J Clin Aesthet Dermatol. 2023;16(12):39–44.
J Clin Aesthet Dermatol. 2023;16(12):39–44.
by Prof. Lili Legiawati, MD, PhD; Shannaz Nadia Yusharyahya, MD, PhD; Prof. Irma Bernadette, MD, PhD; Endi Novianto, MD, PhD; Mufqi Handaru Priyanto, MD; Keneyzia Carla Gliselda, MD; Septiana Iriyanty, MD; and Rizka Mutiara, MD
All authors are with Department of Dermatology and Venereology, Faculty of Medicine, Universitas Indonesia, dr. Cipto Mangunkusumo National General Hospital in Jakarta, Indonesia.
FUNDING: This research was funded by Universitas Indonesia through KEMENDIKBUDRISTEK TA 2022 Grant with contract number NKB-13/H2.RST/HKP.05.00/2022. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
DISCLOSURES: The authors have no conflicts of interest relevant to the contents of this article.
ABSTRACT: Objective. Platelet-rich plasma (PRP) is widely known as an alternative therapy for androgenetic alopecia (AGA); however, there is no standardized method for its preparation and application. This study aims to compare the thrombocyte count elevation and clinical AGA improvements between single- and double-spin PRP preparation methods.
Methods. This preliminary, double-blind, randomized clinical trial included 30 male subjects with AGA aged 25 to 59 years with Hamilton-Norwood stages III to VI. Subjects were divided into a single-spin group (3,000rpm for 15 minutes) and a double-spin group (first spinning at 1,500rpm for 6 minutes, continuing at 2,500rpm for 15 minutes). The study was conducted for six weeks, with a two-week visit interval. Baseline and PRP thrombocyte counts were assessed on the initial appointment. A total of 1cc of PRP was intradermally injected into a 6×4cm predetermined area, administered at Weeks 0, 2, and 4. At every visit, clinical progress was assessed by overall hair appearance, photography, trichoscopy, and trichoscan. All subjects were instructed to use minoxidil twice daily during the study. This study has been registered at clinicaltrials.gov (ID No. NCT05681897).
Results. Both groups increased thrombocyte counts by 4 to 5 times from their initial levels; however, the increase in the single-spin group was more significant. Significant improvements were observed in both groups, including hair density, hair rate, and hair count of anagen, telogen, vellus, and terminal hair.
Limitations. Limitations include lack of placebo or vehicle control.
Conclusion. Both PRP preparation methods significantly raise thrombocyte counts, substantially improve nearly all hair parameters, and have tremendous therapeutic promise for treating AGA. Clinicians may designate one of the two techniques.
Keywords: Androgenetic alopecia, centrifugation, platelet-rich plasma, thrombocyte
Androgenetic alopecia (AGA) is the most common cause of hair loss and baldness.1 AGA is characterized by a noncicatricial progressive miniaturization of the hair follicle and shortening of the anagen phase. This process occurs with a specific distribution, resulting in a pattern formation.2 Although the condition is not life-threatening, patients’ self-consciousness about their appearance and cosmetic concerns have become common issues. According to Huang et al,3 AGA was strongly associated with decreased health-related quality of life (HRQoL) and emotional disturbances. Without treatment, AGA is known to be progressive. Currently, the preferred treatment options work by halting the progression of hair loss and include topical and oral medications and invasive surgery.4
Platelet-rich plasma (PRP) is an autologous plasma concentrate containing 4- to 7-times the thrombocyte concentration of whole blood. PRP consists of numerous growth factors that can enhance skin and hair follicle rejuvenation.5 Although PRP has been widely used as an alternative therapy for AGA, there is no standardized method for its preparation and application.6
According to Marx et al,7 the thrombocyte concentration in 5mL of PRP ought to be as high as 1,000,000/µL. Stevens et al8 proposed a PRP preparation and application standard based on single-spin centrifugation, with thrombocyte concentrations 3- to 6-times higher than whole blood, using calcium chloride or calcium gluconate as an activator. A study by Gkini et al,9 which was done using a single-spin centrifugation method, discovered that hair density increased and hair loss reduced significantly at three months without remarkable major side effects. On the other hand, Kurita et al10 found that double-spin centrifugation yields higher thrombocyte concentrations than single-spin centrifugation and that the double-spin method is preferred for PRP preparation. This claim was supported by Sharma et al,11 who discovered that the single-spin method cannot produce “pure” PRP. Single-spin centrifugation is only recommended for diagnostic purposes; it is not recommended for PRP production.11 A study conducted by Jo et al12 using the double-spin centrifugation method revealed a greater increase in thrombocyte count after the second centrifugation, compared to the thrombocyte concentration after the first centrifugation.
Currently, the standard operating procedure of PRP preparation for treating AGA at Dr. Cipto Mangunkusumo Hospital (RSCM) is 15 minutes of single-spin centrifugation at 3,000rpm. This study aims to evaluate the effects of two different PRP preparation methods on thrombocyte count elevation and clinical improvement of AGA.
Methods
This preliminary, randomized, double-blind clinical trial was conducted at the Department of Dermatology and Venereology Outpatient Clinic of RSCM from September to November 2022. The study has been approved by the Health Research Ethical Committee (No. KET-876/UN2.F1/ETIK/PPM.00.02/2022), the Faculty of Medicine, Universitas Indonesia, and RSCM. This study has been registered at clinicaltrials.gov with the ID No. NCT05681897.
This study included 30 male subjects between the ages of 25 and 59 years who had AGA with Hamilton-Norwood stages III to VI and stopped taking topical anti-androgens or minoxidil for at least one month or oral anti-androgens or minoxidil for at least three months. Subjects with alopecia of any kind, history of keloid or blood coagulation disorders, and nonsteroidal anti-inflammatory drug (NSAID) use seven days prior to the study were excluded. Growth factor treatments, such as PRP and microneedling, within the six months prior to the study were also excluded.
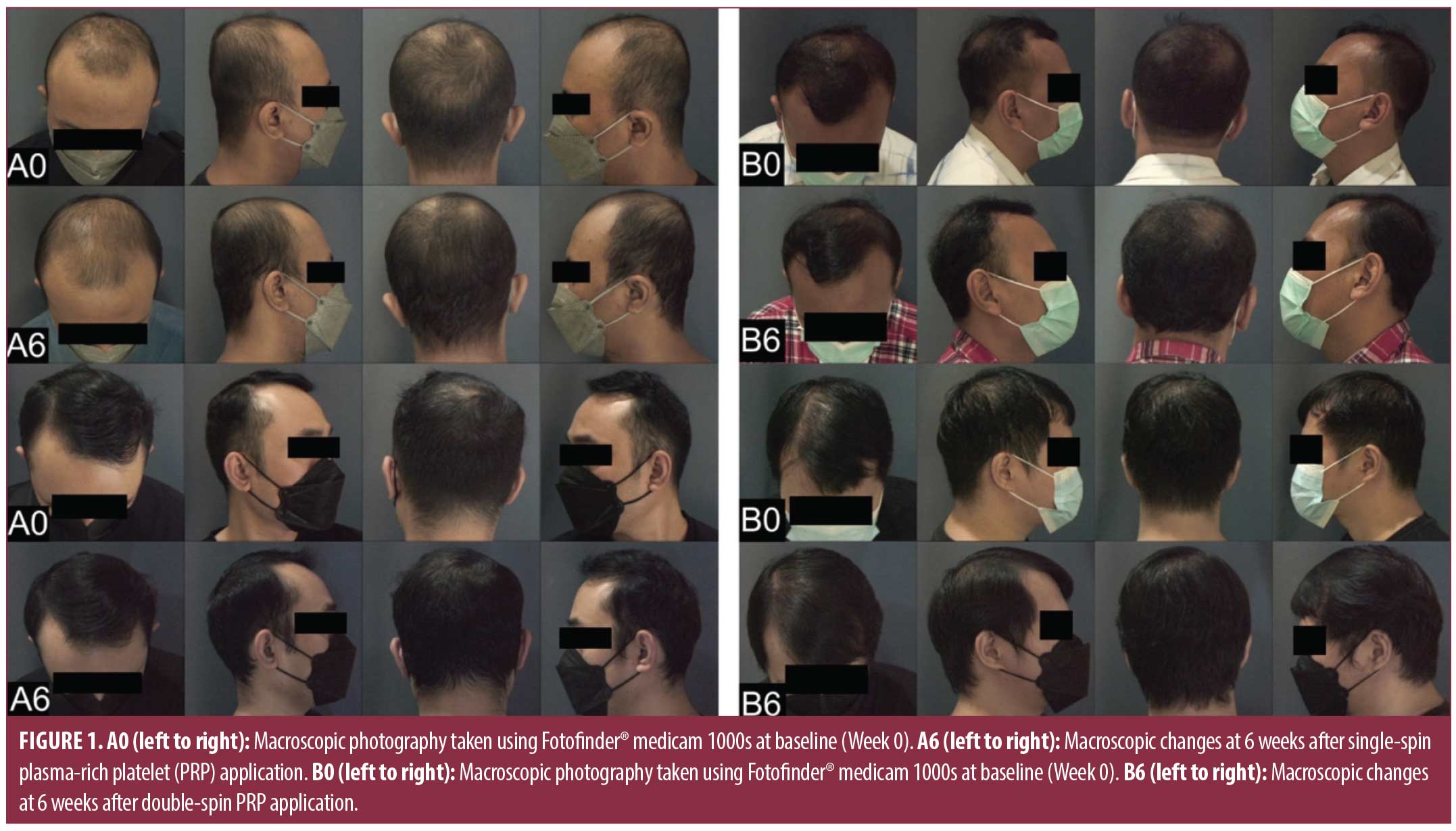
Study setting. All 30 subjects were divided into two groups of 15 at random. One group was allocated to receive PRP treatment by single-spin centrifugation, and another group was allocated to receive double-spin PRP centrifugation. The study was conducted for six weeks with a two-week visit interval (Weeks 0, 2, 4, and 6). On the first visit, all consented subjects had laboratory evaluations (a complete blood count) to measure thrombocytes for baseline and PRP. PRP was administered at Weeks 0, 2, and 4, and final evaluations were done at Week 6. Several examinations were performed on every visit, including history taking, physical examination, photography, trichoscopy, and trichoscan. Furthermore, each subject was instructed to use 5% topical minoxidil twice daily as primary therapy throughout the study. Patients were instructed to report any adverse reactions to topical minoxidil and PRP.
Intervention. PRP was made by drawing 13.5cc of venous blood into a vacutainer tube containing 3.2% citrate as an anticoagulant. The single-spin centrifugation was done at 3,000rpm for 15 minutes. The PRP layer was then carefully aspirated with a 1cc syringe. In the group allocated to receive the double-spin centrifugation method, after the blood was centrifuged at 1,500rpm for six minutes, PRP layers were centrifuged for the second time at 2,500rpm for 15 minutes in a plain tube. The PRP was then aspirated with a 1cc syringe. An additional 15cc of blood was drawn during the first visit. An ethylenediaminetetraacetic acid (EDTA) tube was used to collect 3cc of blood for routine hematology examination to establish baseline thrombocyte count. Another 13.5cc of blood was used to produce an extra 1cc of PRP, which was examined for thrombocyte count. The centrifugation device used was Kubota/S300T, SN:Y262262- MOOO.
To reduce pain, topical anesthetic cream was applied to the scalp for 45 to 60 minutes prior to the PRP injection. After cleaning the scalp with an alcohol swab, 1cc of PRP was injected intradermally at the designated scalp area. In this study, the surface area was 6x4cm, forming a rectangle. The area was defined by measuring 10cm from the right brow’s apex at the pupil’s midpoint to the indentation in the hair regression limit on the right parietal.
Statistical analysis. We presented descriptive data as mean±standard deviation (SD) for continuous variables and as frequency (%) for categorical variables. To compare the demographic and clinical factors of the two groups, independent t-tests and Mann–Whitney U tests were used. The demographic characteristics of both groups were compared using the Kolmogorov-Smirnov test. The paired t-test and Wilcoxon’s signed-rank test were used to compare each group’s pre- and postintervention outcome variables. We also utilized linear regression to examine the mean changes between Weeks 0 and 6 for the two groups. Version 24 of SPSS was used to analyze all the data (IBM; Armonk, New York, United States). A p-value of less than 0.05 was considered statistically significant.
Results
We approached 57 potential candidates, of whom four refused to participate and 23 did not meet the inclusion criteria. Randomization was applied to divide the 30 chosen subjects into two groups of 15. One group was given PRP treatment by single-spin centrifugation, whereas the other received PRP treatment using double-spin centrifugation. All subjects in both groups completed the trial.
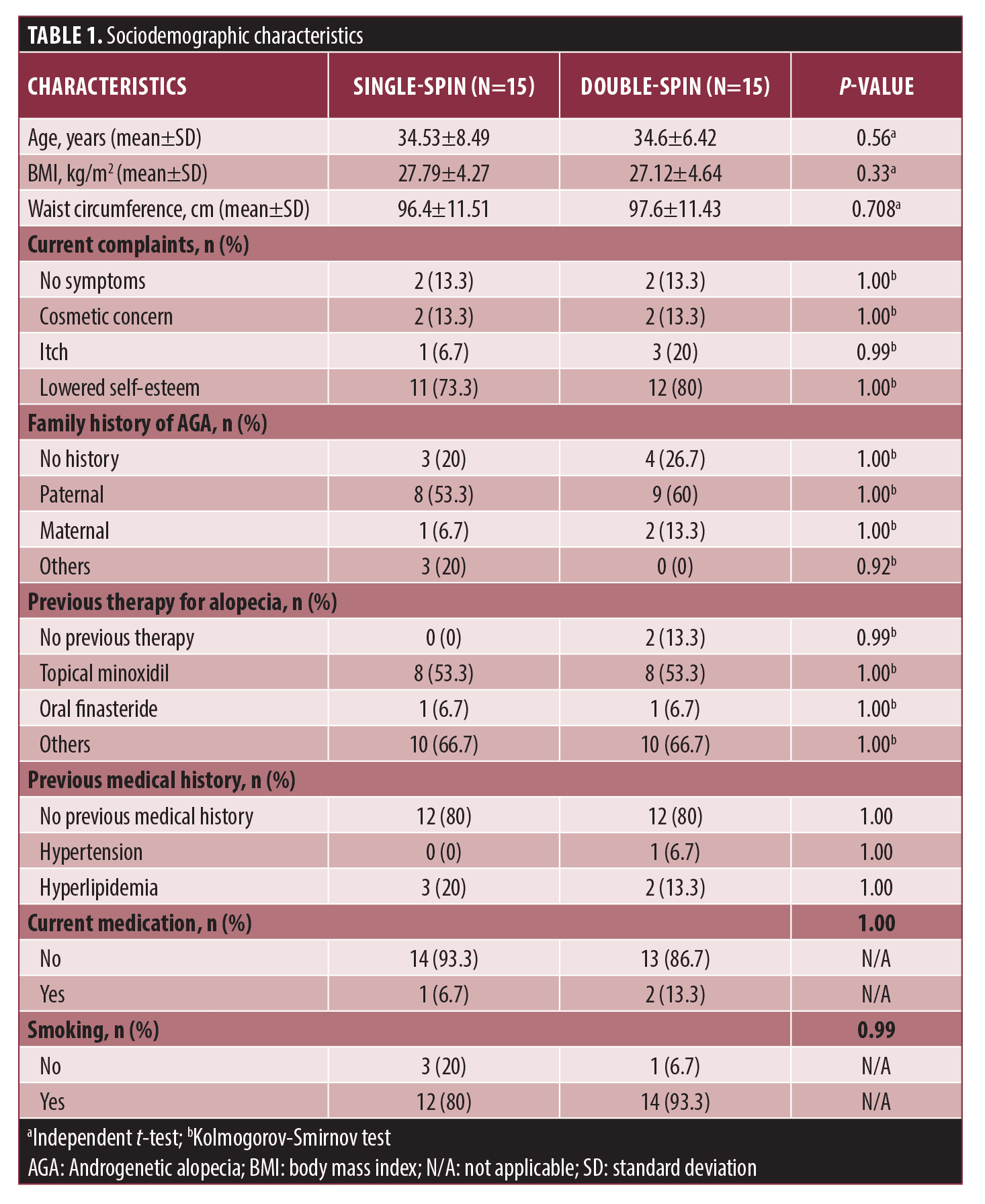
Sociodemographic characteristics. Sociodemographic characteristics can be found in Table 1. All subjects in both groups had a mean age of 34.57±7.4 years. Based on the history taking of onset of baldness, we found that almost all subjects (80%) had early onset AGA. The majority of patients (66.7%) had a body mass index (BMI) of 23kg/m2 or greater. The double-spin group had a greater average waist circumference (p>0.05).
Subjects presented with a variety of complaints, with poor self-esteem being the most prevalent (76.7%). A total 56.7 percent of participants had family history of baldness originating in the paternal line. The majority of subjects had received multiple treatments for alopecia; 66.7 percent of subjects had a history of therapies other than topical minoxidil and oral finasteride, such as nonpharmaceutical topical agents and adipose-derived stem cell-conditioned medium injection (>6 months prior to data collection).
Of the total, only six subjects (20%) had a previous medical history. One subject with hypertension routinely took antihypertensive drugs, while subjects with dyslipidemia were not on routine medication. Based on these results, we found no significant differences (p>0.05) in the sociodemographic characteristics between the two groups.
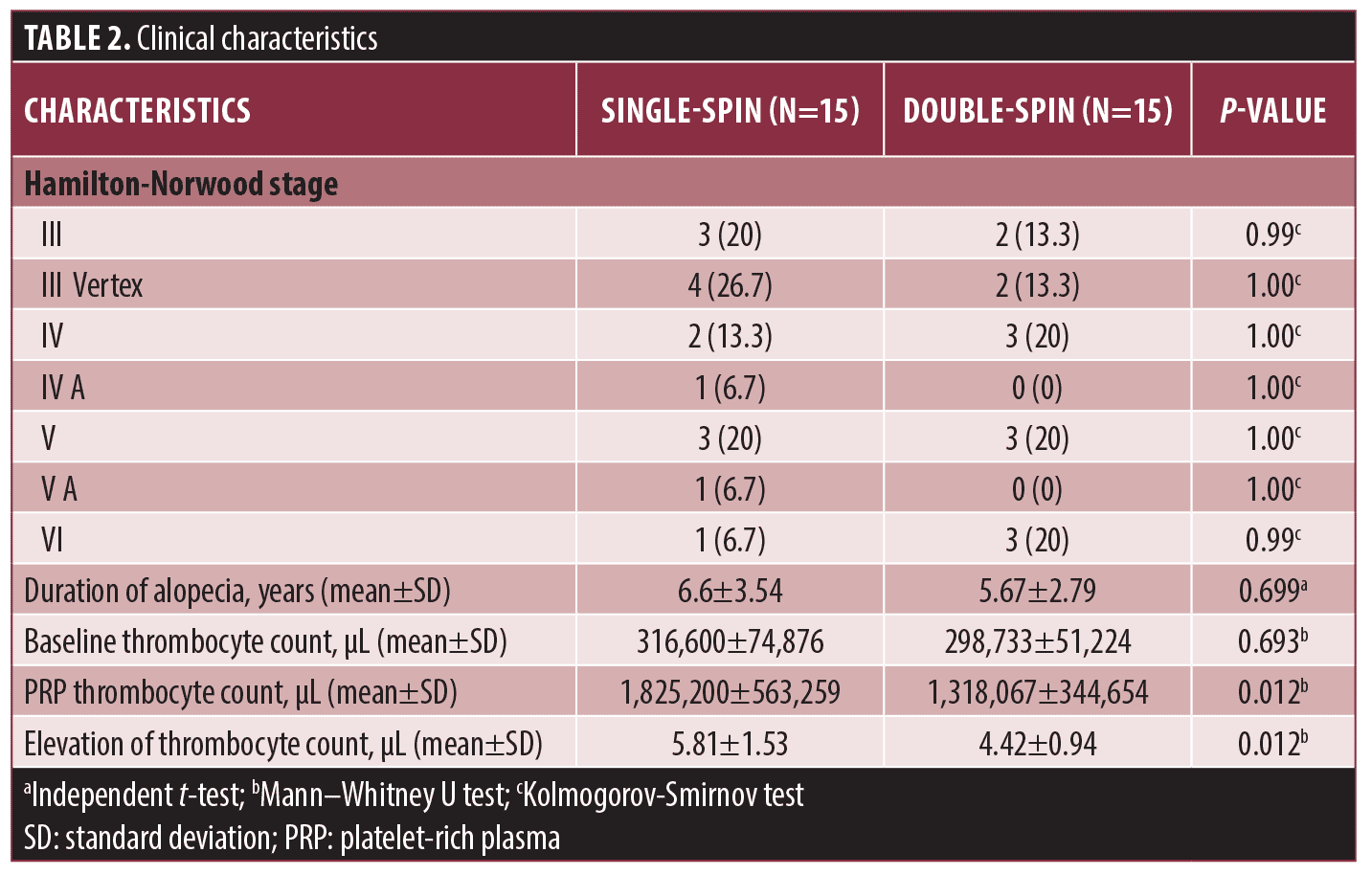
Clinical characteristics. The clinical signs of Hamilton-Norwood stages III and V predominated in both groups. On average, participants manifested AGA 6.13±3.17 years before the commencement of the study. The mean baseline thrombocyte count of the participants was 307,666/µL. In addition, the mean thrombocyte count of PRP was 1,571,633/µL, representing a 5.11-fold increase from baseline levels. The single-spin group showed a higher PRP thrombocyte count and a significant increase in thrombocyte count, compared to the double-spin group (p=0.012). Subjects’ clinical characteristics are shown in Table 2.
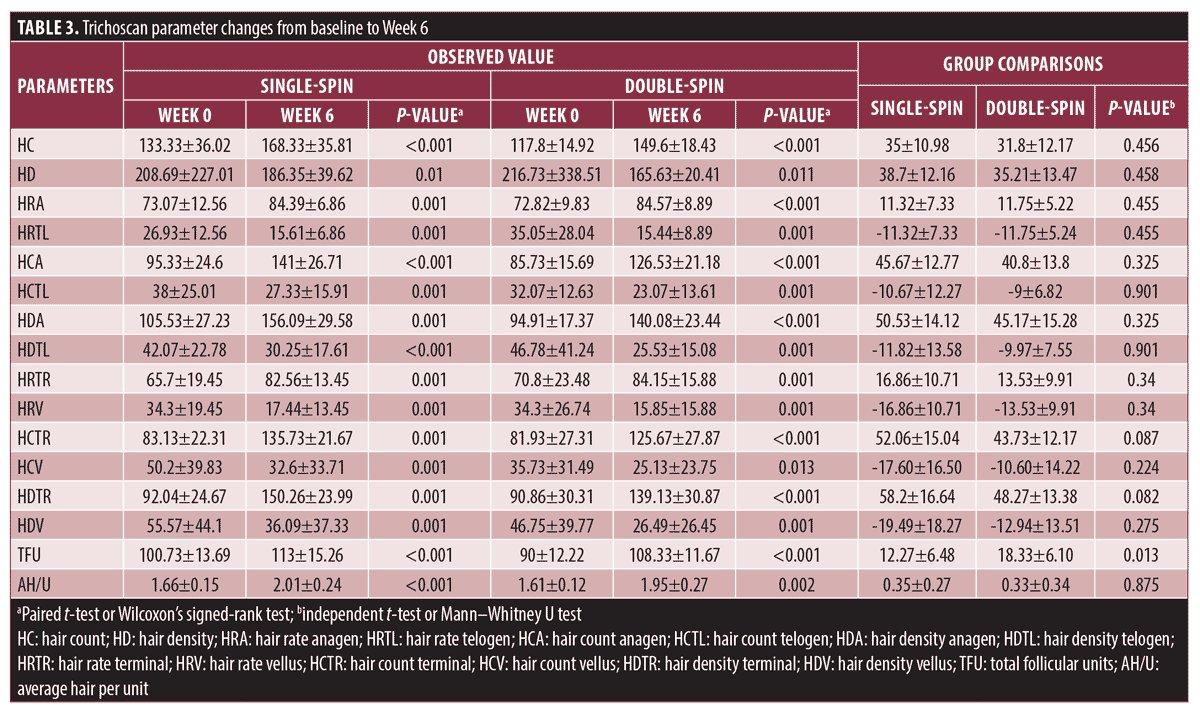
Hair growth parameters. Single-spin group. Over the course of the six weeks, we found significant clinical improvement in both groups (Table 3). All hair parameters showed significant differences in the single-spin group (p<0.05). After the intervention, the anagen hair rate increased, and the telogen hair rate considerably decreased (p=0.001). Additionally, we found that the total number of follicles in the single-spin group increased (p<0.05), and the average number of hairs per unit also improved significantly to 2.01±0.24 (p<0.05).
Double-spin group. We found significant improvements in nearly all hair growth parameters, except for mean length and mean thickness, in the six weeks after PRP treatment. Anagen and terminal hair count, hair rate, and hair density increased significantly (p<0.05). Vellus hair and telogen phase parameters were significantly decreased (p<0.05). Although the mean length and average thickness were reportedly increased by approximately 0.04±-0.16cm and 0.002±0.008mm/cm2, respectively, these were not statistically significant (p>0.05).
Comparison between treatment groups. The single-spin group revealed better enhancements in several parameters, including hair count, hair density, and average hair per unit. However, the comparison remained statistically insignificant (p>0.05). The double-spin group also demonstrated better results in terms of increasing anagen hair rate and decreasing telogen hair rate, although it was not statistically significant (p>0.05). Despite the fact that both treatments raised the average number of hairs per unit, we found that the total number of follicular units in the double-spin group was substantially higher than that of the single-spin group (p=0.013).
Safety evaluation. Itch, discomfort, and hair loss were the most common complaints following PRP therapy and topical minoxidil use. Good compliance with topical minoxidil application was seen in 83.3 percent of subjects. Four subjects reported discomfort at the injection site for about two hours to one day following the first and second PRP therapies. Five subjects complained of hair loss (100 hairs per day) during the second week of examination. However, these symptoms subsided without treatment by Week 6 (2 weeks post-last PRP treatment) and had a minimal effect on daily activities.
Discussion
The incidence and prevalence of AGA vary by age and ethnicity.13 AGA in patients 35 years of age or younger is considered early-onset AGA.14 In the current study, the mean age was 34.53±8.49 years for the single-spin group and 34.6±6.42 years for the double-spin group; thus, the average age of these subjects corresponds with early-onset AGA. Previous clinical studies found a correlation between the occurrence of early-onset AGA and obesity, metabolic syndrome, insulin resistance, cardiovascular illness, and family history of AGA.15
According to the World Health Organization (WHO), waist circumference over 94cm in men and over 80cm in women increases the risk of metabolic complications.16 In this study, the average waist circumference of subjects was 96.4±11.51cm in the single-spin group and 97.6±4.43cm in the double-spin group. Both groups are considered to have a high risk of developing metabolic syndrome.
No significant difference in BMI was observed between the two groups (p>0.05). Fourteen patients (46.7%) were categorized as overweight (23–24.9kg/m2), and six patients (20%) were categorized as having obesity (>25kg/m2). Kamal et al17 also found that 62.6 percent of the 246 patients with AGA had obesity and 26.42 percent had overweight. The correlation between male-pattern AGA and obesity is uncertain. Possible causes include insulin resistance and insulin-like growth factor-1 (IGF-1) overexpression in subjects with obesity that may increase the conversion of testosterone to dihydrotestosterone, the primary androgen responsible for male pattern baldness.18
In this study, 76 percent of subjects had a family history of AGA, predominantly on the paternal side. This was consistent with Su et al,19 who found a statistically significant correlation between moderate or severe AGA and family history of AGA on the paternal side of the family (odds ratio [OR]: 12.69; 95% confidence interval [CI]: 4.65–34.60), but not on the maternal side of the family (OR: 3.0; 95% CI: 0.01–31.32).
PRP production and administration for AGA has been studied extensively. However, the best method has yet to be discovered.20 Raziani et al21 discovered that the optimal thrombocyte increase for PRP was between 2.5- and 3-times baseline level. However, Giusti et al22 observed that 1.5million/mL (5–7x baseline) thrombocyte concentration was best for endothelial cell growth.
The objective of centrifugation in the preparation of PRP is to acquire more thrombocytes in a given volume. The number of thrombocytes produced in PRP depends on centrifugal acceleration, centrifugal duration, number of centrifugation, and others.23 It is known that a greater concentration of thrombocytes results from an increase in rotational force. However, excessive centrifugal forces or duration may lead to the loss of growth factors in the supernatant plasma, hence diminishing the therapeutic efficacy of PRP.24,25
Kramer et al20 advised a mean spin rate of 2,480rpm and a mean spin time of 12 minutes for the preparation of PRP. In contrast, Cervantes et al26 suggested a double-spin centrifugation approach for PRP preparation, with the first spin at 1,500 to 1,700rpm for 6 to 10 minutes and the second spin at 2,500rpm for 10 to 15 minutes. Numerous studies that employed a single-spin approach (ranging from 460–1,500g/580rpm for 5–8 minutes) also revealed favorable results.26
In this study, we discovered that the single-spin method produced a 5.81±1.53-fold rise in thrombocyte concentration from baseline, which was substantially higher than the double-spin method (p=0.012). This result was congruent with that of Bhatia et al,27 who discovered that a single centrifugation of 100g for 10 minutes produced a higher increase in thrombocyte count (2.19x more than baseline) than a double-spin centrifugation method. In contrast, Perez et al28 reported that double-spin centrifugation, 100g for 10 minutes followed by 400g for 10 minutes, raised thrombocyte concentration by a factor of five. These significant differences in thrombocyte increase can be attributed to various factors, including thrombocyte aggregation, and thrombocyte volume variation, among others.27,28
In this study, all trichoscan-observed hair parameters in the single-spin group yielded positive results (p<0.05). The number of hairs increased by 35±10.98 (p<0.001), and hair density increased by 38.7±12.16cm2 (p=0.01). This finding was congruent with that of Gkini et al,9 who discovered that PRP treatment with single-spin centrifugation (1,500g for 5 minutes) and three sessions separated by three weeks reduced hair loss to normal amount. In that same study, hair density peaked (at 170.70±37.81cm2) at three months (p<0.001). It was significantly higher (p<0.001), compared to baseline, at six months and one year (56.25±37.75cm2 and 153.70±39.92cm2, respectively).9
In our study, an increase in anagen hair count (45.67±12.77, p<0.001) and a decrease in telogen hair count (-10.67±12.2, p=0.001) were shown to be substantially different in the single-spin group. Ayatollahi et al29 observed that PRP injections every two weeks for five sessions, utilizing a 1,500g spin process for five minutes, resulted in decreased anagen, increased telogen, and decreased anagen/telogen ratio (p=0.003); unfortunately, the study’s thrombocyte concentration was unknown.
We also found that subjects who received double-spin PRP injections showed significant changes in almost all mean differences of hair parameters, except mean thickness (0.002±0.008mm/cm2, p=0.401) and mean length (0.04±-0.16mm, p=0.078). The authors have not been able to explain why this disparity exists. Kacchawa et al,30 in contrast, discovered that hair density and mean thickness significantly increased (p<0.05) in 50 subjects receiving a double-spin PRP injection, with the first spin at 1,200rpm for eight minutes and the second spin at 2,400rpm for four minutes.
We discovered that therapy for AGA using single- and double-spin PRP preparation methods were equally effective in clinical AGA improvement. Both methods can enhance hair count, hair density, anagen hair rate, total follicular units, average hair per unit and reduce telogen hair rate. However, the mean difference of total follicular unit in double-spin centrifugation (18.33±6.10) was statistically higher (p=0.013) than in single-spin centrifugation (12.27±6.48). Overall, both methods had a positive impact on AGA.
In this study, the clinical improvement of AGA did not differ substantially between the two groups, despite the fact that the thrombocyte concentration rise of PRP in the single-spin group was significantly higher than in the double-spin group. Regardless of the fact that PRP contains growth factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), that can stimulate hair development, Kushida et al31 found that the association between thrombocyte concentration and PDGF was not necessarily approximately equal.
Limitations. This study, to the best of our knowledge, is the first to compare the efficacy of single- and double-spin PRP preparation methods in assessing thrombocyte count and their effect on the clinical improvement of AGA, as measured by trichoscan. Our study’s limitations were the small sample size, brief duration of follow-up, and a lack of placebo or vehicle control. More research is required to identify the difference in thrombocyte elevation and its relevance to the clinical improvement of AGA over a longer time period and using a larger number of subjects.
Conclusion
Both single- and double-spin PRP preparation methods can raise thrombocyte counts by 4 to 5 times, although the rise in single-spin PRP thrombocyte count was substantially more significant. In addition, PRP preparation methods, when administered three times during a two-week period, resulted in substantial improvements in nearly all hair parameters. Despite the statistically higher mean difference in total follicular units that was observed in the double-spin group, authors believe that both PRP methods have tremendous potential in the treatment for AGA. No significant cutaneous or systemic adverse effects were reported in either group. Clinicians may designate either of the studied PRP preparation techniques. Further research using different methods, a larger sample size, and a longer duration is required to evaluate the relation between increased thrombocyte count and hair growth.
References
- Blume-Peytavi U, Kanti V. Androgenetic alopecia. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology, 9th edition. McGraw-Hill Education; 2019:1497–1506.
- Peytavi U, Kanti V. Part 16: disorders of hair and nails. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology, 9th edition. McGraw-Hill Education; 2019:1495–1506.
- Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157(8):963–970.
- Ferreira JR, Teixeira GQ, Santos SG, et al. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular preconditioning. Front Immunol. 2018;9:2837.
- Leo MS, Kumar AS, Kirit R, et al. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14(4):315–323.
- Chen JX, Justicz N, Lee LN. Platelet-rich plasma for the treatment of androgenic alopecia: a systematic review. Facial Plast Surg. 2018;34(6):631–640.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228.
- Stevens J, Khetarpal S. Platelet-rich plasma for androgenetic alopecia: a review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2019;5(1):46–51.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7(4):213–219.
- Kurita M, Aiba-Kojima E, Shigeura T, et al. Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg. 2008;12(2)2:438–448.
- Sharma K, Das G, Sarvesh B, Agarwal A. Platelet-rich plasma for degenerative knee joints: what is the evidence? Indian J Pain. 2019;33(3):126.
- Jo CH, Roh YH, Kim JE, et al. Optimizing platelet-rich plasma gel formation by varying time and gravitational forces during centrifugation. J Oral Implantol. 2013;39(5):525–532.
- Salman KE, Altunay IK, Kucukunal NA, Cerman AA. Frequency, severity and related factors of androgenic alopecia in dermatology outpatient clinic: hospital-based cross-sectional study in Turkey. An Bras Dermatol. 2017;92(1):35–40.
- Ding Q, Xu YX, Sun WL, et al. Early-onset androgenetic alopecia in China: a descriptive study of a large outpatient cohort. J Int Med Res. 2020;48(3):300060519897190.
- Bakry OA, Shoeib MAM, Shafiee MK, Hassan A. Androgenic alopecia, metabolic syndrome, and insulin resistance: is there any association? A case-control study. Indian Dermatol Online J. 2014;5(3):276–281.
- World Health Organization. Waist circumference and waist-hip ratio: report of WHO expert consultation. World Health Organization; 2008.
- Kamal A, Jawaria Farzand Raja. Relationship of androgenic alopecia with higher BMI in Pakistan: a cross sectional study. J Pak Assoc Dermatol. 2021;31(1):28–32.
- Yang C-C, Hsieh F-N, Lin L-Y, et al. Higher body mass index is associated with greater severity of alopecia in men with male-pattern androgenetic alopecia in Taiwan: a cross-sectional study. J Am Acad Dermatol. 2014;70(2):297–302.
- Su L, Chen TH. Association of androgenetic alopecia with smoking and its prevalence among asian men: a community-based survey. Arch Dermatol. 2007;143(11):1401–1406
- Kramer ME, Keaney TC. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J Cosmet Dermatol. 2018;17(5):666–671.
- Graziani F, Ivanovski S, Cei S, et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17(2):212–219.
- Giusti I, Rughetti A, D’Ascenzo S, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49(4):771–778.
- Ozer K, Kankaya Y, Colak O, Kocer U. The impact of duration and force of centrifugation on platelet content and mass in the preparation of platelet-rich plasma. Aesthetic Plast Surg. 2019;43(4):1078–1084.
- Hesseler MJ, Shyam N. Platelet-rich plasma and its utilities in alopecia: a systematic review. Dermatol Surg. 2020;46(1):93–102.
- Marques LF, Stessuk T, Camargo IC, et al. Platelet-rich plasma (PRP): methodological aspects and clinical applications. Platelets. 2015;26(2):101–113.
- Cervantes J, Perper M, Wong LL, et al. Effectiveness of platelet-rich plasma for androgenetic alopecia: a review of the literature. Skin Appendage Disord. 2018;4(1):1–11.
- Bhatia A, Ramya BS, Biligi DS, Prasanna BK. Comparison of different methods of centrifugation for preparation of platelet- rich plasma (PRP). Indian J Pathol Oncol. 2016;3(4):535–539.
- Perez AG, Lana JF, Rodrigues AA, et al. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014;2014:176060.
- Ayatollahi A, Hosseini H, Shahdi M, et al. Platelet-rich plasma by single spin process in male pattern androgenetic alopecia: is it an effective treatment? Indian Dermatol Online J. 2017;8(6):460–464.
- Kachhawa D, Vats G, Sonare D, et al. A split head study of efficacy of placebo versus platelet-rich plasma injections in the treatment of androgenic alopecia. J Cutan Aesthet Surg. 2017;10(2):86–89.
- Kushida S, Kakudo N, Morimoto N, et al. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. J Artif Organs. 2014;17(2):186–192.