J Clin Aesthet Dermatol. 2023;16(12):18–30.
J Clin Aesthet Dermatol. 2023;16(12):18–30.
by Mona L. Alqam, MD; Brian C. Jones, PhD; and Thomas M. Hitchcock, PhD
Dr. Alqam is with Medical and Clinical Affairs, Crown Laboratories in Dallas, Texas. Dr. Jones is with Research and Development, Crown Laboratories in Dallas, Texas. Dr. Hitchcock is Chief Science Officer, Crown Laboratories Dallas in Dallas, Texas.
ABSTRACT: Background. The skin, our body’s largest organ, hosts a complex microbiome that plays a pivotal role in maintaining health and protecting against pathogens. Even slight disruptions to this delicate balance can influence skin health and disease. Among the diverse microbial community, Cutibacterium acnes (C. acnes) subspecies defendens is known for its positive contribution to skin health. However, the interaction between living microbe probiotics and wound healing after aesthetic procedures, such as microneedling, remains unexplored.
Methods. Our study included 40 participants with acne scars who underwent four microneedling sessions spaced three weeks apart. They were randomly assigned to Group 1, receiving a regimen with live C. acnes defendens strain XYCM42, or Group 2, following a conventional skincare routine with a cleanser, moisturizer, and sunscreen. Our study assessed various endpoints, including the Clinician’s Global Aesthetic Improvement Scale (CGAIS), clinical safety, improvement in acne scars using Goodman and Baron’s Qualitative and Quantitative Acne Scars Grading Scale and Subject’s Global Aesthetic Improvement Scale (SGAIS).
Results. Our analysis of live and photo grading data for CGAIS unveiled a statistically significant difference between the two groups, with Group 1 (XYCM42-based regimen) showing remarkable improvement. A similar positive trend was observed in the photo grading for CGAIS. Additionally, participant diaries indicated that Group 1 experienced a faster decline in posttreatment parameters, including erythema, swelling, burning/tingling, and itching.
Conclusion. Integrating a microbiome-optimized, probiotic XYCM42-based regimen with microneedling demonstrated a high safety profile and enhanced treatment outcomes. These findings mark a milestone in aesthetic dermatology, supporting innovative microbiome-based approaches to improve skin health and aesthetics.
Keywords: Cutibacterium acnes subsp. defendens, microbiome, skin probiotics, topical skin products, microbiome health, acne scars, microneedling
In aesthetic medicine, there are many examples of treatments where aesthetic improvements are mainly driven by the way in which the body heals. In real-world practice, many of these aesthetic procedures are paired with topical products or even whole product regimens, often applied postprocedure, as a means to alleviate procedure-associated sequelae and discomfort, as well as to reduce any social downtime by allowing for faster or more efficient superficial wound healing.1 However, as more is being discovered about the intricate relationship between the human skin and the skin’s microflora, it has become essential to consider what effects both aesthetic treatments and any topicals applied pre- and/or postprocedure have on the skin microflora and what effects that dynamic can have on the overall success of the procedure’s aesthetic outcome and overall skin health.
In the past, it was believed that creating a sterile wound bed would allow for better healing outcomes, as the microbial burden of chronic wounds would delay or impair healing and increase risk of infection.2 However, it is now known that while similar taxa of skin microbiota are found on chronic wounds versus normal skin, normal skin has more species and strain diversity than are found in chronic wounds.3 This discrepancy might be considered a dysbiosis within the wound bed, which can contribute to delayed or impaired healing, with the absence of some microbes being as critical as the presence or overgrowth of others. This is also seen in surgical wounds, as they tend to have a microbial composition that contains fewer strains of the skin commensal species, such as Cutibacterium acnes (C. acnes) and Staphylococcus epidermidis (S. epidermidis), and higher counts of Staphylococcus aureus (S. aureus), a species that tends to be frequently associated with pathogenicity and infection.4 Dysbiosis during the initial phases of surgical wound healing is likely the result of significant changes to the skin environment in the wound versus the skin environment typical to intact, healthy skin. While it is still being elucidated as to how such temporary dysbiosis can affect wound healing, it has been observed that some microbes, such as strains of the S. aureus species, have been implicated in impaired wound healing.5 Strains of other skin native species, such as C. acnes and S. epidermidis, have been suggested to contribute to improved healing. Such a protective interaction has been observed from the metabolites produced by protective, commensal strains through the regulation and repair of the skin barrier, promotion of epidermal differentiation and function, suppression of pathogenic microbes, and regulation of the immune responses.6–11
Given that many aesthetic treatments can disrupt the skin barrier at least temporarily, it is important to note that there is increasing evidence that points to the relationship between a healthy skin barrier and the commensal microbes on and in the skin.12,13 While not all of the ways in which commensal microflora benefit the skin are yet known, there are some key pathways that have been elucidated. One of these is the activation of the aryl hydrocarbon receptor (AhR), which is a xenobiotic receptor that plays a critical role in skin health and barrier homeostasis.11 This pathway is important in regulating many important barrier genes, which are hallmarks of a healthy skin barrier. These include the genes involved in the acceleration of epidermal terminal differentiation, cell turnover, and increasing the thickness of the stratum corneum.11,14–16 Activation of this pathway by topical AhR agonists (e.g., coal tar) has been shown to be effective in the treatment of skin barrier issues, dysbiosis, and inflammation.17 This observation suggests that modulation of the skin commensal microflora that activate the AhR may also have therapeutic benefits to barrier repair and wound healing. Interestingly, certain strains of C. acnes and S. epidermidis have been shown to activate the AhR.11,18
Another way in which commensal microflora of the skin may potentially affect the outcomes of aesthetic procedures is via their metabolites, namely short-chain fatty acids (SCFAs). These SCFAs, such as propionate and butyrate, have been shown extensively to contribute to reductions in systemic inflammation via the microflora of the gastrointestinal system and have become an indicator of human/microbiome symbiosis.19,20 Additionally, SCFAs such as butyrate and propionate have been successfully used as treatments for inflammatory skin issues, including atopic dermatitis (AD) and psoriasis.21
Our previous research described the benefits of daily topical application of live strains of the C. acnes defendens strain XYCM42.6 Given the observed benefits of having a healthy balance of commensal skin flora and their metabolites, especially during wound healing when transient dysbiosis may occur, we conducted a clinical study to ascertain whether we might obtain better clinical outcomes when performing skin microneedling in conjunction with pre- and postprocedure daily application of a regimen containing live cultures and ferments of the skin commensal C. acnes defendens strain XYCM42.
Methods
Study design. This single-center, two-arm clinical study was conducted at the Crown Clinic in Dallas, Texas, to evaluate the safety and efficacy of a XYCM42-based topical probiotic as a pre- and postmicroneedling procedure regimen. Adult male and female patients of all Fitzpatrick skin types between the ages of 22 and 60 years in generally good health were eligible to be screened for this study. Subjects with atrophic acne scars (boxcar, rolling, and ice pick) that met the eligibility criteria were enrolled in the study. The primary endpoints of this study were improvement in the Clinician’s Global Aesthetic Improvement Scale (CGAIS) and assessment of clinical safety endpoints. Secondary endpoints included improvement in acne scars based on Goodman and Baron’s Qualitative and Quantitative Acne Scars Grading Scale at two- and six- month follow-up visits, compared to the baseline visit. Additional secondary endpoints included supplemental clinical grading, bioinstrumentation measurements, Subject Global Aesthetic Improvement Scale (SGAIS), and patient satisfaction questionnaires.
Individuals with known allergies to facial or general skin care products, active systemic or local skin diseases affecting wound healing, or sensitivities to lidocaine and stainless steel were excluded to ensure participant safety and the integrity of the study. Participants with recent facial trauma, hypertrophic or keloid scars, cancerous or precancerous lesions in the treatment areas, active infections, planned surgeries or invasive face procedures, and pregnancy were also excluded.
The study design and protocol adhered to the ethical considerations expected for a clinical study and was approved by the relevant Institutional Review Board (IRB; Allendale IRB, 30 Neck Road Old Lyme, Connecticut, 06371; IRB study number CL-XYC-21-05). Informed consent was obtained from all subjects before participating, and participants were required to sign a photography/digital photo release form.
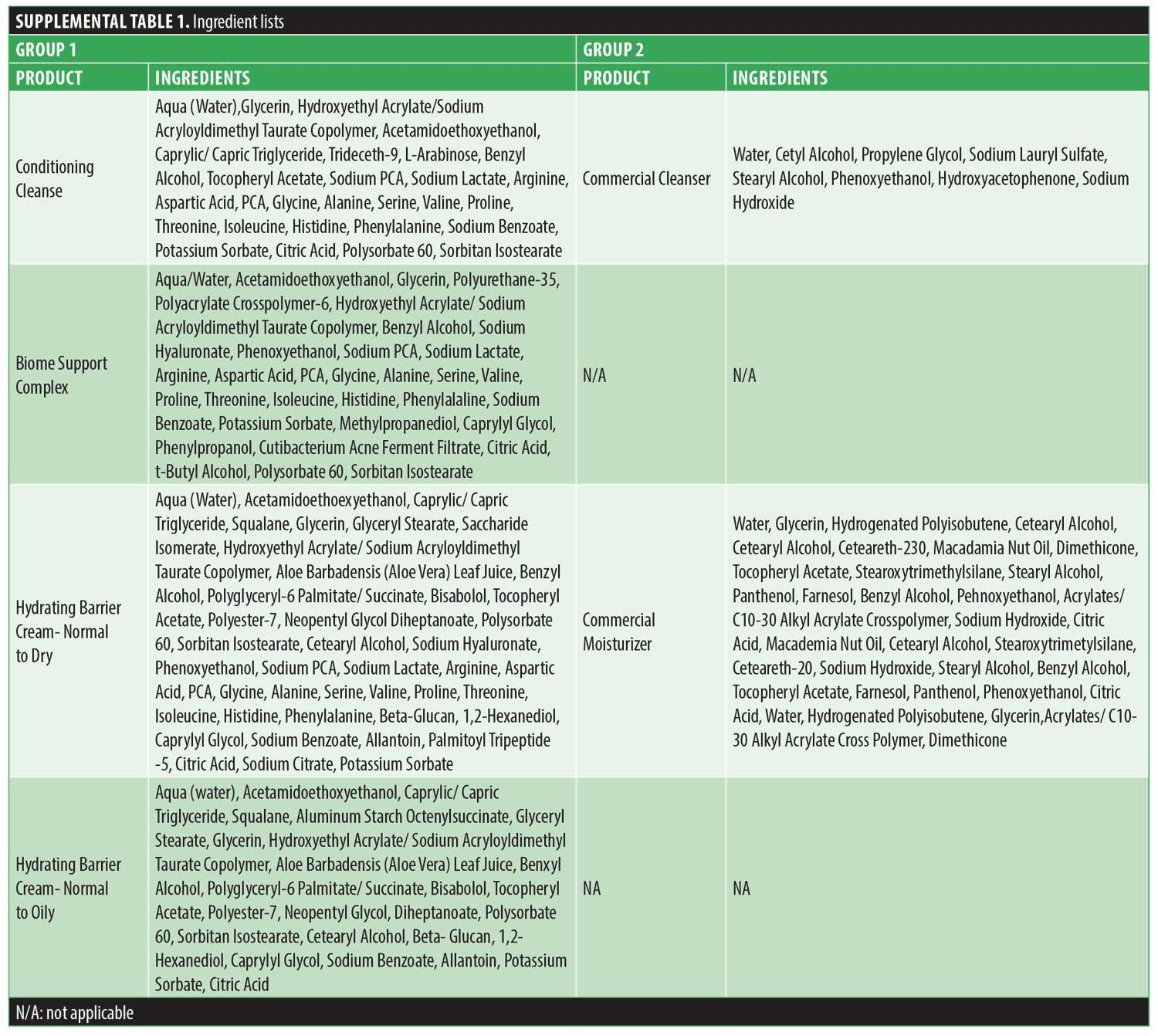
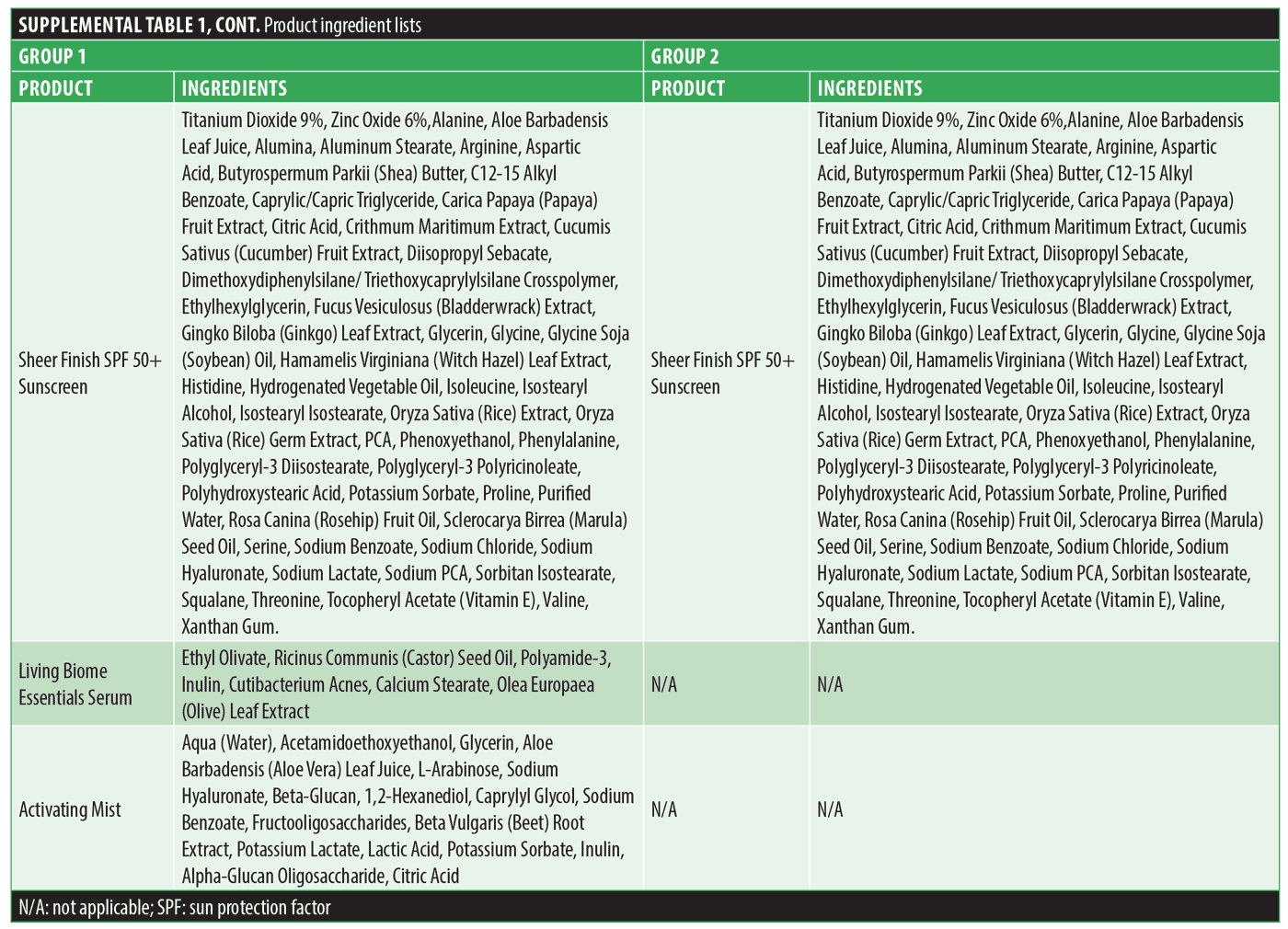
The study involved a 14-day preconditioning phase before the first microneedling treatment was performed at Visit 2 (V2). At their baseline visit (V1), the subjects were randomly assigned to Group 1 (G1) or Group 2 (G2). Subjects in G1 used the XYCM42-based topical probiotic regimen, including sunscreen (BIOJUVE, Crown Aesthetics; Dallas, Texas), while subjects in G2 used a traditional moisturizer-based skincare regimen that included a cleanser, moisturizer, and sunscreen (Equate, Walmart, Inc.; Bentonville, Arkansas). A complete ingredient list is shown for all products in Supplemental Table 1. All study participants were trained in the proper application of products, and instructions were provided regarding product usage requirements. Study participants agreed to discontinue their typical skincare regimens and refrain from any aesthetic procedure to the face throughout their participation in this clinical study.
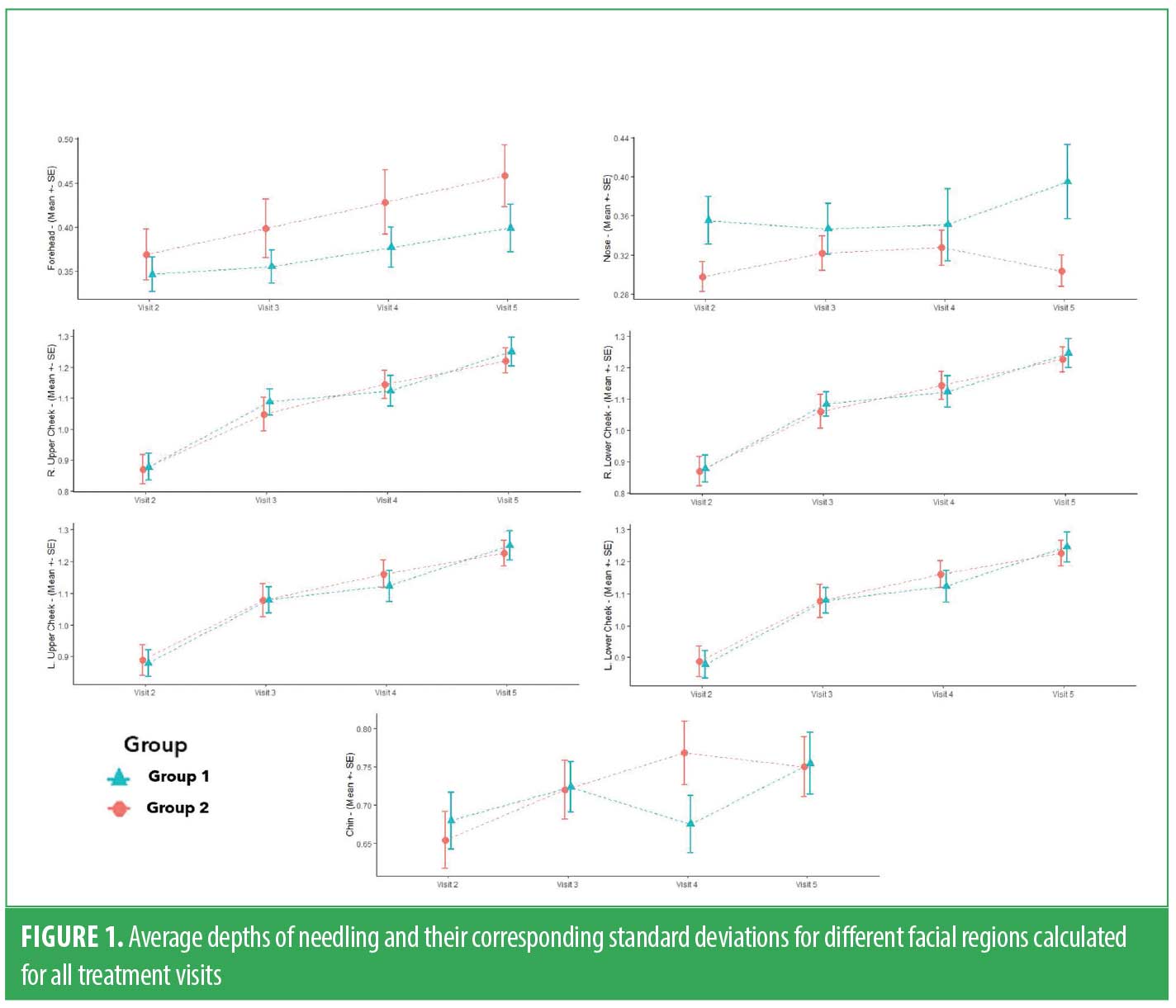
The study consisted of four microneedling treatments (V2, V3, V4, and V5), performed at three-week intervals using an automated microneedling device (SkinPen Precision medical device, Crown Aesthetics; Dallas, Texas). Each subject was instructed to attend their treatment visits without products applied to the face. A compounded numbing cream containing 20% benzocaine, 6% lidocaine, and 4% tetracaine was applied to the subject’s face to enhance the tolerability of the microneedling procedure. After a 20-minute exposure, the numbing cream was thoroughly removed by applying a facial cleanser (SkinFuse®, Crown Aesthetics; Dallas, Texas). The cleanser was gently massaged into the skin and subsequently wiped off using damp gauze. To ensure complete cleansing and drying of the subject’s face, multiple passes were made using the gauze. To safeguard against abrasion and friction during the microneedling procedure, a nonmedicated hydrogel wound dressing (SkinFuse® Lift HG, Crown Aesthetics; Dallas, Texas) was applied to the treatment area. The microneedling procedure was performed by the clinician, spanning all facial regions. The needle depths were adjusted for each treatment area, with the clinician performing three passes on each treatment region. The depths of all treatments were documented for each facial region. The minimum treatment depth recorded in this study was 0.25mm, and the maximum was 2.00mm. The mean value of treatment depths for each region is shown in Figure 1. Subjects in both groups were instructed to follow the microneedling device instructions for use (IFU) and apply the nonmedicated hydrogel for the first 24 hours postprocedure. After this period, subjects were instructed to reintroduce their assigned topical regimen.
Clinical assessments were conducted at all visits. These included qualitative and quantitative grading of acne scars, clinical grading assessments for skin quality, and collection of the safety endpoint data. Bioinstrumentation procedures were performed to assess several parameters using a multiparameter skin analysis system (DermaLab, Cortex Technology; Denmark). Standard photography was performed before and after each microneedling treatment using the Canon EOS80 camera (Tokyo, Japan). Product use compliance and additional safety evaluations were conducted through phone calls at specified intervals.
To assess acne scar improvement, two medically trained clinical evaluators used the Goodman and Baron’s scale at three key time points: V1, two-month follow-up (V6), and six-month follow-up (V7). Additionally, clinical grading and CGAIS assessments were conducted at V6 and V7, while the SGAIS assessment was performed by subjects from V2 through V7.
Safety assessments. Safety diaries. Participants were provided with a safety diary from V1 to V6 to record any anticipated or unanticipated events. Patients were to document any aftereffects of the procedure, such as erythema, swelling, burning/tingling, dryness, peeling, and/or itchiness. The daily scores for each day postmicroneedling treatment were averaged across treatments from V2 to V5. The average values between groups were compared, and graphs were plotted for each parameter per visit to show the average values and standard deviations (SD) for both groups during the above visits. The primary objective of this analysis was to determine which group experienced decreased severity and duration of these transient procedure sequalae.
After completing each treatment, the clinician conducted a safety assessment. This assessment encompassed parameters including erythema, scaling/peeling, edema, burning/stinging, itching, and dryness immediately postprocedure. The scores for these safety parameters were then averaged across all subjects. From V2 to V7, subjects were asked to complete a questionnaire, obtaining subject feedback on their microneedling treatments and regimen application.
Clinical assessments. Efficacy assessments. During V6 and V7, the clinician conducted live and photo CGAIS assessments. A t-test was subsequently utilized to compare improvements between these time points and identify the group that displayed a statistically significant aesthetic improvement. Participants’ completed SGAIS at V5, V6 and V7 was used for analysis. The acne scars were assessed using Goodman and Baron’s Qualitative and Quantitative scale, collected at V6 and V7, and then compared to the baseline between the groups. A paired t-test was employed to identify which group exhibited a significant improvement. Additionally, the clinician also assessed clinical grading parameters, such as texture, lack of evenness of skin tone, mottled pigments, discrete pigments, and fine lines and wrinkles. Both groups had their V6 and V7 scores compared to baseline to determine which group exhibited more significant improvement in these parameters.
Results
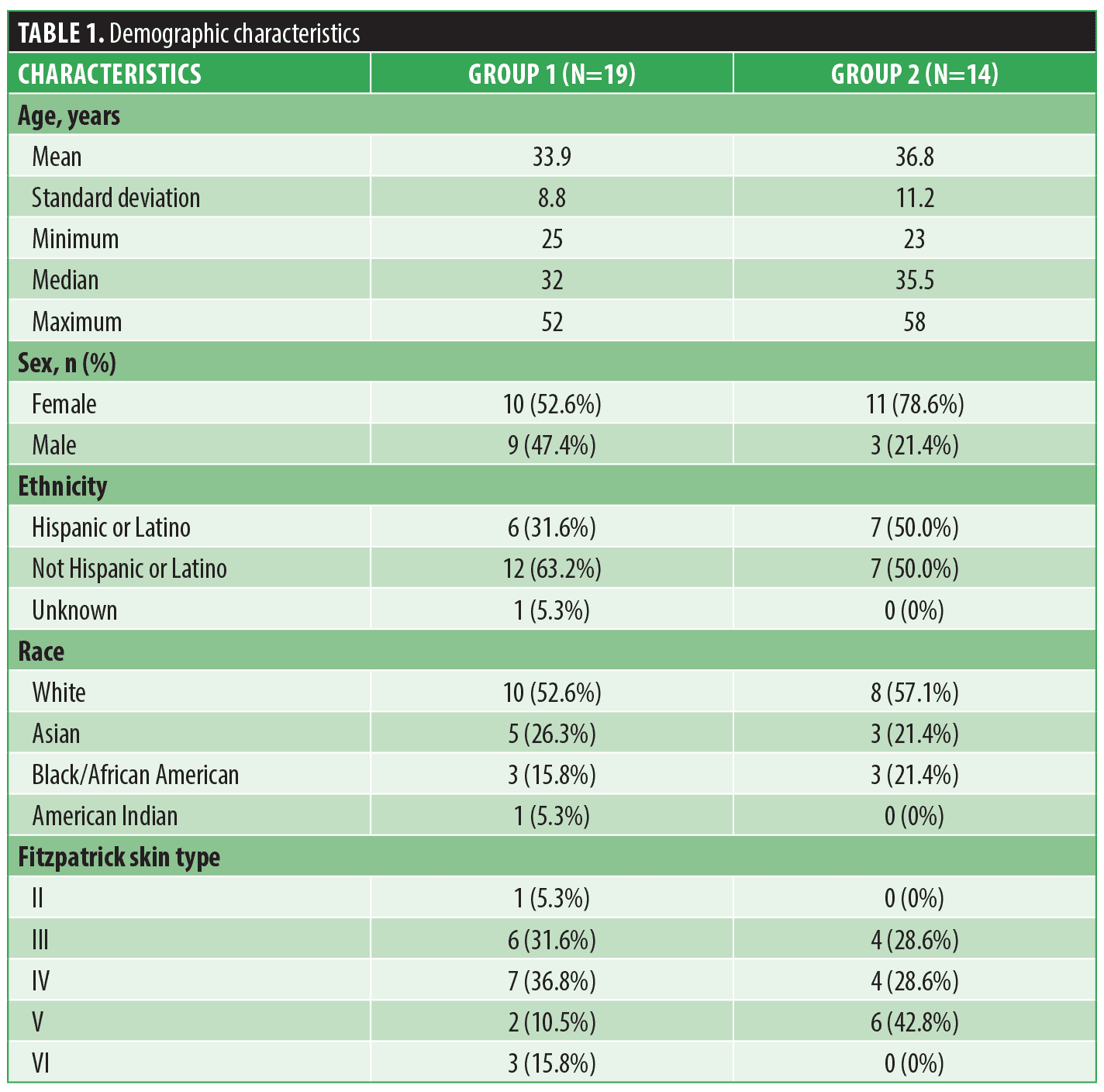
Subject demographics. A total of 45 subjects signed the IRB-approved informed consent and were screened according to the approved protocol. Five subjects did not meet the eligibility criteria and were not enrolled in the study. The remaining 40 subjects were randomly assigned to either G1 or G2, with an equal split of 20 subjects in each group. Even though all subjects completed the study in G1, we excluded one subject from data analysis due to noncompliance with product application requirements throughout the whole study. In G2, six subjects were discontinued, with five of them being lost to follow-up and one being noncompliant. See Table 1 for descriptive statistics for demographics by group.
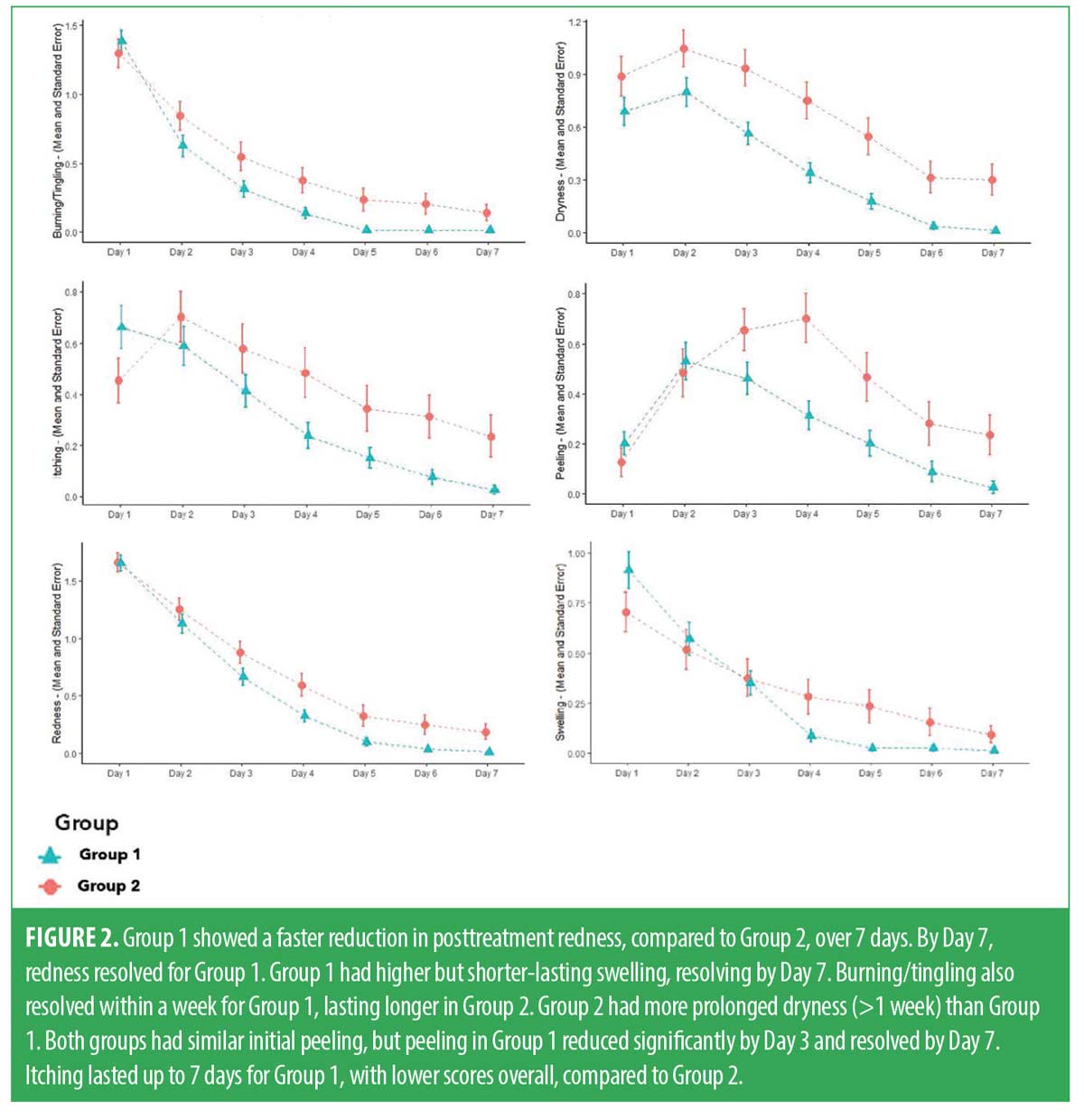
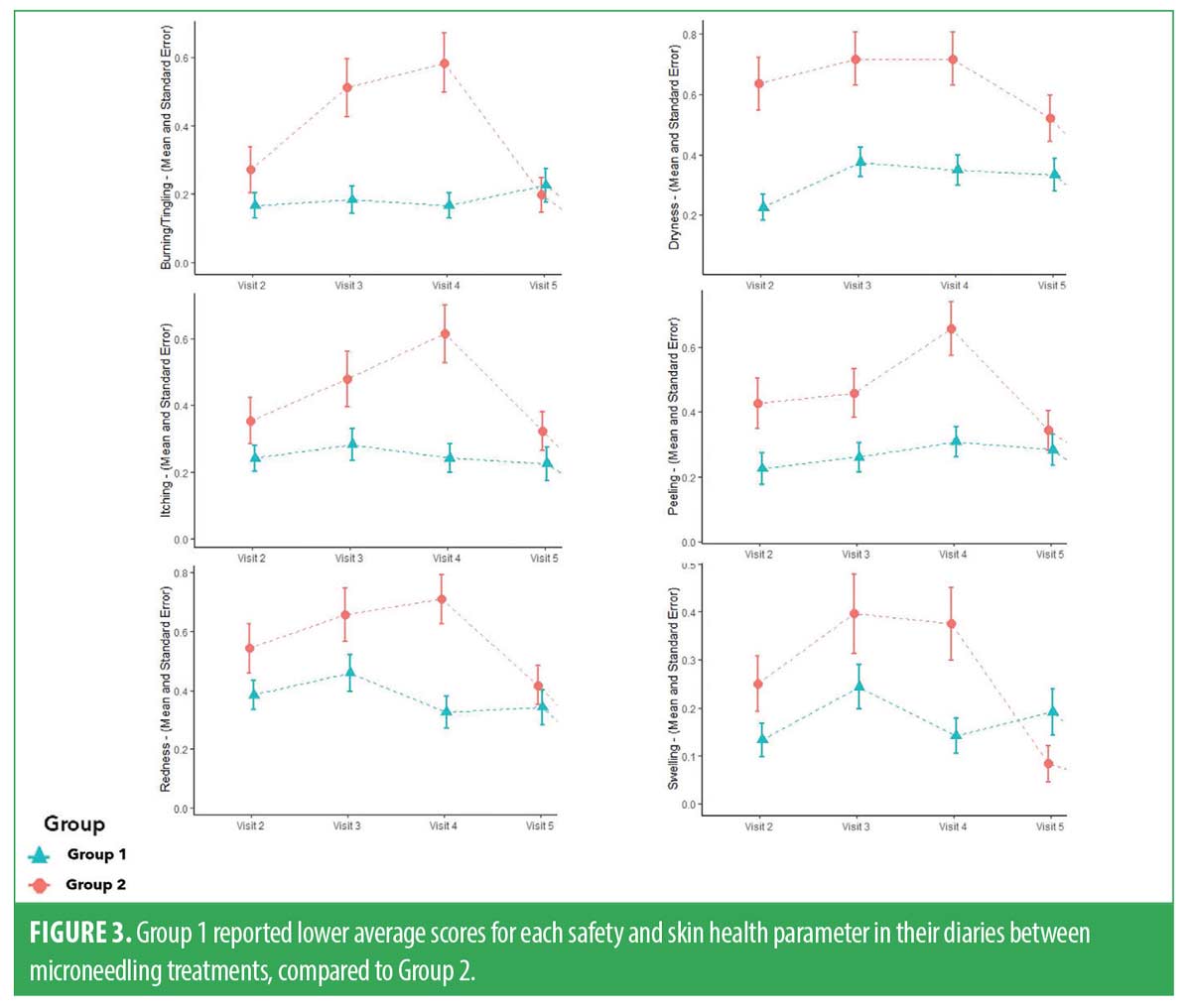
Safety assessments. Throughout the study, participants maintained diaries between treatment visits. Subjects were required to return diaries at every clinic visit, and research staff reviewed these diaries with participants to ensure accuracy. Anticipated transitory posttreatment sequalae, such as redness, swelling, burning/tingling, dryness, and itching, were captured to assess skin health and measure safety. In this study, all anticipated events generally occurred within seven days posttreatment. Diary data showed that both groups typically experienced the onset of anticipated events at the same rate. However, the topical probiotic group showed a faster decline in these parameters, not only in severity, but in duration as well. In G1, swelling and burning/tingling resolved within five days, redness and dryness cleared within six days, and itching resolved within seven days after treatment. In contrast, the severity of these anticipated events persisted at higher levels and these events lasted longer than seven days for subjects in G2. Both groups had an increase in peeling that resolved by Day 7 for G1 and beyond seven days for G2. Reduction of all parameters was statistically significant between groups (p<0.05). The daily trends of these safety parameters are presented in Figure 2. We then obtained the daily average for each parameter and generated mean values with SD graphs for each visit over the course of this study (Figure 3).
After each treatment session, the investigator evaluated participant skin response to microneedling. The results showed that 47 percent of subjects in both groups exhibited mild erythema after treatment, while 45 percent displayed moderate erythema consistently across all treatment visits. Instances of severe erythema were rare, occurring in one subject in G1 and two subjects in G2. The subjects did not require any medical attention, and erythema resolved within 24 hours. In terms of edema, 56 percent of subjects had no posttreatment swelling, while 43 percent experienced mild edema. Additionally, one subject encountered moderate edema during a single treatment visit. Approximately one-half of subjects reported mild burning/stinging sensations, and 19 percent indicated a moderate level of discomfort. As for itching, 14 percent of subjects experienced mild itching following microneedling, and four percent encountered a moderate degree of itching. Lastly, 44 percent of subjects had mild skin dryness immediately after the procedure, and five percent exhibited moderate dryness postmicroneedling.
During this study, 14 adverse events (AEs) were documented. These incidents varied in severity from mild to moderate. None of these AEs were attributed to microneedling treatment or application of the products used. It is noteworthy that all reported AEs resolved before the study’s conclusion.
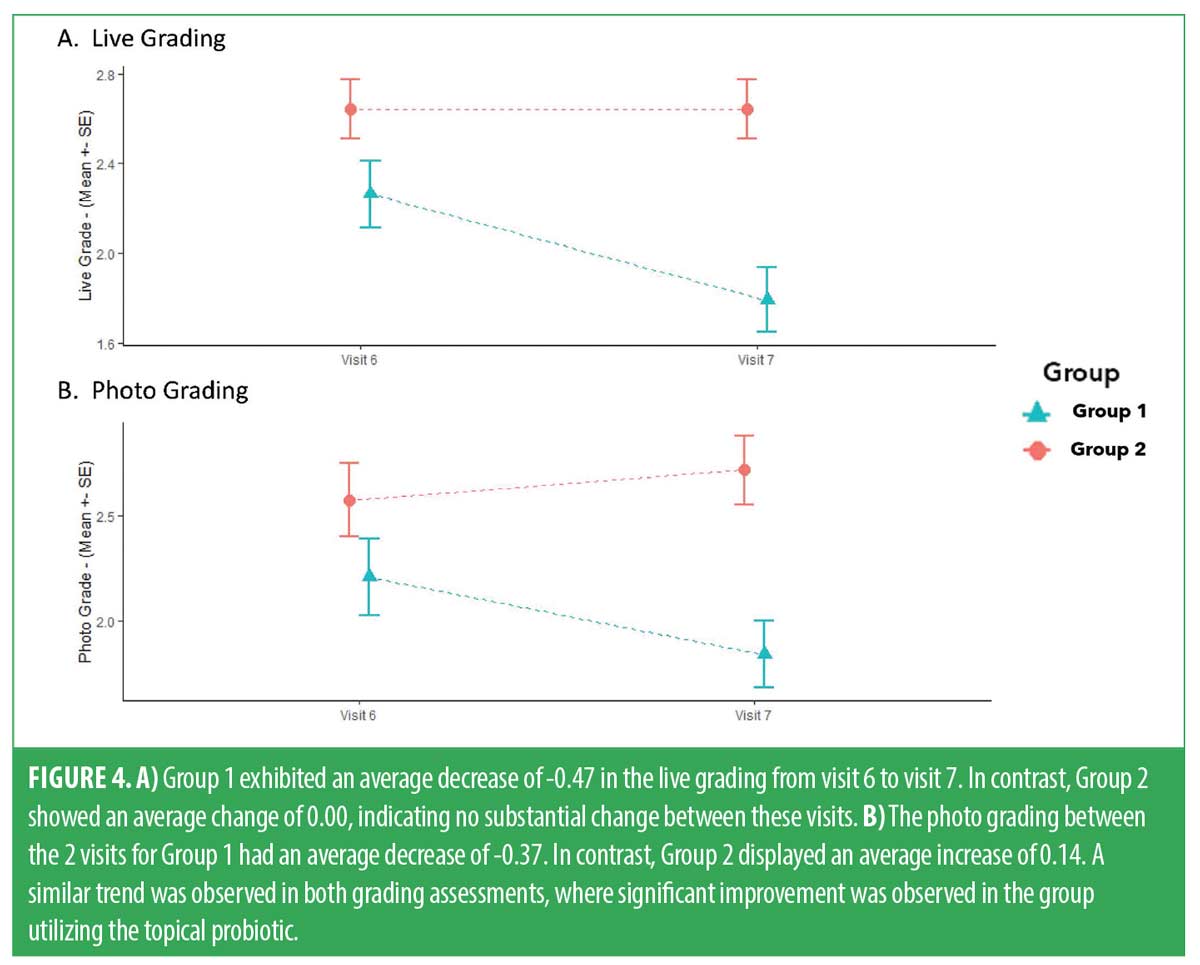
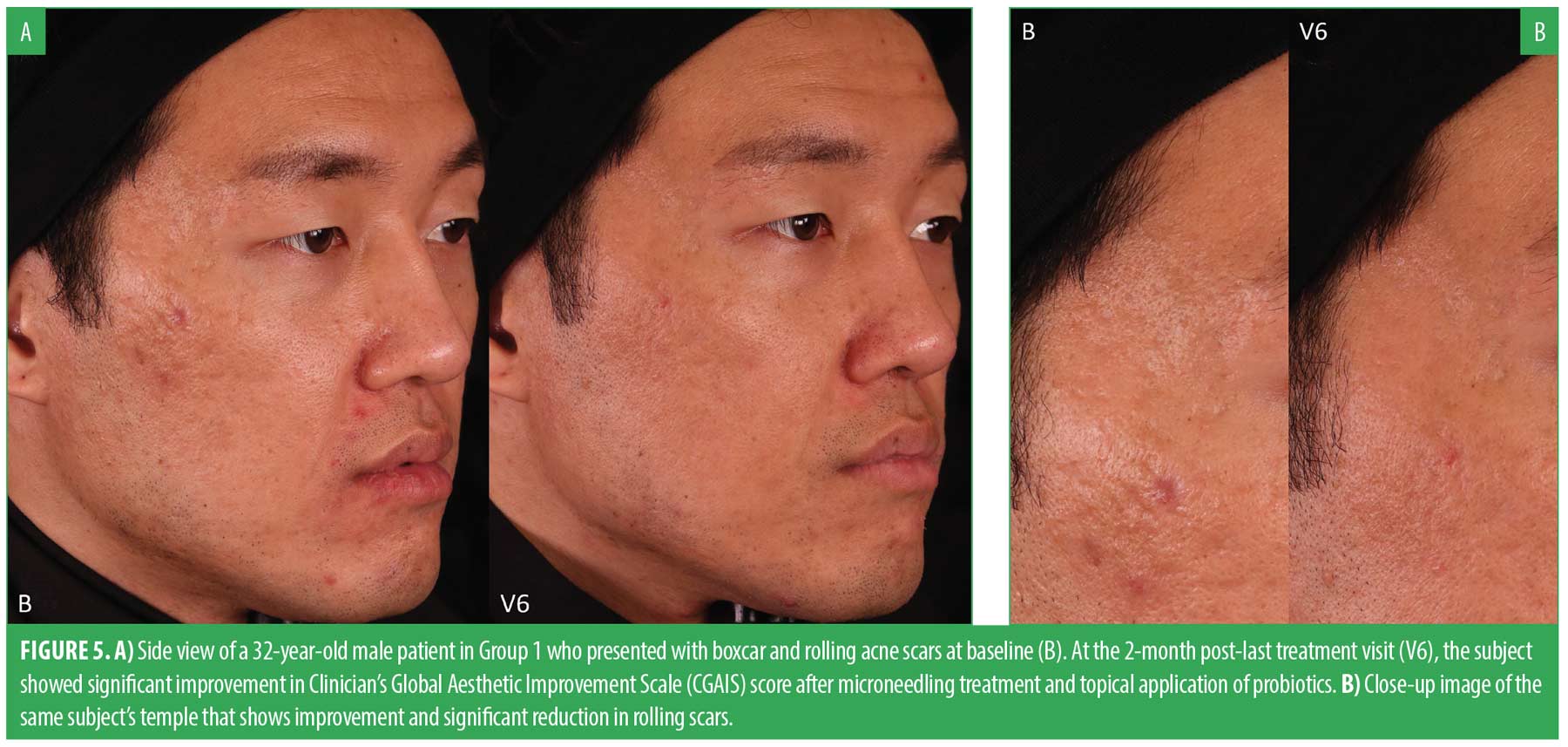
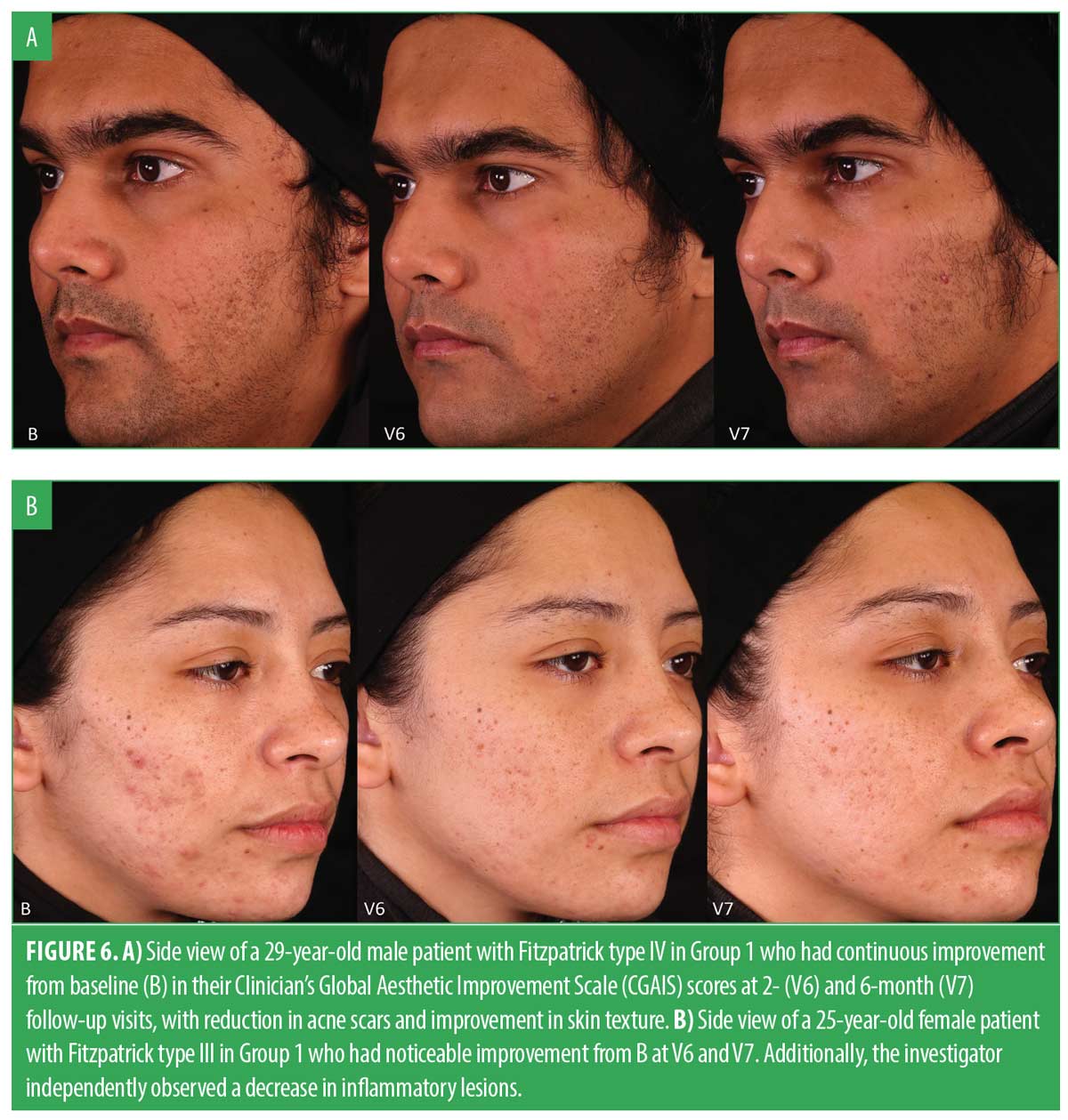
Efficacy results. The CGAIS scores were utilized to analyze the mean changes in both live and photo grading for both groups between V6 and V7. For the live grading, G1 experienced an average grade decrease of -0.47 (SD: 0.51), while G2 showed no overall change (SD: 0.39). The difference between the groups was statistically significant (p<0.05). Similarly, in terms of photo grade change, G1 exhibited an average grade decrease of -0.37 (SD: 0.50), compared to an average increase of 0.14 (SD: 0.36) in G2. The t-test revealed statistical significance for G1, with a p-value less than 0.05. Both the live and photo grading t-test results indicated that G1 had a greater, statistically significant improvement over G2. Figure 4 shows the mean score reduction in both groups. Figures 5 and 6 demonstrate a clear aesthetic improvement from baseline to follow-up visits among individuals in G1.
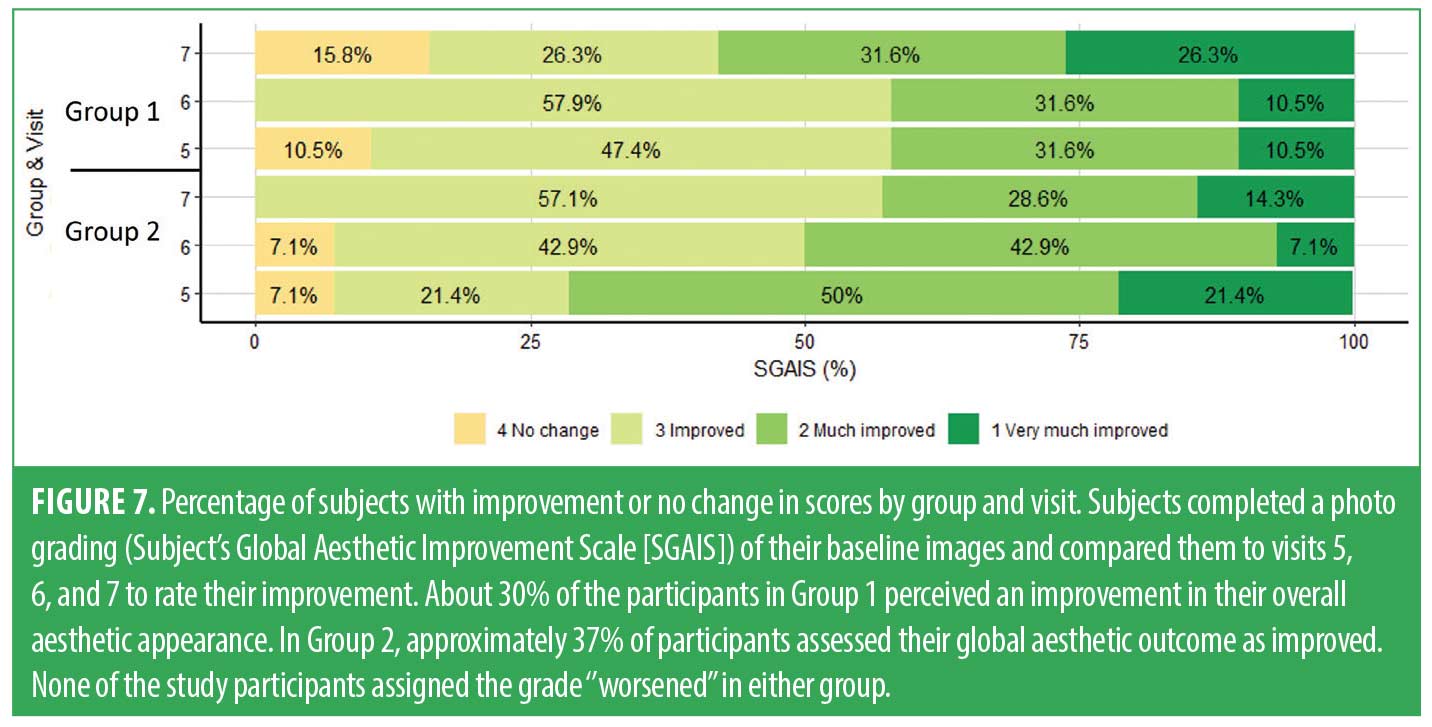
Participants’ SGAIS assessment results were determined by comparing the photographs from baseline with those from V5, V6, and V7. The results are shown in Figure 7. Overall, there were no statistically significant differences between the two groups.
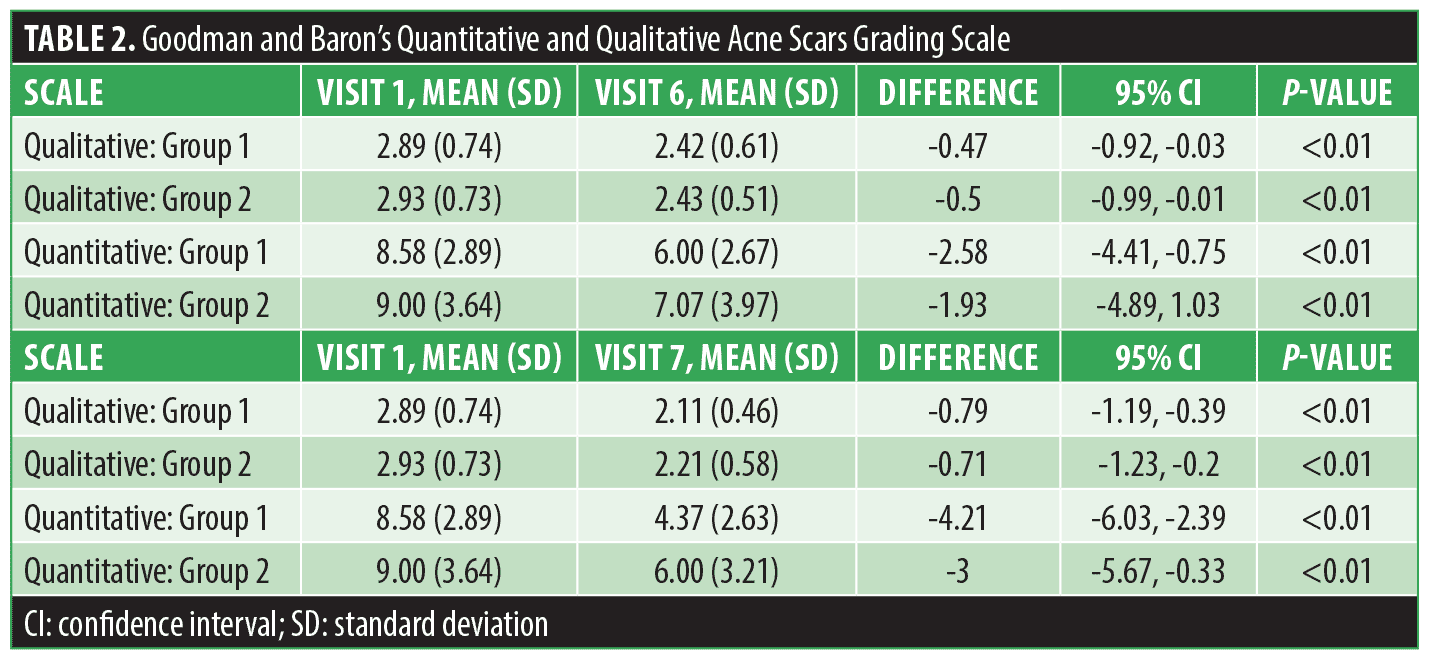
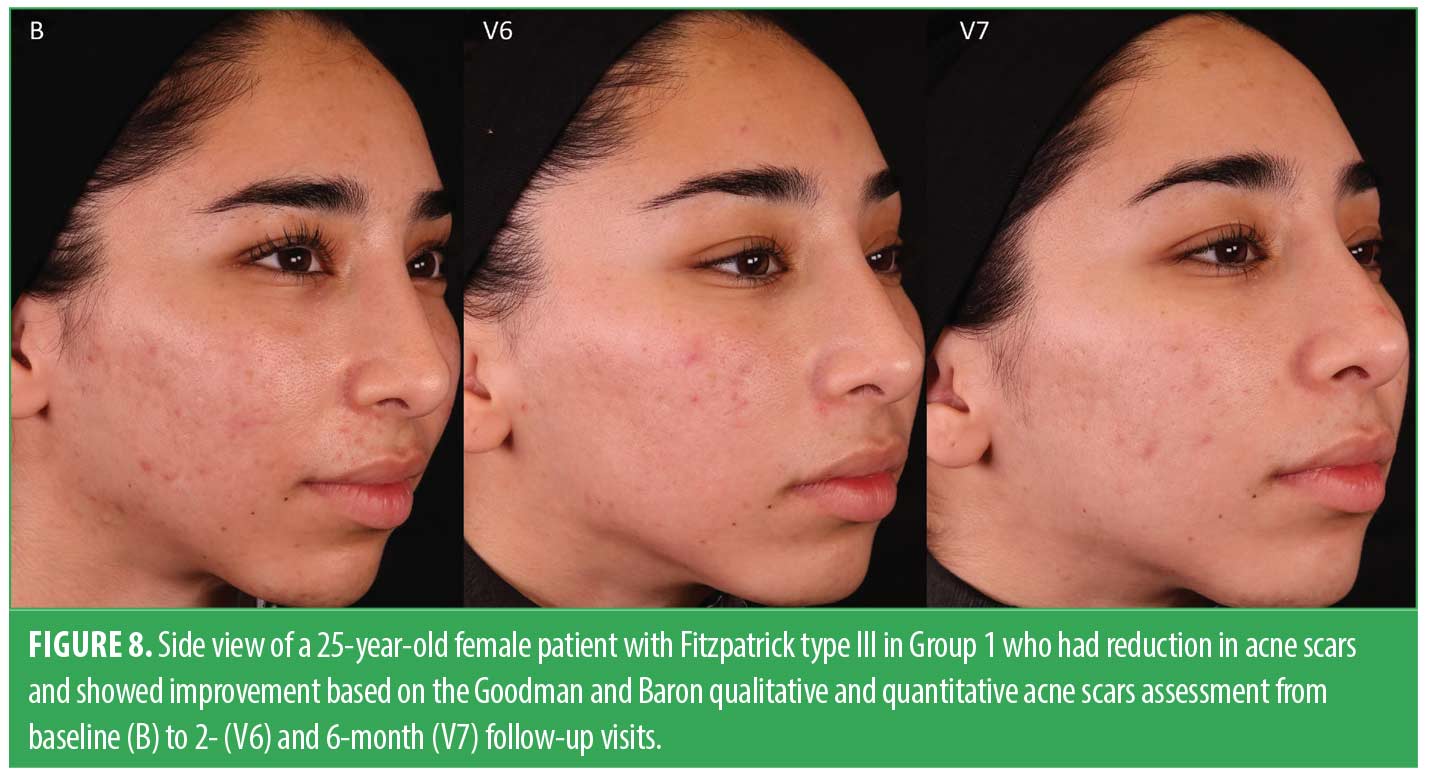
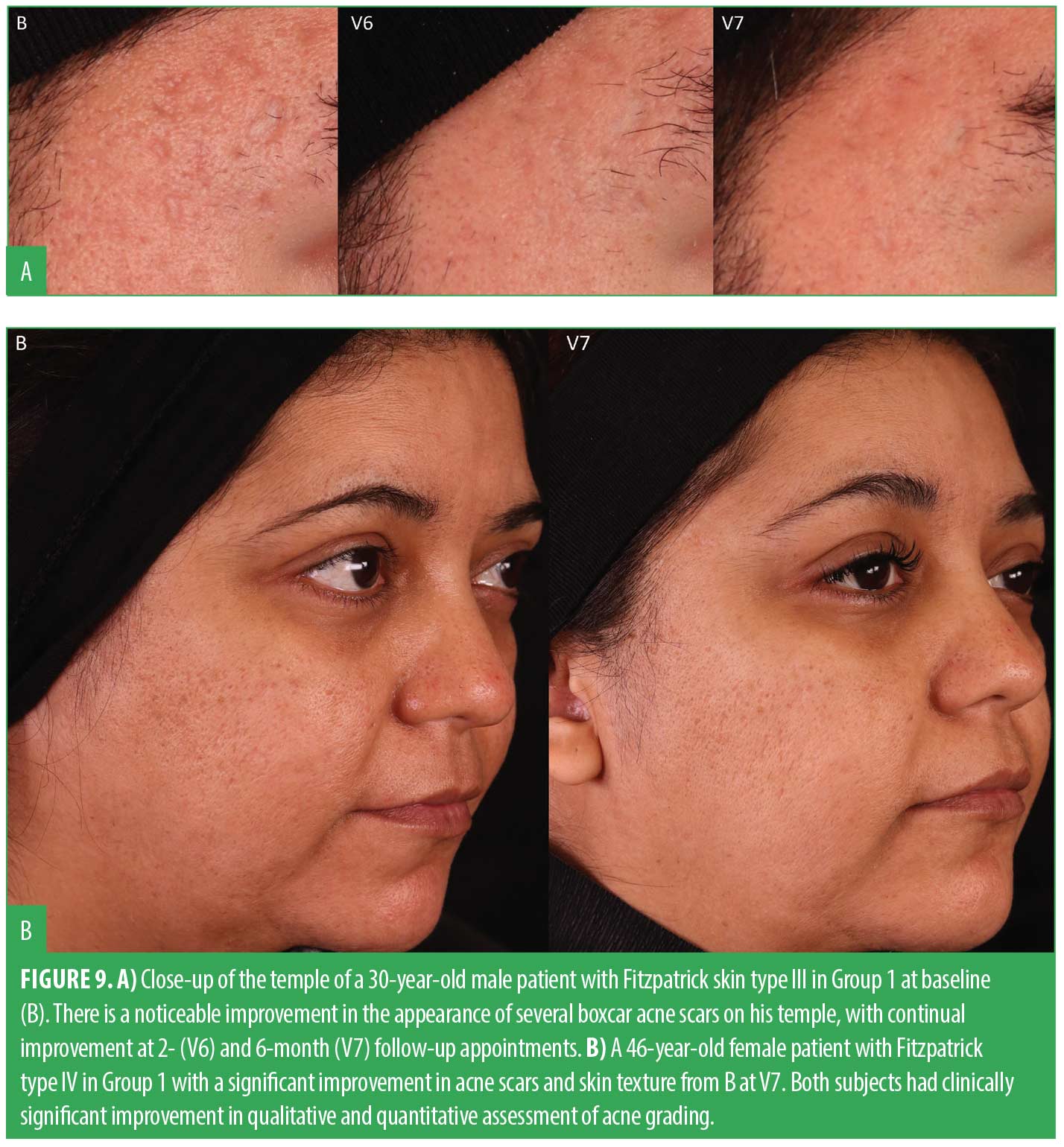
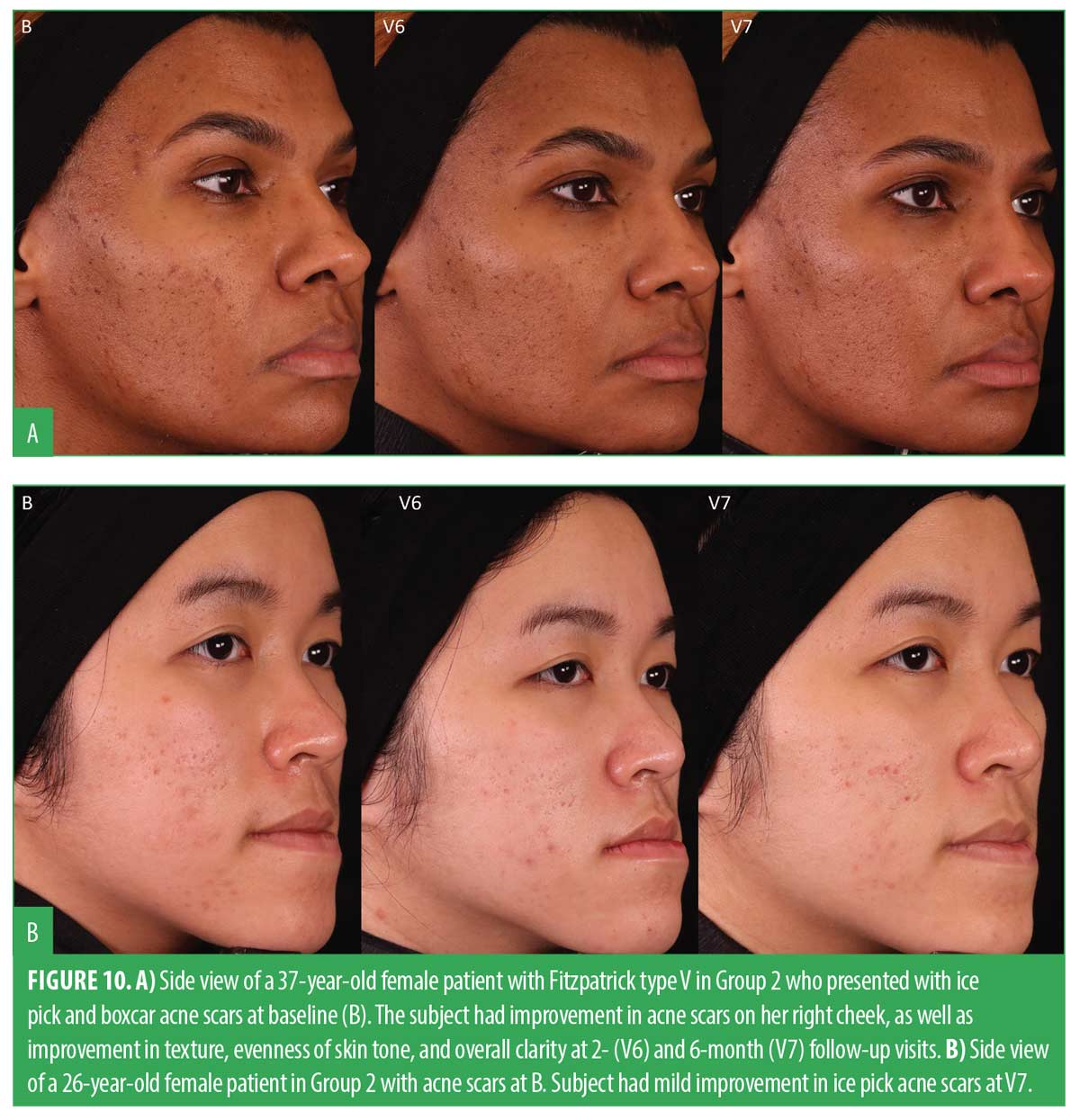
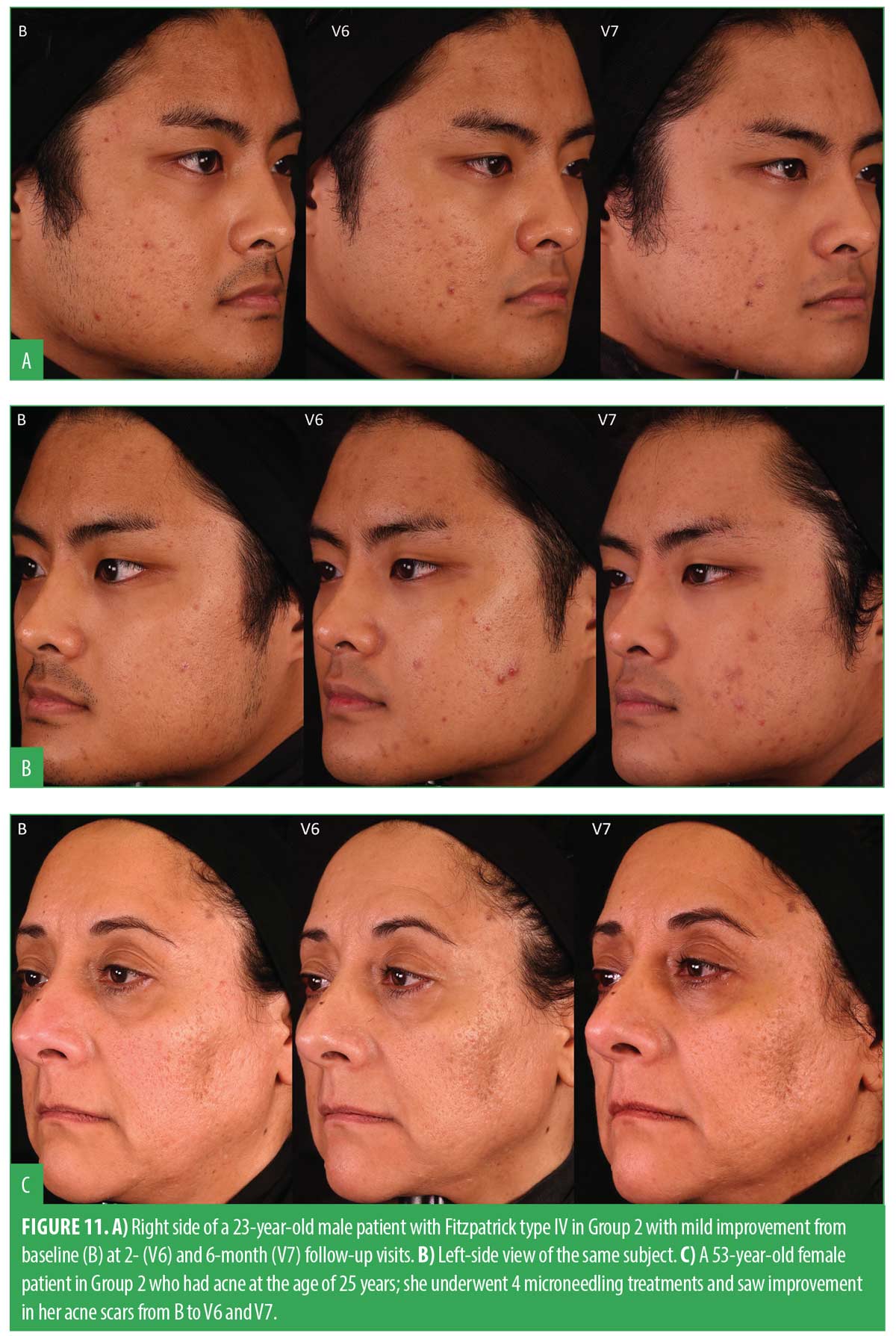
Acne scar gradings were completed at baseline, V6, and V7 using the Goodman and Baron’s Qualitative and Quantitative Acne Scar Grading Scale. Table 2 shows the results from a paired t-test comparing both visits to baseline. The results demonstrated that both groups exhibited a statistically significant improvement in the reduction of acne scars, compared to their initial baseline gradings. This improvement was seen in both the quantitative and qualitative scales, indicating positive progress for both groups over time. This extended follow-up corroborated the evidence supporting the efficacy of microneedling in the treatment of acne scars. Figures 8 and 9 show a noticeable reduction in acne scars in subjects in G1, and Figures 10 and 11 show reductions in subjects in G2.
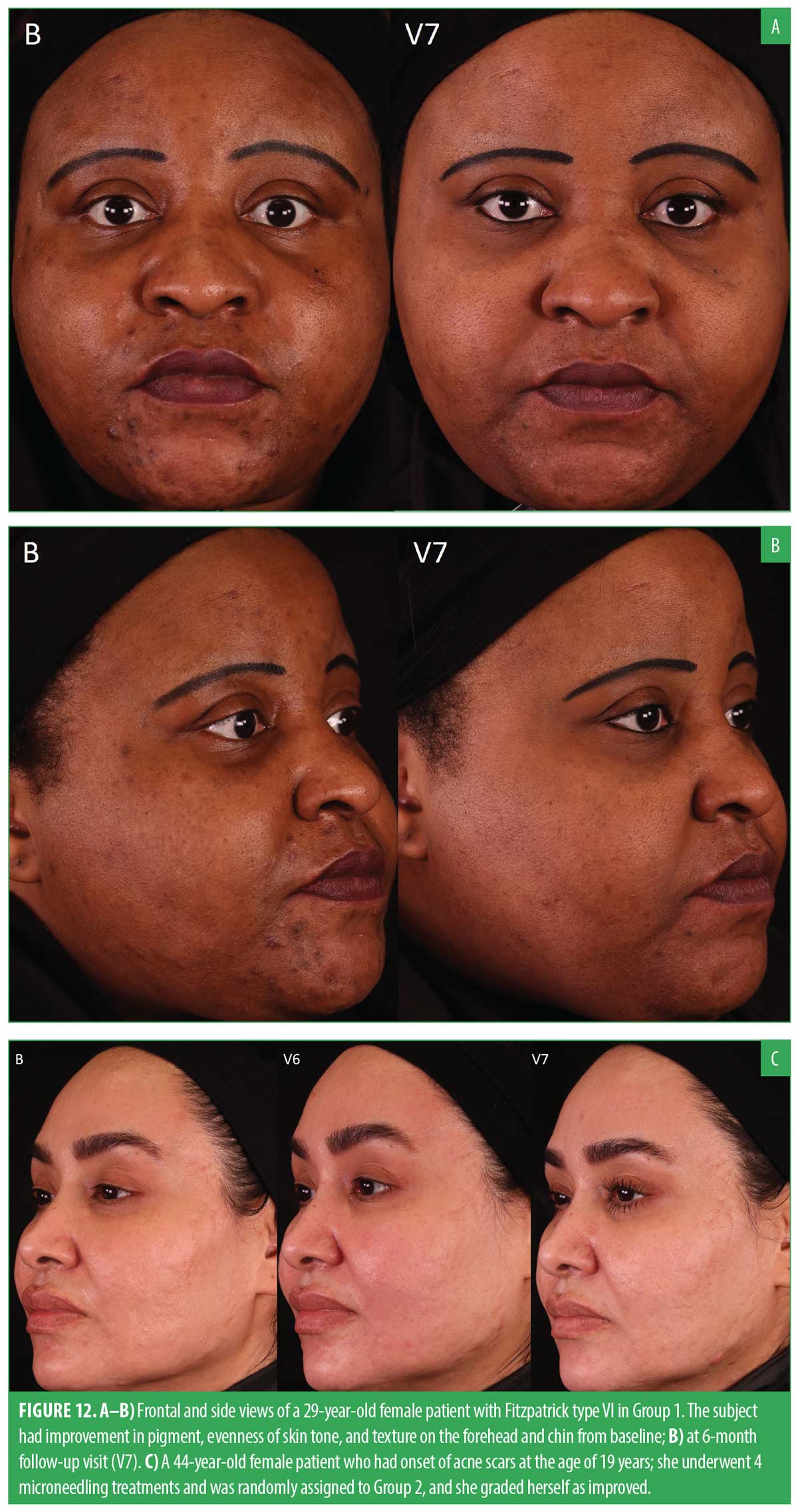
All subjects had statistically significant improvements in evenness of skin tone and texture when comparing clinical grading parameters taken at V6 and V7 to baseline. Notably, at V7, a significant improvement in the discrete pigment parameter for G1 was observed, whereas G2 exhibited this improvement at the V6 follow-up visit. The results for clinical grading assessments showed that participants in G1 achieved statistically significant clinical improvements in parameters including evenness of skin tone, discrete pigment, fine lines and wrinkles, and texture. G2 had similar significant improvements, apart from discrete pigment at V7 and wrinkles at V6. Figures 8 and 12A shows noticeable improvement in clinical grading for a subject in the XYCM42 group.
Discussion
Historically, the species C. acnes has often been associated with a negative impact on the overall health and wellbeing of the skin and acne vulgaris. Additionally, certain phylotypes of C. acnes have been reported as potential opportunistic pathogens in postoperative and implant-related orthopedic transplants.22 However, as has been reported by multiple studies, there is an emerging body of evidence pointing to the abundant benefits of having the correct strains and balance of C. acnes on the skin for individuals with healthy skin as well as those with skin conditions.6,23 With the ever-increasing research into the diverse array of microorganisms found within the human skin microbiome, it has become crucial to understand the role of microorganisms, and in particular, the role of certain phylotypes of C. acnes, as it pertains to their association with skin health. This becomes especially salient when considering use of topical skin probiotic products on broken or compromised skin, as it may seem counterintuitive to add a living strain of C. acnes to a medical procedure, such as microneedling.
To support the hypothesis that concurrent application of the XYCM42-based skin probiotic during a series of microneedling procedures will lead to better aesthetic outcomes, we conducted this study. We demonstrated that the incorporation of the living C. acnes subspecies defendens XYCM42-based skin biome care regimen with a regular series of microneedling procedures is safe and effective for the treatment of acne scars and improvement in aesthetic outcome, compared to microneedling alone. Additionally, the XYCM42-based regimen was shown to provide improved safety postmicroneedling, as demonstrated by a reduction in the magnitude and duration of reactive skin responses noted by patients, such as redness, swelling, and burning, compared to the use of a traditional skincare routine, consisting of a cleanser, moisturizer, and sunscreen.
Certain C. acnes strains, including the XYCM42 bacterium in this regimen, have previously been shown to produce significant quantities of substances to calm posttreatment skin, such as antioxidants and SCFAs.6,23,25 These molecules may be responsible for the attenuated resolution time for the known side effects of microneedling, such as erythema and edema. C. acnes strains are classified as facultative anaerobic bacteria that are generally found in low oxygen environments. The production of antioxidants by C. acnes may be a protective mechanism for the bacteria when exposed to an oxygen-rich environment, when topically applied to skin. This environment-induced production of antioxidants by C. acnes can subsequentially provide benefits to the patient in inflammatory conditions, such as microneedling.
It is also known that C. acnes strains produce significant amounts of the SCFA propionic acid from the metabolism of free fatty acids found in sebum. Production and release of propionic acid helps to maintain the acid mantle of the skin, thus improving the skin barrier properties. Propionic acid production by C. acnes strains also has been shown to reduce both the population of bacteria that prefer higher skin pH, such as S. aureus strains, as well as certain species of Malassezia.23 It is also known that SCFAs can activate AhR-mediated responses, including induction of regulatory T cells, anti-inflammatory markers, and induction of interleukin.22,26 Furthermore, SCFAs enhance AhR ligand-induced responses via their activities as histone deacetylase (HDAC) inhibitors. In skin models, HDACs exert an effect on both the epidermal morphology and differentiation process of human skin, such as thickening of the stratum corneum. SCFAs also improve the skin barrier by altering mitochondrial metabolism and function in keratinocytes. They are metabolized by epidermal keratinocytes, which in turn enhances the synthesis of long-chain fatty acids (LCFAs) and very long-chain fatty acids (VLCFAs) in keratinocytes, which are necessary to the generation of ceramides and are required for full keratinocyte maturation to establish skin barrier function.27
During microneedling treatment, millions of microchannels are created through the stratum corneum and into the epidermis and dermis layers of the skin. Dependent on the device setting, these channels may be over 2mm in depth and stay open for many hours, with a corresponding increase in transepidermal water loss (TEWL) values.28 It is well established that C. acnes bacteria are the predominant strain of bacteria found on the face, principally in the subsurface pores. The normal cleansing process prior to microneedling does not remove all the bacteria from the skin, especially those found within the pores, such as C. acnes strains. Given that the surface area of the hair follicles is estimated to be upward of 10-times greater than that of the skin surface,23 it would be reasonable to consider that these bacteria could potentially have a greater influence on the postmicroneedling response than that of bacteria only found on the skin surface. It has recently been shown in human keratinocytes, human skin constructs, and SKH-1 hairless mice that increases in skin lipids were mediated by SCFAs produced by C. acnes, and this was regulated by lipid synthesis genes.24 This increase in keratinocyte lipid content corresponded with improved innate barrier function, including a decrease in TEWL. It should be noted that in the referenced study, the types of lipids were not determined, and that mice keratinocytes produce a preponderance of sphingosine-based lipids. In contrast, humans with impaired skin barrier function predominately require phytosphingosine-based lipids.27 Further support to the benefits of SCFAs has been correlated with the severity of AD.
In regard to the potential to induce acneic lesions, clinical observations of the subjects in G2 showed a noticeable reduction in inflammatory lesions during the microneedling treatment phase. However, upon returning for two- and six-month postmicroneedling follow-ups, acne severity had returned to baseline and resembled the pattern reported in our previous study that evaluated the treatment of acne by microneedling alone.31 Intriguingly, subjects who received the C. acnes XYCM42-based regimen did not display the same posttreatment flare-up of inflammatory lesions. Their reduction in inflammatory lesions continued throughout the study period. Comparative images vividly illustrate these contrasting trends between the two treatment groups (Figures 6B and 11A).
Retinoids and vitamin C-associated compounds are often considered the gold standard for improving skin damage associated with intrinsic and extrinsic aging. Much of the benefit of these classes of ingredients is attributable to the upregulation of collagen type I (COL1A1) and collagen type 3 (COL3A1) genes and subsequent production of procollagen 1 and procollagen 3. It has also been shown that C. acnes defendens XYCM42 bacterium upregulate collagen 1 and 3 genes in cell culture models, but without the irritation or the stability issues of the other compounds.6 Like retinoids, C. acnes strains also produce components that have demonstrated inhibition of matrix metalloproteinases (MMPs).32,33 MMPs are thought to play an important role in keratinocyte proliferation and turnover.34 The increase in collagen synthesis, decrease in collagen degradation, and cellular turnover after microneedling are important for the remodeling of skin and improvement of acne scars.35 Assessing the improvement of acne scars in this study, both treatment groups showed statistically significant improvements, both qualitative and quantitative, compared to their respective baseline scores. It may be worth noting that there was an observable improvement with the XYCM42-based regimen group, compared to the control group, in the quantitative assessment of acne scars as early as two-months posttreatment, although this was not statistically significant. At the final follow-up visit, both qualitative and quantitative improvement assessments in acne scars were directionally greater in the XYCM42-based regimen group. As the skin remodeling process after microneedling has been reported to last several months,36 the greater difference in improvements in acne scar grading observed in the two groups between two- and six-month follow-ups may have been due to the continual signaling from the XYCM42-based regimen, whereas the control group may have been relying solely on any residual signal from the last microneedling treatment.
Conclusion
We believe that that this is the first study of its kind to combine a living skin biome care regimen with an aesthetic procedure, such as microneedling. This study supports the safety of a skin biome care regimen utilizing a specific living bacterium, XYCM42, based on C. acnes defendens, applied throughout the course of microneedling treatments. Additionally, it also demonstrates improved recovery of patients after microneedling treatments, compared to a traditional cleanser and moisturizer routine. Any concern regarding potential for opportunistic infection of barrier-impaired microneedled skin by the bacteria was not substantiated. No infections were seen in any patient after any of the microneedling procedures or during the use of the probiotic product line. We conclude that the use of a probiotic skin biome care regimen addresses the needs of the skin before and after microneedling and provides significant, early, demonstratable benefits to the patient. Additional studies should be undertaken to assess if these study findings translate to other aesthetic treatments, such as laser skin resurfacing, laser hair removal, intense pulse light (IPL) photorejuvenation, microdermabrasion, chemical peels, and facial cleansing procedures. The evidence generated here suggests the key role of preprocedural microbiome optimization in the mitigation of postprocedural anticipated events, which may impact optimization of the clinical outcomes of aesthetic interventions.
Acknowledgments
We want to acknowledge Jose Maldonado, PhD, who helped prepare the manuscript, and John Harbell, PhD, for providing edits and comments.
Author Contributions
MLA was the lead investigator on the study. All authors contributed to study design and collectively wrote the body of the manuscript.
References
- Cachafeiro T, Escobar G, Maldonado G, et al. Comparison of nonablative fractional erbium laser 1,340 nm and microneedling for the treatment of atrophic acne scars. Dermatol Surg. 2016;42(2):232–241.
- Loesche M, Gardner SE, Kalan L, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(1):237–244.
- Park J-U, Oh B, Lee JP, et al. Influence of microbiota on diabetic foot wound in comparison with adjacent normal skin based on the clinical features. Biomed Res Int. 2019;2019:7459236.
- Gupta S, Poret AJ, Hashemi D, et al. Cutaneous surgical wounds have distinct microbiomes from intact skin. Microbiol Spectr. 2023;11(1):e0330022.
- Kirker KR, Secor PR, James GA, et al. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen. 2009;17(5):690–699.
- Rhee MS, Alqam ML, Jones BC, et al. Characterization of a live Cutibacterium acnes subspecies defendens strain XYCM42 and clinical assessment as a topical regimen for general skin health and cosmesis. J Cosmet Dermatol. 2023;22(3):1031–1045.
- Juarez VM, Montalbine AN, Singh A. Microbiome as an immune regulator in health, disease, and therapeutics. Adv Drug Deliv Rev. 2022;188:114400.
- Gimblet C, Meisel JS, Loesche MA, et al. Cutaneous leishmaniasis induces a transmissible dysbiotic skin microbiota that promotes skin inflammation. Cell Host Microbe. 2017;22(1):13–24.e4.
- Tax G, Urbán E, Palotás Z, et al. Propionic acid produced by Propionibacterium acnes strains contributes to their pathogenicity. Acta Derm Venereol. 2016;96(1):43–49.
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14(1):2096995.
- Uberoi A, Bartow-McKenney C, Zheng Q, et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe. 2021;29(8):1235–1248.e8.
- Lu Q, Stappenbeck TS. Local barriers configure systemic communications between the host and microbiota. Science. 2022;376(6596):950–955.
- Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143–155.
- Esser C, Bargen I, Weighardt H, et al. Functions of the aryl hydrocarbon receptor in the skin. Semin Immunopathol. 2013;35(6):677–691.
- Furue M, Takahara M, Nakahara T, Uchi H. Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res. 2014;306(9):769–779.
- Sutter CH, Bodreddigari S, Campion C, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol Sci. 2011;124(1):128–137.
- van den Bogaard EH, Bergboer JGM, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123(2):917–927.
- Cao K, Chen G, Chen W, et al. Formalin-killed Propionibacterium acnes activates the aryl hydrocarbon receptor and modifies differentiation of SZ95 sebocytes in vitro. Eur J Dermatol. 2021;31(1):32–40.
- Tian X, Hellman J, Horswill AR, et al. Elevated gut microbiome-derived propionate levels are associated with reduced sterile lung inflammation and bacterial immunity in mice. Front Microbiol. 2019;10:159.
- Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13(20):2826–2832.
- Anshory M, Effendi RMRA, Kalim H, et al. Butyrate properties in immune-related diseases: friend or foe? Fermentation. 2023;9(3):205.
- Mayslich C, Grange PA, Dupin N. Cutibacterium acnes as an opportunistic pathogen: an update of its virulence-associated factors. Microorganisms. 2021;9(2):303.
- Rozas M, Hart de Ruijter A, Fabrega MJ, et al. From dysbiosis to healthy skin: major contributions of Cutibacterium acnes to skin homeostasis. Microorganisms. 2021;9(3):628.
- Almoughrabie S, Cau L, Cavagnero K, et al. Commensal Cutibacterium acnes induce epidermal lipid synthesis important for skin barrier function. Sci Adv. 2023;9(33):eadg6262.
- Allhorn M, Arve S, Brüggemann H, Lood R. A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci Rep. 2016;6:36412.
- Wu J, Pang T, Lin Z, et al. The key player in the pathogenesis of environmental influence of systemic lupus erythematosus: aryl hydrocarbon receptor. Front Immunol. 2022;13:965941.
- Trompette A, Pernot J, Perdijk O, et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022;15(5):908–926.
- Kalluri H, Kolli CS, Banga AK. Characterization of microchannels created by metal microneedles: formation and closure. AAPS J. 2011 Sep;13(3):473–481.
- Gallo RL. Human skin is the largest epithelial surface for interaction with microbes. J Invest Dermatol. 2017;137(6):1213–1214.
- Kawana M, Miyamoto M, Ohno Y, Kihara A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J Lipid Res. 2020;61(6):884–895.
- Alqam ML, Jones BC, Hitchcock TM. Study to determine the safety and efficacy of microneedling as an effective treatment for acne vulgaris. Skin Health Dis. 2023;3(5):e264.
- Xiao X, Hu X, Yao J, et al. The role of short-chain fatty acids in inflammatory skin diseases. Front Microbiol. 2023;13:1083432.
- Kawamura T, Andoh A, Nishida A, et al. Inhibitory effects of short-chain fatty acids on matrix metalloproteinase secretion from human colonic subepithelial myofibroblasts. Dig Dis Sci. 2009;54(2):238–245.
- Michopoulou A, Rousselle P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing? Eur J Dermatol. 2015;25 Suppl 1:33–42.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8(7):36–42.
- Sitohang IBS, Sirait SAP, Suryanegara J. Microneedling in the treatment of atrophic scars: a systematic review of randomised controlled trials. Int Wound J. 2021;18(5):577–585.