between the immunologic response and mycobacteria with certain human leukocyte antigen phenotypes, suggesting a genetic component might contribute to anergy.6
Cell-mediated immunity is known to be critical for host defense against TB, while antibodies have been shown to not be protective. A new paradigm for the pathogenesis of cell-mediated immunity has been suggested by Hunter,7 which takes into account the different host responses in primary and postprimary TB. The development of disseminated TB has largely been attributed to the bursting of postprimary caseous cavities into blood or lymph vessels rather than a bronchus communicating with the exterior in an immunocompetent patient.7
In addition to HIV and immunosuppressive therapies (e.g., corticosteroid, biological), advanced medical interventions (e.g., ureteral catheterization, extracorporeal shockwave lithotripsy, laser lithotripsy, cardiac valve homograft replacement, intravesical bacillus Calmette-Guerin [BCG] therapy for urinary bladder carcinoma) might also be responsible for dissemination of the infection.4,5
The efficacy of the BCG vaccine varies from 0 to 80 percent in various studies and is at least known to protect from the severe and disseminated forms of TB. Its efficacy also depends upon human and mycobacterial genetics, previous exposure to environmental mycobacteria, coinfections with viruses or parasites, geographical location, and socioeconomic and nutritional factors.8
Specific cutaneous lesions in the present patient led to the diagnosis of two distinct forms of TB—scrofuloderma and metastatic cutaneous abscess—the coexistence of which is extremely rare. Metastatic TB abscesses might also occur with progressive organ TB or in miliary TB; however, there are reports showing that it can occur without any underlying focus.
In the present case, the underlying organs involved were the bones, joints, and thyroid gland. The spectrum of skeletal involvement in TB includes the vertebrae, knee, hip, elbow, and smaller joints; virtually no bone is immune to the bacilli. The spine is the most common form of skeletal TB (50%).3,4 Flat bones like the ribs, scapula, skull, and mandible are rarely involved in TB. There have been a few reported cases of isolated flat bone involvement including the ribs, scapula, and skull, but involvement of these bones, combined with such a florid manifestation of the infection, in an immunocompetent patient is rare.9–13
Similarly, TB can affect any endocrine gland, with frequencies of 55, 20, and 1.15 percent, for example, in the adrenal, pituitary, and thyroid glands, respectively. Thyroid TB is a very rare entity even in the TB endemic areas and was first reported in a patient with disseminated TB. It can occur via a hematogenous or lymphogenous route or rarely via direct invasion from the larynx or cervical lymph nodes. Both focal caseous TB and miliary tubercles in the thyroid gland have been reported. It can present as an acute infectious thyroiditis, subacute granulomatous thyroiditis (De Quervain’s), or chronic nonsuppurative thyroiditis. Rarity of TB of thyroid gland has been attributed to the bactericidal properties of colloid, high vascularity, an excess of iodine, and possibly the antituberculous properties of thyroid hormones. Thyroid function tests are normal with an occasional association with hypothyroidism or hyperthyroidism. Ultrasonography and contrast-enhanced computed tomography help in diagnosis, which is confirmed by fine needle aspiration cytology or biopsy.14,15
The patient described here had involvement of the skin, subcutaneous tissues, lungs, bones, joints, spine, lymph nodes, and thyroid gland, which led to extensive multisystem disseminated disease. The obvious cause of dissemination could not be elucidated in this apparently immunocompetent patient; however, the role of transient immunosuppression due to intercurrent viral, bacterial, or parasitic infection altering immunity to tubercular bacilli could have led to the dissemination of the infection in our patient. Cytomegalovirus, hepatitis C virus, and human herpes virus 2 have been associated with increased rates of atypical TB, as well as the reactivation of latent TB. Deficiency in natural killer cell cytotoxic activity induced by such chronic viral infections might play an important role in the development of the transient suppression of the immune responses to TB.16 To the best of our knowledge, this is the first report of its kind for disseminated TB in an apparently immunocompetent patient.
References
- Inayat F, Jafar MS, Ali NS, et al. Enigma of extrapulmonary tuberculosis: where do we stand? Cureus. 2017;9(8):e1554.
- Wang JY, Hsueh PR, Wang SK, et al. Disseminated tuberculosis: a 10-year experience in a medical center. Medicine. 2007;86(1):39–46.
- Verma R, Patil TB, Lalla R. Disseminated tuberculosis manifesting as pulmonary, meningeal and spinal tuberculosis in an immunocompetent patient. BMJ Case Rep. 2012;2012. pii: bcr2012007778.
- Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res. 2012;135(5):703–730.
- Kerkhoff AD, Barr DA, Schutz C, et al. Disseminated tuberculosis among hospitalised HIV patients in South Africa: a common condition that can be rapidly diagnosed using urine-based assays. Sci Rep. 2017;7(1):10931.
- Nash DR, Douglass JE. Anergy in active pulmonary tuberculosis. A comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest. 1980;77(1):32–37.
- Hunter RL. Tuberculosis as a three-act play: a new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis (Edinb). 2016;97:8–17.
- Moliva JI, Turner J, Torrelles JB. Prospects in Mycobacterium bovis Bacillus Calmette et Guérin (BCG) vaccine diversity and delivery: Why does BCG fail to protect against tuberculosis? Vaccine. 2015;33(39):5035–5041.
- Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011;34(5):440–454.
- Sezgin B, Atilganoglu U, Yigit O, et al. Concomitant cutaneous metastatic tuberculous abscesses and multifocal skeletal tuberculosis. Indian J Dermatol. 2008;53(3):149–153.
- Singhal S, Arbart A, Lanjewar A, et al. Tuberculous dactylitis: a rare manifestation of adult skeletal tuberculosis. Indian J Tuberc. 2005;52:218–219.
- Sharma R, Tyagi I, Kumar R, et al. Tuberculosis of the skull; a case report and review of the literature. Neurosurg Rev. 2000;23(2):104–106.
- Chang JH, Kim SK, Kim SK, et al. Tuberculosis of the ribs: a recurrent attack of ribs caries. Yonsei Med J. 1992;33(4):374–378.
- Arya V. Endocrine dysfunctions in tuberculosis. Int J Diabetes Dev Ctries.1999;19:71–77.
- Terzidis K, Tourli P, Kiapekou E, et al. Thyroid tuberculosis. Hormones (Athens). 2007;6(1): 75–79.
- Karalian MA. The influence of chronic viral infections on tuberculosis. Georgian Med News. 2009;(168):63–67.

