J Clin Aesthet Dermatol. 2023;16(12):32–38.
J Clin Aesthet Dermatol. 2023;16(12):32–38.
by Paula Resende Salomão, MD; Matheus Resende Costa Pimenta, MD; Alberto Julius Alves Wainstein, MD; and Ana Paula Drummond-Lage, PhD
All authors are with Faculdade Ciências Médicas de Minas Gerais, Post-graduation Department, Belo Horizonte, Minas Gerais, Brazil.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors have no conflicts of interest relevant to the contents of this article.
ABSTRACT: Introduction. The recurrence of cutaneous melanoma is one of the main reasons for surveillance after primary tumor treatment, and there is still little data on melanoma recurrence related to the Brazilian population.
Objective. We sought to evaluate the profile of patients with cutaneous melanoma recurrence within five years of (early) and five years after (late) initial diagnosis.
Methods. Patients diagnosed between 2006 and 2014 in a private reference service in Belo Horizonte, Brazil, were included. Demographic, clinical, histopathological, and disease evolution variables were collected and analyzed using the R version 4.0.0 program. A p-value less than 0.05 was considered significant.
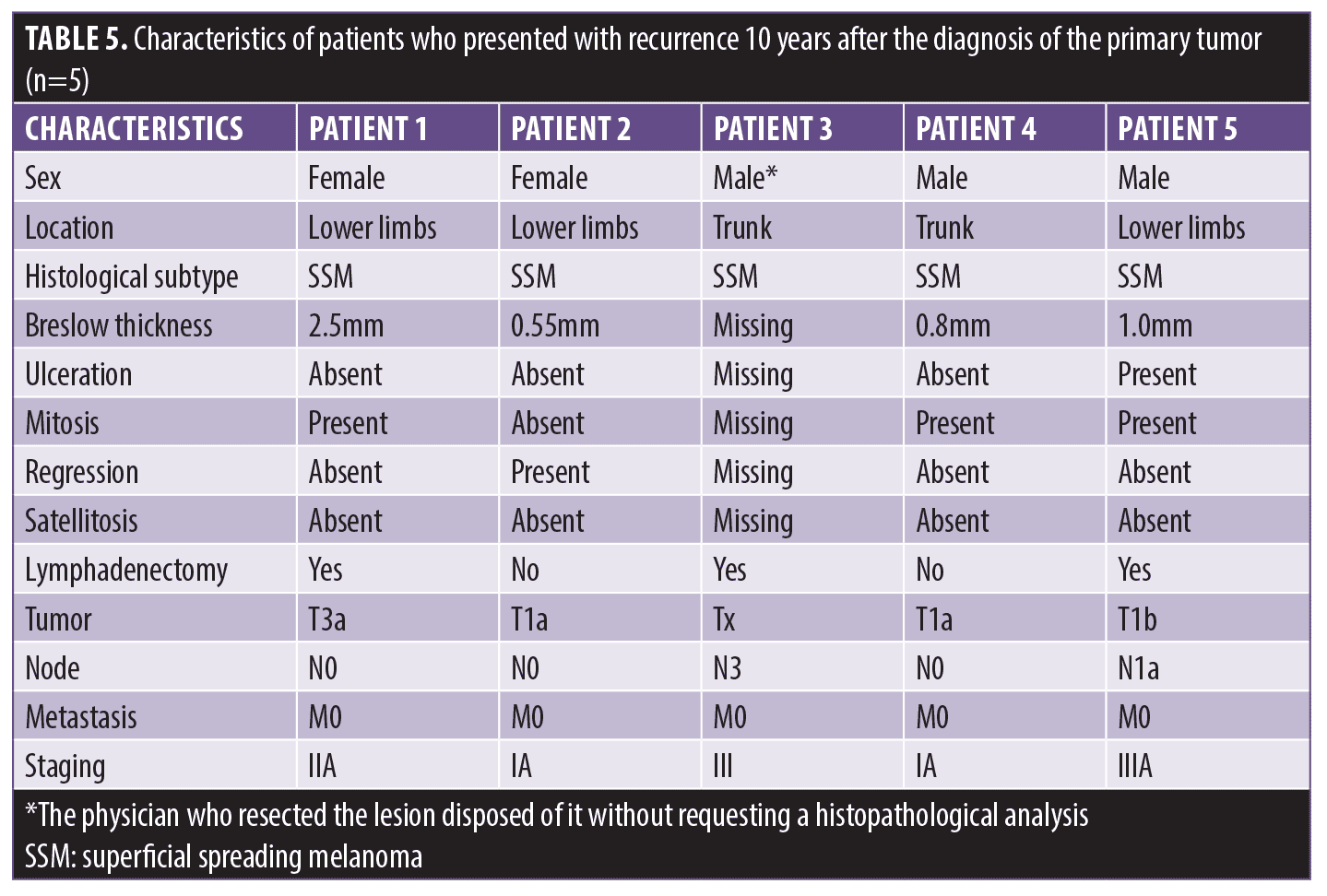
Results. The sample was composed of 331 patients with melanoma, and the 43 patients with recurrence presented with higher mean age (p=0.049), male predominance (p=0.030), a lower proportion of Breslow thickness under 0.8mm (p<0.001), and a more significant presence of mitosis (p=0.007). The 29 patients (8.8%) with early recurrence presented with tumors with ulceration (p<0.018). Late recurrence occurred in 14 patients (4.2%). Five patients relapsed after 10 years; most of them had tumors up to 1mm thick, without ulceration, regression, or satellitosis, but with the presence of mitosis.
Conclusion. The possibility of cutaneous melanoma recurrence after five, and even 10, years, although rare, might indicate the need for longer medical follow-up. Multicenter studies may better characterize Brazilian patient profiles of those with early and late recurrence of melanoma.
Keywords: Brazil, cutaneous melanoma, relapse, follow-up
Although not the most prevalent type of skin cancer, cutaneous melanoma is responsible for most deaths related to malignant neoplasms that affect this organ.1,2 Improved prevention, early diagnosis, and new treatments are related to increased survival, especially in countries where incidence is higher.3,4 The increased prevalence of cutaneous melanoma has a direct effect on the financial and structural burden of health systems due to the high treatment costs in metastatic disease.5,6
According to the American Joint Committee on Cancer (AJCC) staging system, the survival rate and stratification of the risk of progression are mainly based on the clinical and histopathological characteristics of primary melanoma.7 Although it is the most validated prognostic prediction tool, this classification does not define individual risk for disease recurrence.7,8 Other clinical, histopathological, immunological, genetic, and molecular factors can be considered alongside staging for a more personalized definition of treatment and prognosis.9,10 The panels of genetic mutations and gene expression allow for the stratification of patients by the possibility of recurrence, which is fundamental for indicating more intensive follow-up in the high-risk group, in addition to reducing costs and unnecessary exposure to ionizing radiation from imaging studies in the low-risk group.11–13
The importance of knowing the factors related to melanoma recurrence has become particularly important after the development of target therapies and immunotherapy, which are more effective with a precise indication.14 Genetic, serum, and tumor biomarkers may contribute to the change in follow-up protocols; however, clinical and histopathological characteristics are still the basis for assessing the recurrence risk for surviving melanoma patients.13
The data on melanoma recurrence in Brazil are limited; as such, this study evaluated the temporal pattern of cutaneous melanoma recurrence in a series of cases treated at a single center in Belo Horizonte, Brazil.
Methods
A retrospective, observational study was conducted based on the analysis of medical records of patients diagnosed with primary cutaneous melanoma between 2006 and 2014, attended by a single oncology surgeon at a referral center for cutaneous oncology.
Demographic data, histopathological parameters of the primary lesion, data from surgical treatment, staging,7 and tumor recurrence in a minimum period of five years were evaluated. In the case of recurrence, tumor data was collected. Recurrence was classified as early (occurring within 5 years of initial diagnosis) or late (occurring 5 years or more after initial diagnosis). In the late group, cases of recurrence after 10 years were also identified.
The Institutional Research Ethics Committee of Faculdade Ciências Médicas–Minas Gerais approved the project under number 96345218.6.0000.5134.
Qualitative variables were presented as absolute and relative frequencies and quantitative variables as mean±standard deviation (median). Quantitative variables were submitted to the Shapiro-Wilk normality test. The association between qualitative variables was assessed using Fisher’s exact test, Chi-squared test, and binary and ordinal logistic models. To compare quantitative variables between two groups, the Mann–Whitney U test was used for independent samples, and to compare between three groups, the Kruskal Wallis test was used. The analyses were performed using the free program R version 4.0.0, and p-value less than 0.05 was considered significant.
Results
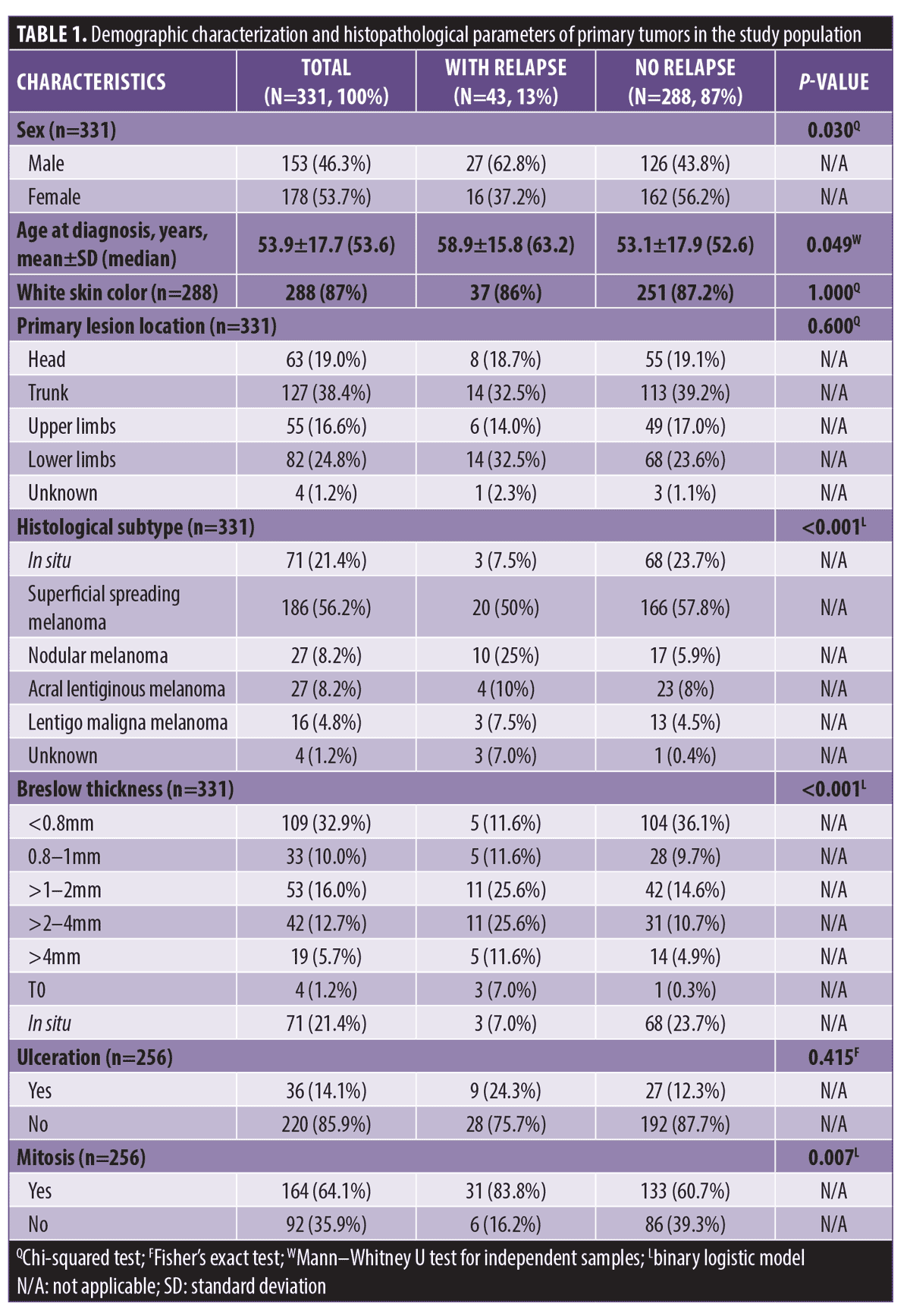
The study included 331 patients, 46.3 percent of whom were male, with a mean age of 53.9±17.7 years at primary tumor diagnosis. Eighty-seven percent of patients were White. The primary lesion occurred at the trunk in 38.4 percent of the patients. The most prevalent histological subtype was superficial spreading melanoma (56.2%), and Breslow thickness was less than 0.8mm in 32.9 percent of cases. The presence of mitosis was observed in 64.1 percent of cases and ulceration in 14.1 percent of cases. Among the 43 patients (13%) who relapsed, there was a higher mean age (p=0.049), higher proportion of male patients (p=0.030), and the presence of mitosis (p=0.007). They also had lower frequency of in situ histological subtype (p<0.001). The proportion of patients with Breslow thickness less than 0.8mm was significantly lower in the group of patients who had tumor recurrence (p<0.001; Table 1).
Other histopathological parameters analyzed were peritumoral inflammatory infiltrate, which was present in 40.8 percent of the sample; intratumoral inflammatory infiltrate, present in 26.5 percent; vascular invasion, present in eight percent; perineural invasion, present in 0.4 percent; regression, present in 7.5 percent; and satellitosis, present in 2.8 percent. Most patients had a free surgical margin (70.7%).
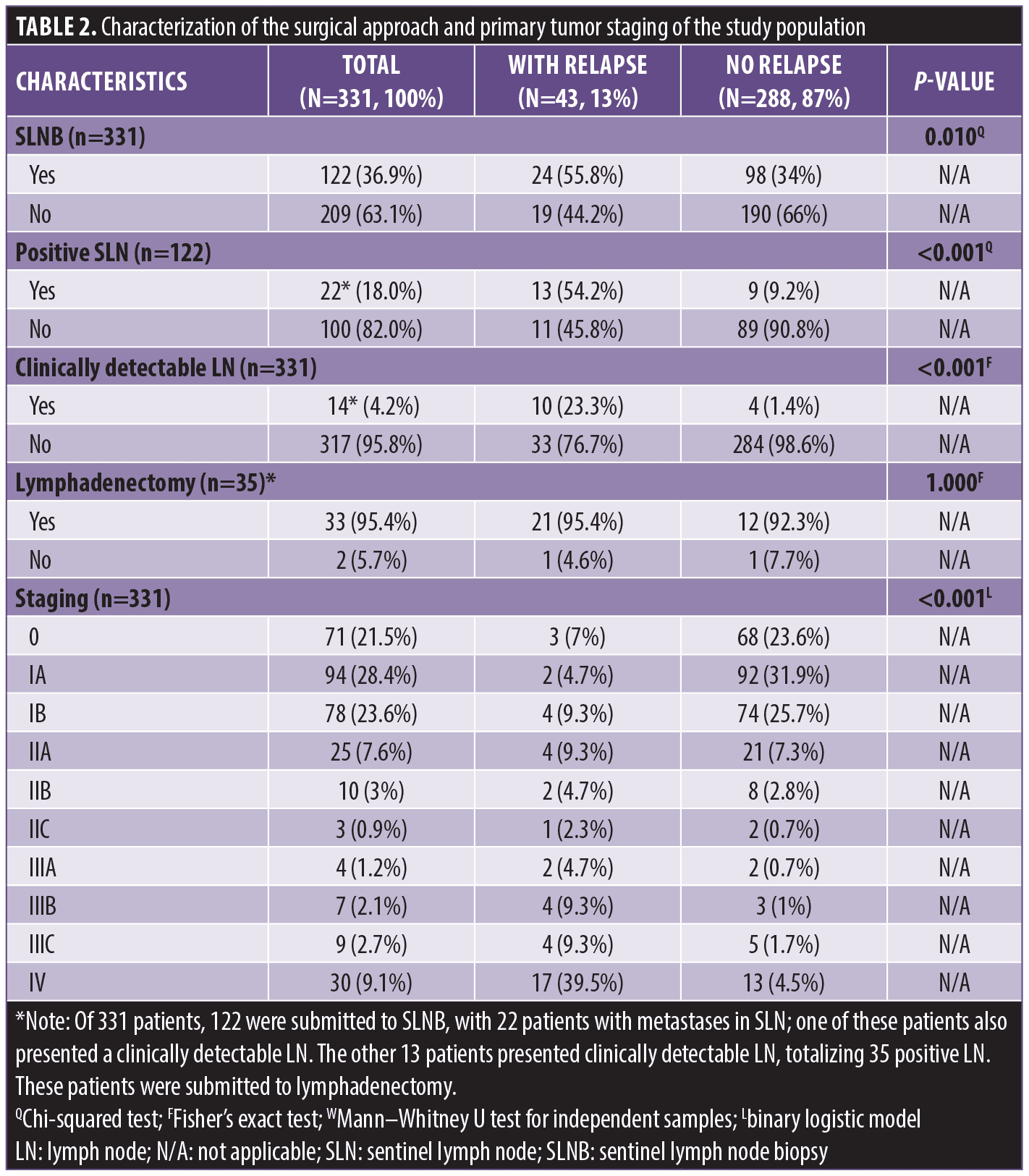
Regarding surgical treatment, patients who relapsed had a higher frequency of sentinel lymph node biopsy (SLNB; p=0.001), positive SLN (p<0.001), and clinically detectable lymph node (p<0.001; Table 2). Most patients who did not relapse had Stage 0 or IA melanoma (p<0.001).
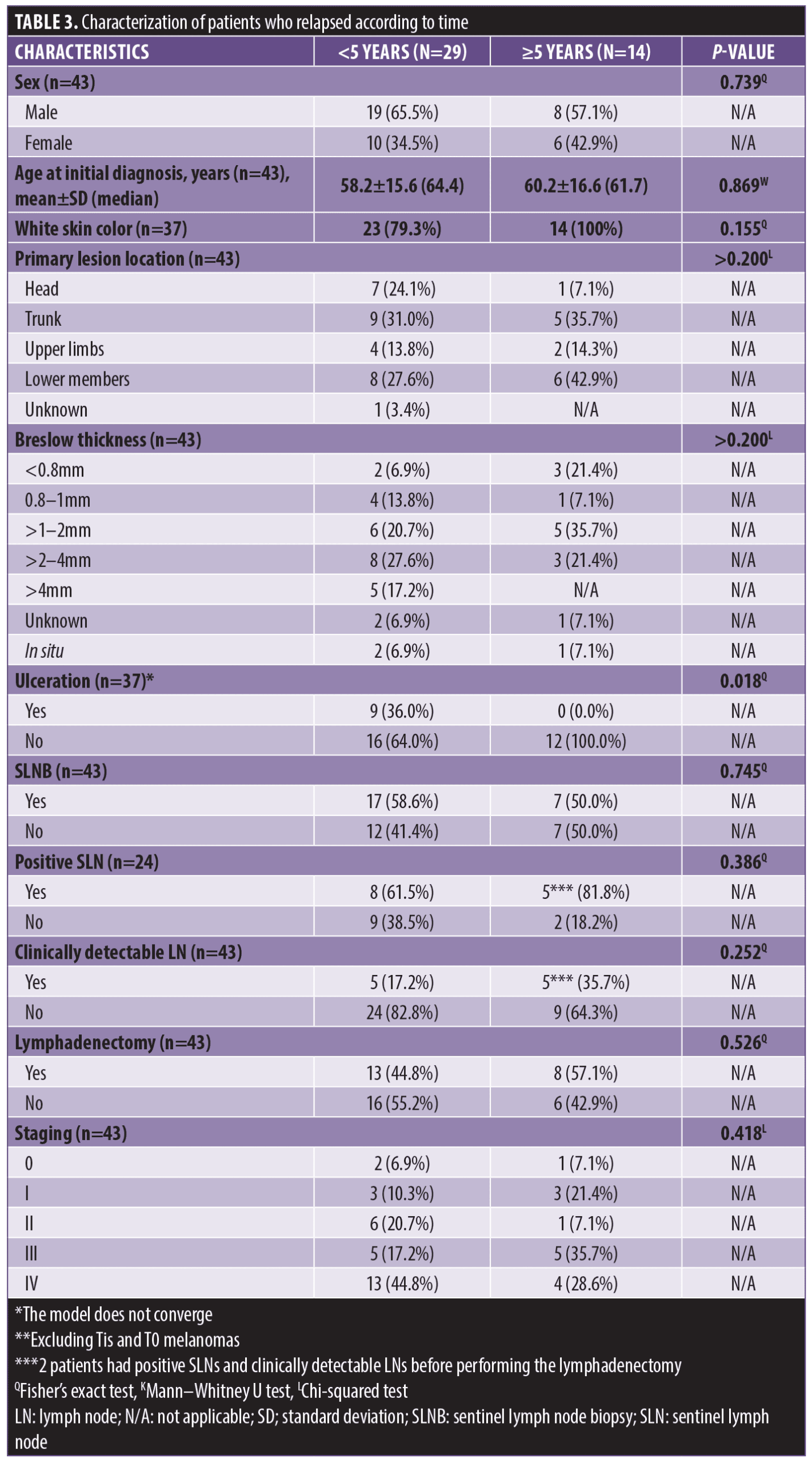
When recurrences were stratified as a function of time, it was observed that 29 patients (8.8%) relapsed within five years, and 14 (4.2%) relapsed after five years. The only statistical difference between the two groups is that patients with early recurrence had tumor ulceration (p<0.018; Table 3).
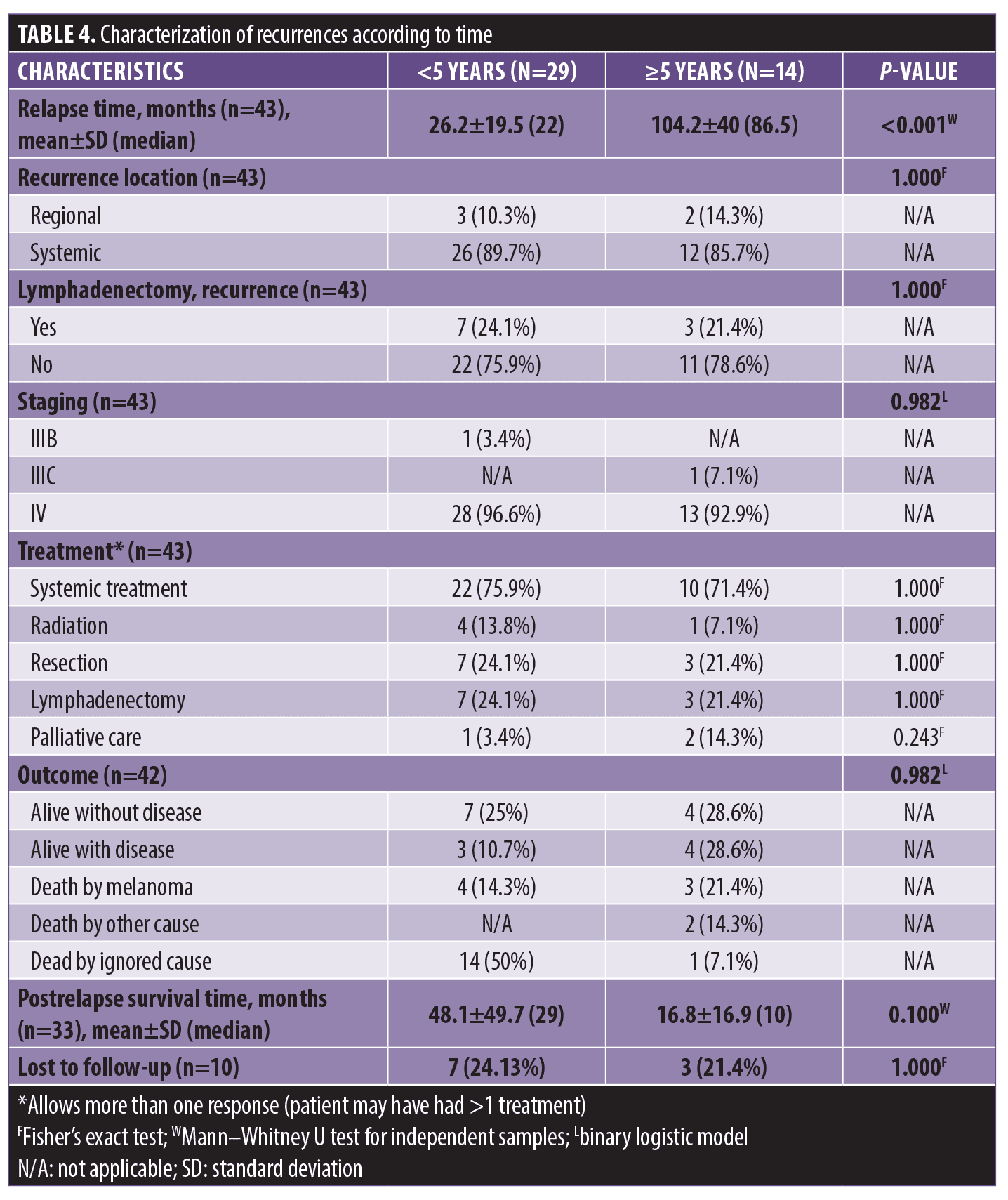
There was no association between recurrence times (early and late) and location of recurrence, lymphadenectomy, staging, outcome, or survival. The median time to recurrence was 22 months within five years and 86.5 months after five years (Table 4).
Among the 14 patients (4.2%) who relapsed after five years, five of them had relapsed at least 10 years (152.2±11.58 months) after diagnosis of the primary tumor. Most of the patients had thin tumors without ulceration, regression, satellitosis, or lymph node involvement in this group. In three of the five cases, mitosis was reported (Table 5).
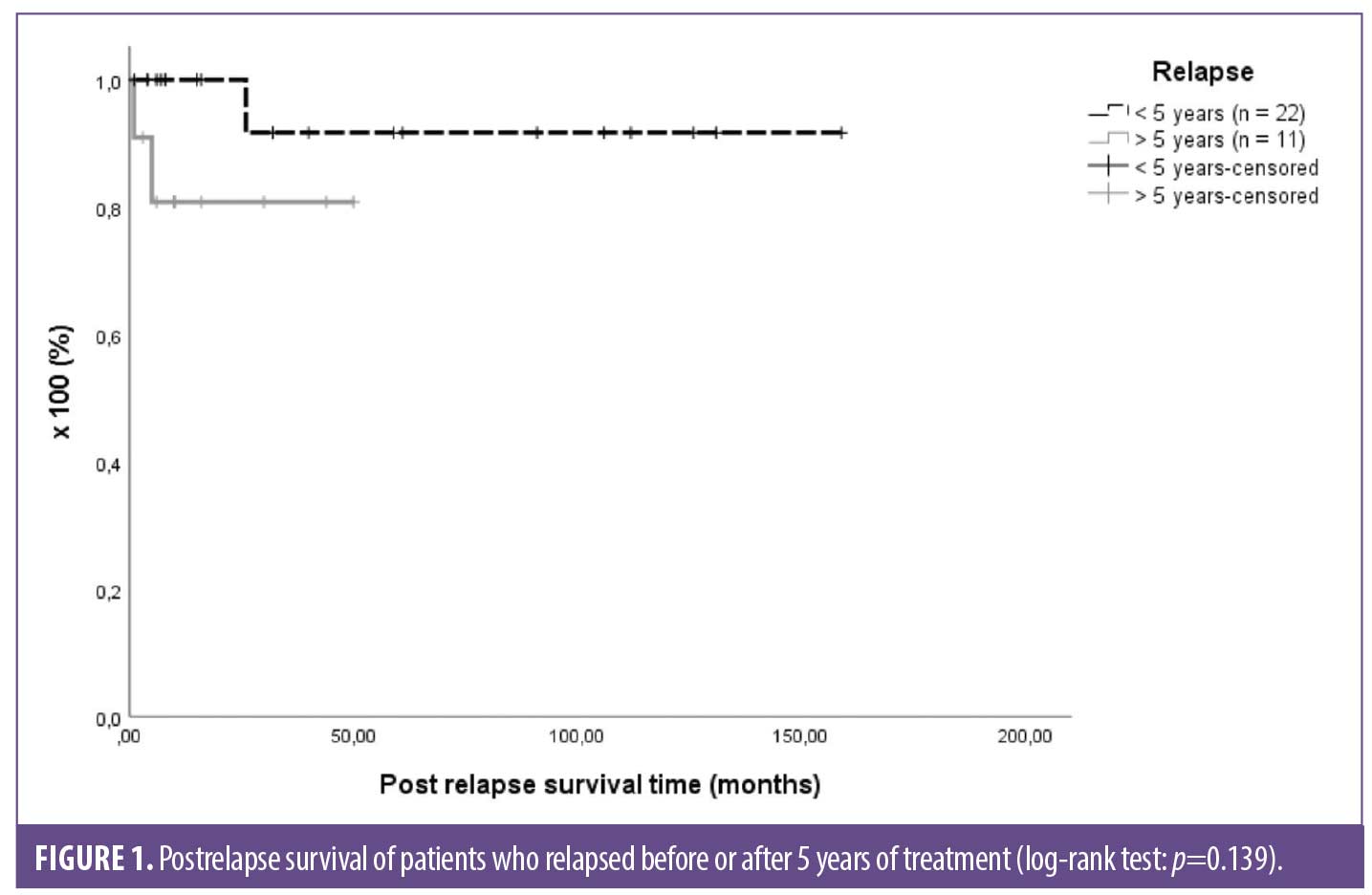
Comparing the postrelapse survival time among groups, there was no difference (p=0.139; Figure 1).
Discussion
Brazilian studies report the demographic, clinical, and prognostic characteristics of patients with melanoma, but data on recurrence are limited.15,16 In this study, the main objective was to identify the characteristics associated with patients who experienced cutaneous melanoma recurrence, according to time interval of recurrence.
In the evaluated sample, there was a predominance of female (53.7%) and White (75%) patients, with a mean age of 53.9±17.7 years, and the most frequent location of melanoma was the trunk; this data is corroborated by other studies carried out in the Brazilian population. De Melo et al17 found that, among about 28,000 patients of cutaneous melanoma, 51.9 percent were women, 75 percent were White, and many patients were between 40 and 69 years of age. Wainstein et al,18 evaluating patients across the country, found that 57 percent of patients were female and 91 percent were White, with a mean age of 52 years, in a cohort of 1,567 cases.
In the present study, the superficial extensive histological subtype had a higher prevalence (56.2% of cases), which is similar to data also reported by other authors in Brazil, the United States, and Europe.16,17,19,20
Patients with recurrence had a higher mean age (p=0.049) and were more often male (p=0.030), which are considered independent risk factors for cutaneous melanoma recurrence. Feigelson et al20 found that, in a population of 1,931 patients with melanoma, advanced age and male sex were associated with recurrence in the multivariate analysis, suggesting that male patients might benefit from more intensive follow-up with shorter intervals between consultations. In a Swedish population study, the recurrence risk was also significantly higher in men than in women, and in older patients.21 Two studies on the Brazilian population identified, through multivariate analysis, male sex as one of the worst prognosis factors for melanoma.16,22
In this cohort, tumor thickness and presence of mitosis (p<0.001 and p=0.007, respectively) were related to recurrence. Tumor thickness is considered the main histopathological feature of AJCC staging, which is evident in the T-stage survival curves.7 The thickness of the tumor, (Breslow thickness) is considered the strongest predictor of worsening survival and increased risk for disease recurrence.7,23,24 Contrarily, the mitotic index is a less reproducible factor and has less impact than thickness and ulceration. It was removed as a prognostic criterion in the eighth edition of the AJCC classification since it must be registered as previously reported as an important recurrence predictor.7,25 Ulceration (the second most important prognostic factor), vascular invasion, perineural invasion, intratumoral inflammatory infiltrate, regression, satellitosis, and free surgical margins were not associated with recurrence in this study. This finding can be justified by the limited sample size or lack of information about these histopathological reports’ parameters. Among almost 5,000 patients with cutaneous melanoma in Germany, Leither et al23 identified the importance of tumor thickness and ulceration as risk factors for reduced recurrence-free survival.23 Increase in tumor thickness, which is associated with the risk of nodal metastasis, is an essential criterion for the indication of SLNB.7 In addition to Breslow thickness, Mitra et al26 found that the highest mitosis count was associated with SLN positivity in a multicenter study comparing clinical and pathological characteristics of 198 cases submitted to SLNB.
In the current study, patients who relapsed were more frequently submitted to SLNB and had greater lymph node involvement. These findings are per the AJCC criteria that include SLNB as a standard for confirming pathological staging in patients with primary tumors above T1b.7 Sartore et al,27 although observing a significant decrease in relapse-free survival and overall 10-year survival in patients with positive SLNB, only confirmed Breslow thickness greater than 2mm as an independent predictive factor of relapse and mortality in 10 years in the multivariate analysis. Portinari et al28 also reported that positive SLNB proved to be less relevant than Breslow thickness greater than 2mm as a predictor of increased risk of recurrence and mortality after the fifth year of follow-up. Lymphadenectomy, which was performed in 33 of 331 patients, can be indicated for better local control of the disease, even if there is no gain in overall survival (OS) or disease-free survival (DFS). This procedure should be considered particularly for patients to whom follow-up and systemic adjuvant therapy are not available, such as those who belong to Brazil’s public health system.29,30
Stratifying melanoma recurrence according to time, we observed that the recurrence within five years was related to the presence of ulceration, as previously described by other authors. Von Schuckman et al30 reported increases in tumor thickness and ulceration as independent prognostic factors for recurrence in the first two years among 700 patients defined as high risk (T1b to T4b), in addition to positive SLNB, mitotic index above 3/cm², and location on the head and neck.
When comparing recurrence data according to time, no statistically significant differences were found in the location of recurrence (regional or systemic), tumor staging, treatment performed, and outcome, probably due to the limited sample size. These data differ from those found in the literature, which point to a progressive increase in recurrence risk at a distance, with increased staging.3,31,32 In general, patients with more advanced melanoma recur more frequently and in a shorter time. Park et al33 found variations in the frequency of systemic recurrences according to staging (Stage II: 48%, Stage III: 68%, Stage IV: 77%) after surgical resection. Lo et al31 reported an inverse relationship between the time interval for recurrence and tumor thickness, so that thicker tumors tended to recur in a shorter time interval, and thinner tumors may recur many years after diagnosis. According to Balch et al,25 the first relapse was related to the primary tumor’s staging and treatment.
In the small group of patients (1.51%) who relapsed after 10 years, most tumors had a thickness up to 1mm, without ulceration, regression, or satellitosis, but with the presence of mitosis. The primary lesions were located on the lower limbs and trunk, and distant metastasis predominated. Faries et al34 assessed 4,731 patients and described melanoma recurrence after 10 years in 6.9 percent of cases; recurrence was related to younger age at diagnosis, lesser thickness of the primary tumor, less frequent location on the head and neck, negative SLNB, and predominance of distance recurrence. This data was corroborated by Sarac et al35 in a study of 1,537 patients who relapsed within 10 years and after 10 years.
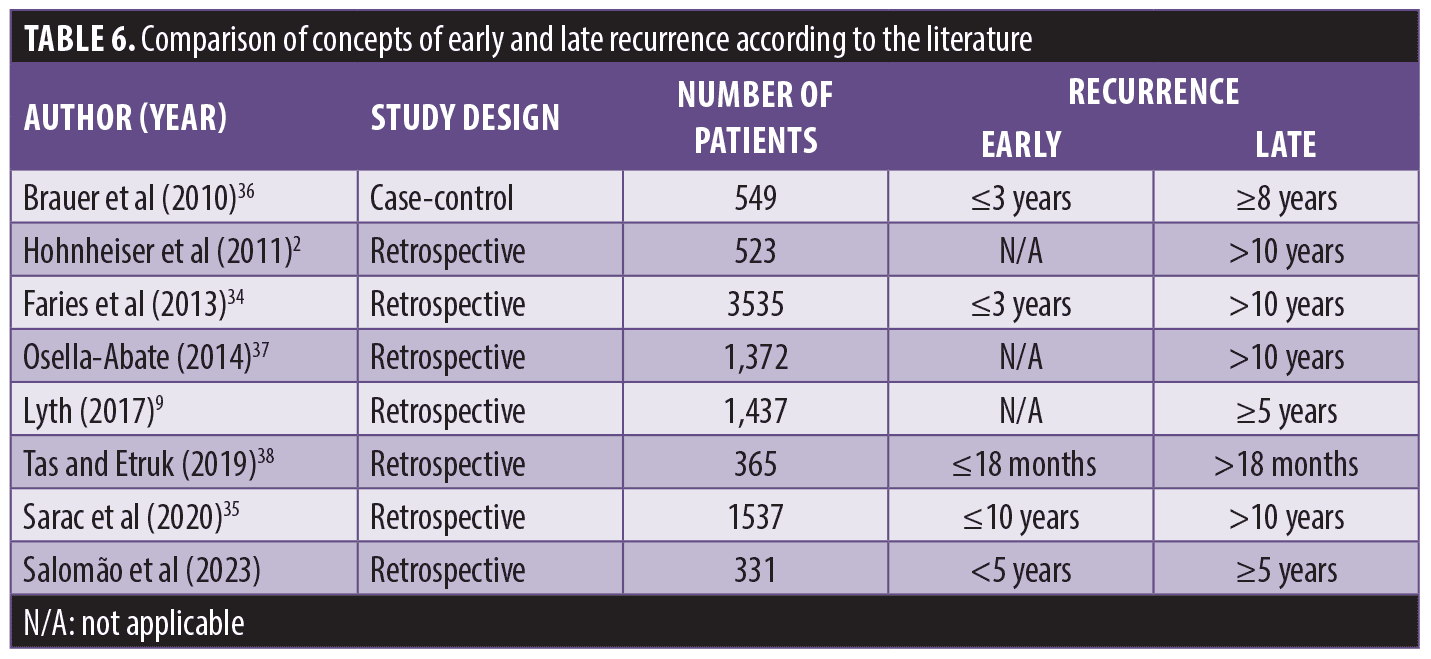
The lack of consensus on the concept of early and late recurrence is a factor that makes it difficult to compare data from different studies, as some authors have considered early recurrence as relapse between 2 to 5 years, and late recurrence after 5 to 10 years (Table 6).2,9,34–38 The temporal standardization of recurrence could facilitate communication and improve the understanding of the factors that lead to variation in the recurrence time results.
Strengths and limitations. This study was carried out in a single reference center for cutaneous oncology, which allowed for the collection of detailed information and standardized tests for patients whose diagnoses, treatments, and follow-ups were relatively uniform, since all patients were followed for a minimum of five years at the same center. Also, the evaluated patients had similar socioeconomic conditions because they were treated in the supplementary health system. This work’s primary relevance is that there are still few records on the recurrence of cutaneous melanoma in Brazil, making it informative to study the temporal pattern related to recurrence risk factors in this population. This study’s main limitations are the retrospective analysis format, loss of data not found in the medical records, and small sample size.
Conclusion
The possibility of cutaneous melanoma recurrence after five or even 10 years, although rare, might suggest the need for a longer medical follow-up of patients with a lower risk of recurrence within the first five years. It is essential to highlight the need to carry out large, multicenter studies to better define the profiles of Brazilian patients who experience early and late recurrence of melanoma.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by PRS, MRCP, and APD-L. The first draft of the manuscript was written by PRS, AJAW, and APD-L, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136(6):1161–1171.
- Hohnheiser AM, Gefeller O, Göhl J, et al. Malignant melanoma of the skin: long-term follow-up and time to first recurrence. World J Surg. 2011;35(3):580–589.
- Miller R, Walker S, Shui I, et al. Epidemiology, and survival outcomes in stages II and III cutaneous melanoma: a systematic review. Melanoma Manag. 2020;7(1):39–53.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1)7–30.
- Deschner B, Wayne JD. Follow-up of the melanoma patient. J Surg Oncol. 2019;119(2):262–268.
- Deckers EA, Hoekstra-Weebers JEHM, Damude S, et al. The MELFO study: a multicenter, prospective, randomized clinical trial on the effects of a reduced stage-adjusted follow-up schedule on cutaneous melanoma IB–IIC patients—results after 3 years. Ann Surg Oncol. 2020;27(5):1407–1417.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492.
- Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18(8):775–784.
- Lyth J. Conditional recurrence-free survival in patients with primary stage I-II cutaneous malignant melanoma – a population-based study. Melanoma Res. 2018;28(6):637–640.
- Lo SN, Ma J, Scolyer RA, et al. Improved risk prediction calculator for sentinel node positivity in patients with melanoma: the Melanoma Institute Australia nomogram. J Clin Oncol. 2020;38(24):2719–2727.
- Aderhold K, Wilson M, Berger AC, et al. Precision medicine in the treatment of melanoma. Surg Oncol Clin N Am. 2020;29(1):1–13.
- Keller J, Diggs LP, Hsueh EC. Prognostic molecular testing in melanoma: ready for prime time? Melanoma Manag. 2017;4(4):171–174.
- Dubin DP, Dinehart SM, Farberg AS. Level of evidence review for a gene expression profile test for cutaneous melanoma. Am J Clin Dermatol. 2019;20(6):763–770.
- Verver D, van Klaveren D, van Akkooi ACJ, et al. Risk stratification of sentinel node-positive melanoma patients defines surgical management and adjuvant therapy treatment considerations. Eur J Cancer. 2018;96:25–33.
- Da Costa LMM, Crovador CDS, De Carvalho CEB, Vazquez VDL. Characteristics of Brazilian melanomas: eeal-world results before and after the introduction of new therapies. BMC Res Notes. 2019;12(1):10–14.
- Vazquez VDL, Silva TB, Vieira MDA, et al. Melanoma characteristics in Brazil: Demographics, treatment, and survival analysis. BMC Res Notes. 2015;8(1):1–9.
- De Melo AC, Wainstein AJA, Buzaid AC, Thuler LCS. Melanoma signature in Brazil: epidemiology, incidence, mortality, and trend lessons from a continental mixed population country in the past 15 years. Melanoma Res. 2018;28(6):629–636.
- Wainstein AJA, Duprat Neto JP, Enokihara MY, et al. Demographic, clinical, and pathologic features of patients with cutaneous melanoma: final analysis of the Brazilian Melanoma Group Database. JCO Glob Oncol. 2020;(6):575–582.
- Lattanzi M, Lee Y, Simpson D, et al. Primary melanoma histologic subtype: impact on survival and response to therapy. J Natl Cancer Inst. 2019;111(2):186–188.
- Feigelson HS, Powers JD, Kumar M, et al. Melanoma incidence, recurrence, and mortality in an integrated healthcare system: a retrospective cohort study. Cancer Med. 2019;8(9):4508–4516.
- Rockberg J, Amelio JM, Taylor A, et al. Epidemiology of cutaneous melanoma in Sweden—stage-specific survival and rate of recurrence. Int J Cancer. 2016;139(12):2722–2729.
- Cherobin ACFP, Wainstein AJA, Colosimo EA, et al. Prognostic factors for metastasis in cutaneous melanoma. An Bras Dermatol. 2018;93(1):19–26.
- Leiter U, Buettner PG, Eigentler TK, et al. Hazard rates for recurrent and secondary cutaneous melanoma: an analysis of 33,384 patients in the German Central Malignant Melanoma Registry. J Am Acad Dermatol. 2012;66(1):37–45.
- Stiegel E, Xiong D, Ya J, et al. Prognostic value of sentinel lymph node biopsy according to Breslow thickness for cutaneous melanoma. J Am Acad Dermatol. 2018;78(5):942–948.
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206.
- Mitra A, Conway C, Walker C, et al. Melanoma sentinel node biopsy and prediction models for relapse and overall survival. Br J Cancer. 2010;103(8):1229–1236.
- Sartore L, Papanikolaou GE, Biancari F, Mazzoleni F. Prognostic factors of cutaneous melanoma in relation to metastasis at the sentinel lymph node: a case-controlled study. Int J Surg. 2008;6(3):205–209.
- Portinari M, Baldini G, Guidoboni M, et al. The long-term prognostic impact of sentinel lymph node biopsy in patients with primary cutaneous melanoma: a prospective study with 10-year follow-up. Ann Surg Treat Res. 2018;95(5):286–296.
- Tarhini A, Ghate SR, Ionescu-Ittu R, et al. Postsurgical treatment landscape and economic burden of locoregional and distant recurrence in patients with operable nonmetastatic melanoma. Melanoma Res. 2018;28(6):618–628.
- Von Schuckmann LA, Hughes MCB, Ghiasvand R, et al. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatol. 2019;155(6):688–693.
- Lo SN, Scolyer RA, Thompson JF. Long-term survival of patients with thin (T1) cutaneous melanomas: a Breslow thickness cut point of 0.8 mm separates higher-risk and lower-risk tumors. Ann Surg Oncol. 2018;25(4):894–902.
- Lee AY, Droppelmann N, Panageas KS, et al. Melanoma patients. 2018;24(4):939–946.
- Park TS, Phan GQ, Yang JC, et al. Routine computer tomography imaging for the detection of recurrences in high-risk melanoma patients. Ann Surg Oncol. 2017;24(4):947–951.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2014;23(1):1–7.
- Sarac E, Wilhelmi J, Thomas I, et al. Late recurrence of melanoma after 10 years–is the course of the disease different from early recurrences? J Eur Acad Dermatology Venereol. 2020;34(5):977–983.
- Brauer JA, Wriston CC, Troxel AB, et al. Characteristics associated with early and late melanoma metastases. Cancer. 2010;116(2):415–423.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136(10):2453–2457.
- Tas F, Erturk K. Early and late relapses of cutaneous melanoma patients. Postgrad Med. 2019;131(3):207–211.