 J Clin Aesthet Dermatol. 2020;13(10):12–16
J Clin Aesthet Dermatol. 2020;13(10):12–16
by Brian Berman, MD, PhD; Mark S. Nestor, MD, PhD; Michael H. Gold, MD; David J. Goldberg, MD, JD; Eduardo T. Weiss, MD; and Isabelle Raymond, PhD
Drs. Berman and Nestor are with the Center for Clinical and Cosmetic Research in Aventura, Florida. Dr. Gold is with the Gold Skin Care Center in Nashville, Tennessee. Dr. Goldberg is with the Skin Laser & Surgery Specialists of NY/NJ in New York City, New York. Dr. Weiss is with the Memorial Health System in Hollywood, Florida. Dr. Raymond is with Memorial Health System in Boca Raton, Florida.
FUNDING: This study was sponsored by Sensus Healthcare, Boca Raton, Florida.
DISCLOSURES: Dr. Berman is a consultant and speaker for Sensus Healthcare. Dr. Nestor is a consultant, speaker, and member of the Advisory Board for Sensus Healthcare. Drs. Gold and Goldberg are speakers for Sensus Healthcare. Dr. Raymond is a full-time employee of and owns shares in Sensus Healthcare. Dr. Weiss has no relevant conflicts to disclose.
ABSTRACT: Background: Surgical treatment of keloid scars is associated with an approximately 70% recurrence rate at the excision site.
Objective: We sought to assess keloid recurrence rates when superficial radiation therapy (SRT) was applied following surgical excision.
Methods: Medical records were reviewed of subjects treated for keloid scars followed by SRT (SRT-100™; Sensus Healthcare, Boca Raton, Florida) using a biologically effective dose (BED) of 30Gy and for whom the required retrospective data was available. Eligible subjects (N=61) were treated for 96 keloid scars with SRT. Subjects were male (48%) and female (52%) with a mean age of 38.87 years. Subjects were treated for ?1 keloid scars following removal by sutured excision (93%) or tangential excision with secondary intention technique (7%). Almost all subjects (98%) received BED 30Gy with irradiation scheme of three 6Gy SRT treatments on Days 1, 2 and 3 following surgery. Mean energy of 100KV (73%) or 70KV (27%) were applied.
Results: Ten treated keloidectomy sites (10.4%) had recurrences (i.e., presence of any new tissue growth on the surgical scar) within 12 months increasing to 11 (12.7%) at 18 months. Kaplan-Meier survival probability cure rate was 85.6% from 24 months post-SRT treatment onwards. Transient hyperpigmentation was the most frequent adverse event and there were no malignancies in the treatment area during follow-up evaluations.
Conclusions: SRT with a BED value of 30 Gy delivered to keloidectomy excision sites immediately following excision was well-tolerated and resulted in markedly fewer long-term recurrences than reported following keloidectomy alone. Most keloid scar recurrences occurred within one year. There were no malignancies during follow-up evaluations.
Keywords: Keloids, keloid scars, superficial radiation therapy, recurrence rate
A keloid scar is an abnormal growth of scar tissue that occurs at the site of a cutaneous injury.1 They do not regress and grow beyond the original margins of the scar, which differentiates keloid scars from hypertrophic scars.2 The etiology of keloid scars is not known with certainty, but it appears to involve various cytokines and growth factors, resulting in overactive fibroblasts and excessive collagen formation.1 Keloid scars occur more commonly in genetically susceptible individuals, such as people of African and Asian descent.3,4 Common causes of keloid scars include piercing, surgery and mechanical trauma.5–7 In addition to their unsightly appearance, keloid scars can cause significant pain and pruritis and adversely affect quality of life.7–9
Surgical excision of keloid scars is associated with an unacceptably high rate of recurrence, reported to be in excess of 80 percent.10–12 Consequently, a wide range of other treatments have been used to treat keloid scars, including silicon bandaging, intralesional injections of corticosteroids and other drugs, cryotherapy, and radiation therapy.1,7
The use of radiation therapy is known to affect gene activity. Genes involved in cell proliferation and extracellular matrix production are down-regulated while genes promoting apoptosis are upregulated.13 Thus, the application of radiation therapy following surgery appears to prevent keloid scar recurrence by decreasing fibroblast proliferation, arresting the cell cycle and inducing premature cellular senescence.14 Several studies have demonstrated the therapeutic benefits of surgical removal of keloid scars followed by radiation therapy.15–17 There is little evidence to suggest that exposing keloid scars or surrounding healthy skin to the amount of radiation used causes skin cancer.18–20 The combined use of surgery with radiation therapy is an accepted method of treating keloid scars, although differences in radiation type, dose, fraction, and treatment interval remain.16
One systematic review of the literature found that the recurrence rate of keloid scars treated with combined surgery and various forms of radiation treatments, including brachytherapy, electron beam and x-ray could be less than 10 to 20 percent.21 In order to compare the different radiation therapy doses used, the authors applied the linear-quadratic concept to normalize doses to a biologically effective dose (BED). For keloid scars treated with surgery alone, the rate of recurrence ranged from 50 to 80 percent, while the addition of radiotherapy following keloidectomy with a BED value greater than 30Gy reduced the recurrence rate to less than 10 percent. The best outcomes were achieved with a BED value of 30Gy administered within two days of surgery.21 A more recent meta-analysis of various types of post-surgical radiation treatment using the same BED calculations revealed recurrence rates of 15 percent for isotopic brachytherapy and 23 percent for x-ray and electron beam therapy. However, most x-ray studies reviewed did not employ a beneficial BED.22
To confirm the effectiveness of x-ray therapy following the surgical removal of keloid scars, the following retrospective study assessed the rate of keloid scar recurrence following post-surgical treatment with superficial radiation therapy (SRT) using a therapeutic dose with a BED value of 30Gy.
Materials and Methods
All known clinical centers in the United States treating keloid scars with SRT (SRT-100™, Sensus Healthcare, Boca Raton, Florida) were contacted to participate. To be eligible to participate, sites were required to treat each surgical keloidectomy site with a BED value of 30Gy within the first two days of surgery as defined by Kal et al21 and have at least ten patients with at least 1-year follow-up. Two centers declined/did not respond and four clinical centers agreed to participate. A chart review was performed for each treated patient and information was obtained about demographics, keloid scar characteristics, surgical treatment, SRT treatment parameters, adverse events (AEs) and keloid scar recurrence.
The recurrence rate, based on any new tissue growth on the surgical scar margin, was assessed using Kaplan-Meier survival probability estimates calculated across available follow-up data, reporting recurrence probability estimates, and lower and upper limits of the 95 percent confidence interval by follow-up year. The study protocol was determined to meet the conditions for exemption under 45 CFR 46.101(b)by a commercial IRB (Western Institutional Review Board (WIRB), Puyallup, Washington). ClinicalTrials.gov Identifier: NCT03693924.4
Results
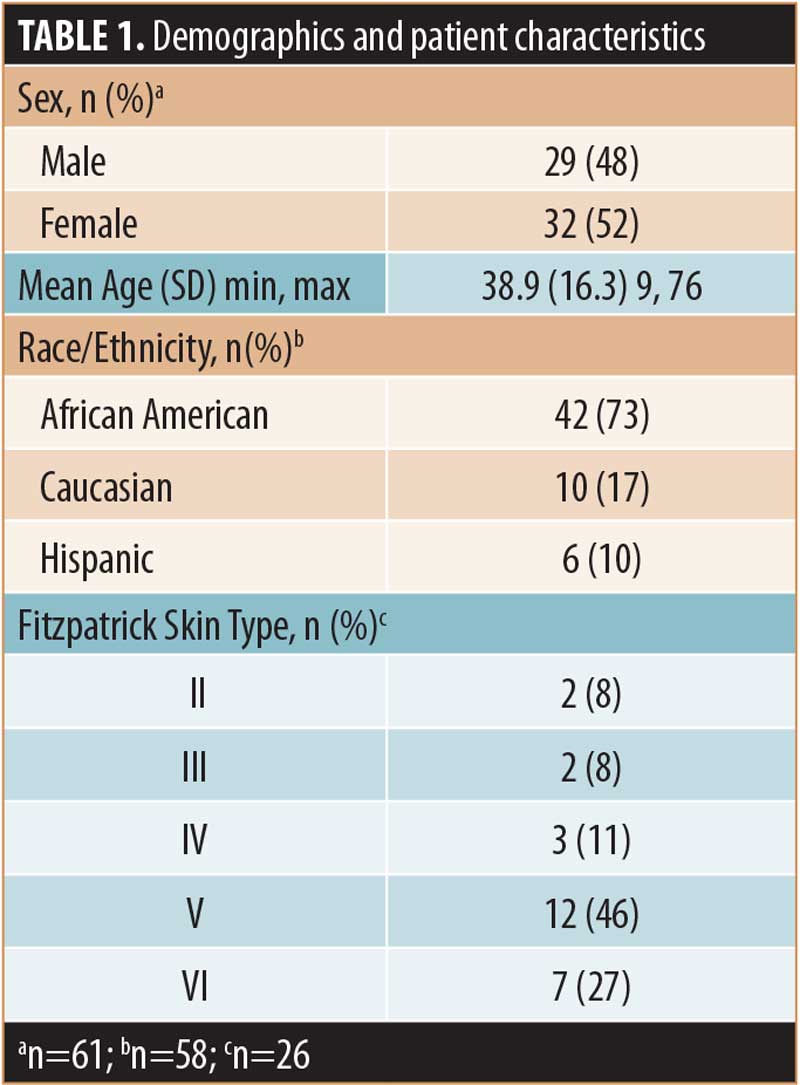
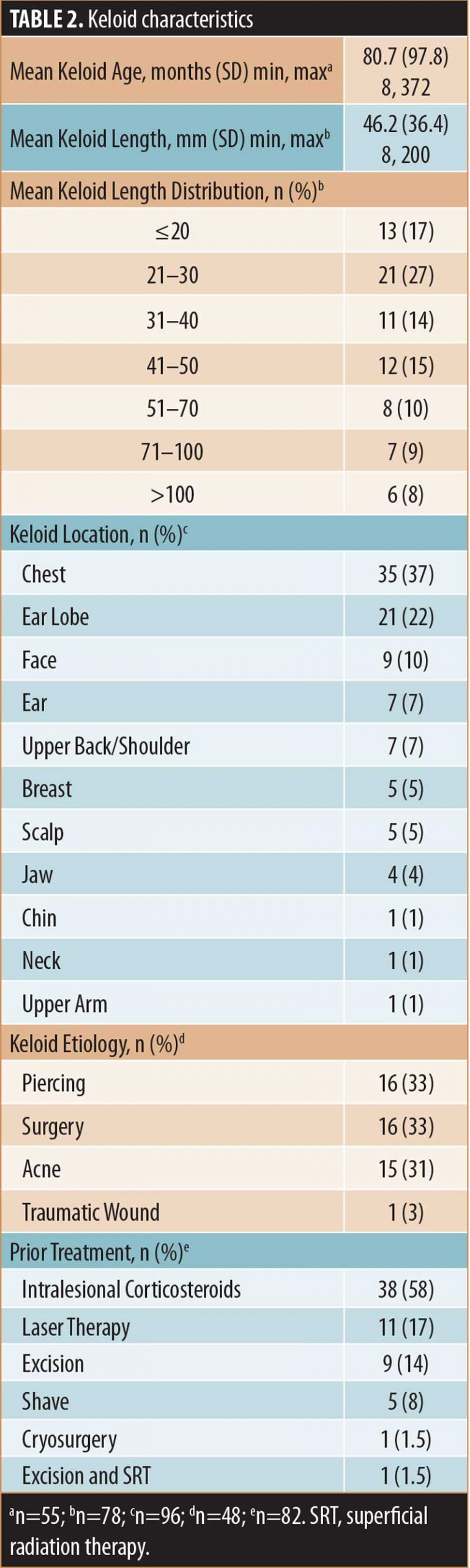
Patients. The demographics and characteristics of the patients included in this analysis (N=61) are summarized in Table 1 and keloid scar characteristics are summarized in Table 2. Treated patients presented with one (n=39, 64%), two (n=13, 21%), three (n=7, 11%), four (n=1, 2%) or six (n=1, 2%) lesions for a total of 96 keloid scars. Approximately one-half of treated lesions (54%), for which information was available (n=63), were recurrent keloid scars and most treated keloid scars, for which information was available (n=82, 79%), had received one or more prior treatments (Table 2).
Procedures. Among the total treated keloid scars, most (n=89, 93%) were excised with a scalpel and the remainder (n=7, 7%) were removed using tangential excision with secondary intention technique. The most commonly applied suture was a simple suture (66%), either alone (57%) or in combination with buried (8%) and running (1%) sutures. The length of the surgical excision was reported for 84 keloid scars (94%) that were removed via excision scalpel surgery (n=89). The mean (SD) length was 62.2 (49.7) mm (range, 10–230 mm). Following excision, sutures were removed after a mean of 11.6 (3.8) days (range, 7–21 days).
Following surgery, the first SRT was administered after a mean of 1.1 (0.36) days (range, 1–3 days), the second after 2.4 (0.81) days (range, 2–5 days), and the third after 3.9 (1.51) days (range, 3–8 days). One keloid scar received two SRT treatments on Post-surgical Days 1 and 2, and one keloid scar received one SRT treatment on Post-surgical Day 3. Mean energy of 100KV (73%) or 70KV (27%) were applied.
Among subjects for whom data are available (n=90), the mean individual SRT dose was 601.8 (2.8) cGy (range, 600.0–611.2 cGy) and the mean total SRT dose was 1805.3 (8.3) cGy (range, 1800.0-1833.6 cGy). Among subjects with available data (n=84), the mean treatment margin was 7.9 (2.9) mm (range, 2–15 mm).
In addition to SRT, other postoperative treatments included silicone (n=19, 23%), antibiotics (n=13, 16%), intralesional corticosteroids (n=7, 8%) and pressure (n=4, 5%). The mean duration of follow-up was 13.2 (10.9) months (range, 1–41.5 months). A large proportion of subjects (n=39, 44%) had follow up beyond one year and 15 (17%) had follow-up beyond two years.
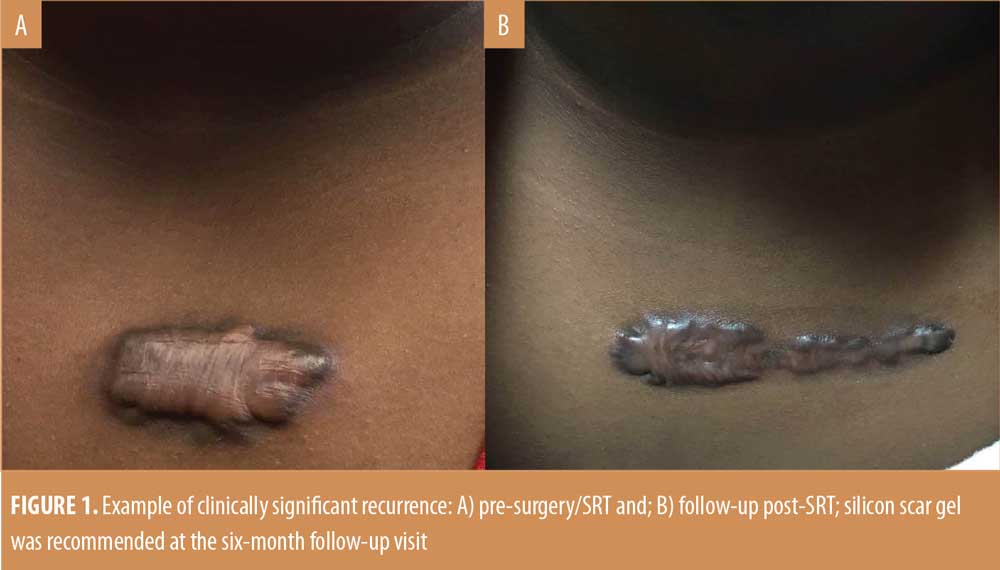
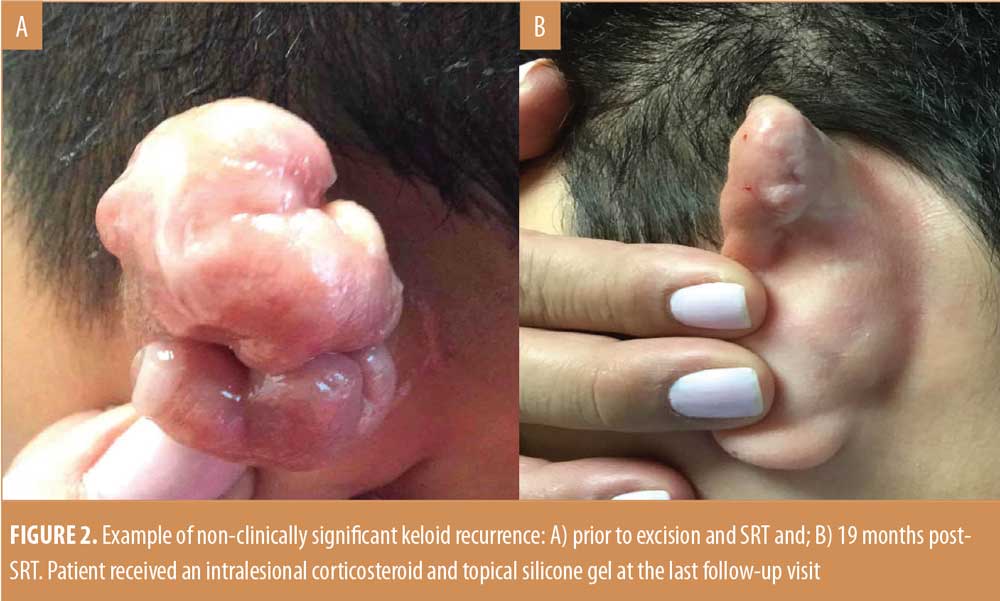
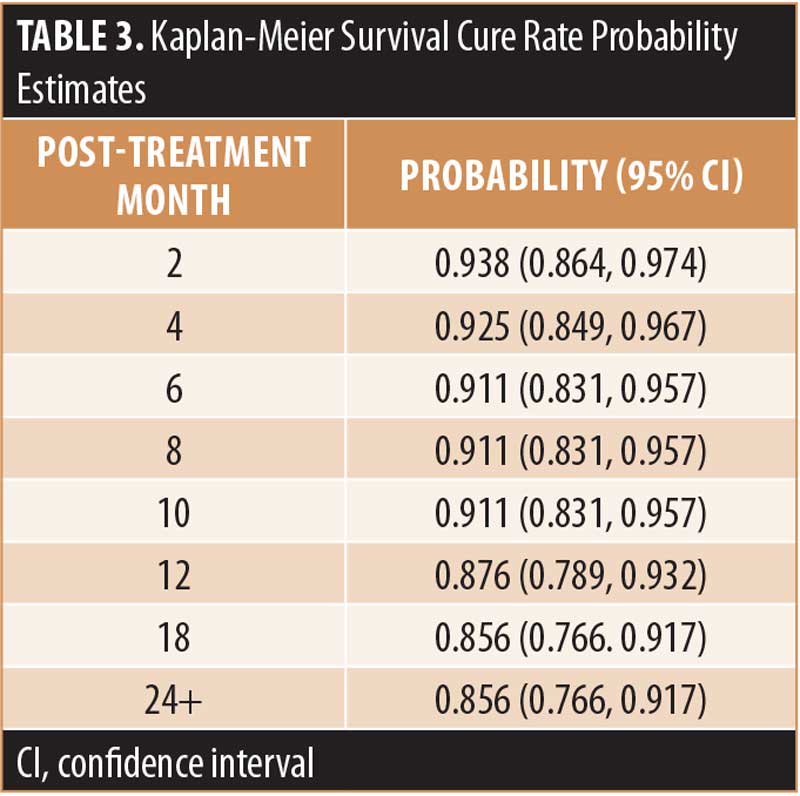
Efficacy. Ten of 96 treated keloidectomy sites (10.4%) had recurrences within 12 months, five of which were considered clinically significant by the treating physician. Assessment for whether a recurrence was clinically significant was based on each individual clinician’s evaluation of the keloid. Examples of clinically significant and non-significant keloid recurrences are shown in Figures 1 and 2. By 18 months, a recurrence was present (n=11; 12.7%) or absent (n=75; 87.2%) at 86 SRT-treated keloidectomy sites. The Kaplan-Meier survival probability, based on all 11 recurrences (both clinically significant and nonsignificant), was 85.6 percent from 24 months post-SRT treatment onwards. The survival cure rate probability estimates and the lower and upper limits of the 95 percent confidence interval for these data are summarized in Table 3.
Recurrent keloid scars had a mean age of 67.9 months, mean length of 44 mm and were located on the torso (n=8) and face (n=1) or site not reported (n=2). Subjects with recurrent keloid scars were female (n=8) and male (n=3), with a mean age of 40.4 years and were of African-American (n=8), Caucasian (n=2) or Hispanic (n=1) descent. For six patients, the recurring keloid scar was the only one treated. Four patients had more than one keloid scar treated, but only one recurred. One patient, a 54-year old African American woman, had two of three treated keloid scars recur. Among nine recurrent keloid scars that had been previously treated, eight had received previous intralesional corticosteroids.
A multivariate Cox regression analysis of the potential influence of sex, age, and keloid scar location, etiological factor, perioperative treatment, and postoperative treatment determined none of the evaluated factors were independent risk factors for lesion reoccurrence (p>0.05).
A comparison of the variables associated with the recurring keloid scars to those same variables as evaluated for the overall study population revealed the following observations:
Keloid scars recurred more frequently among female subjects (73% vs. 52%).
The greatest incidence of keloid scar recurrence was on the chest (78% vs. 41%).
Recurrent keloid scars were more likely to have previously recurred (71% vs. 54%).
The timeframe of suture removal after excision surgery was somewhat longer for recurring keloids (14–17 days vs. 7–14 days).
Safety. SRT-related adverse events. Pigmentation change was noted following SRT treatment for 59 (61%) subjects demonstrating hyperpigmentation (n=33, 56%), hypopigmentation (n=4, 7%) or both (n=4, 7%). Other reported AEs included radiation dermatitis (n=15, 18%), pain (n=14, 19%), pruritus (n=6, 8%) and radiation area pinkness (n=1, 1%). The six instances of localized pruritus occurred at six keloidectomy sites on one subject.
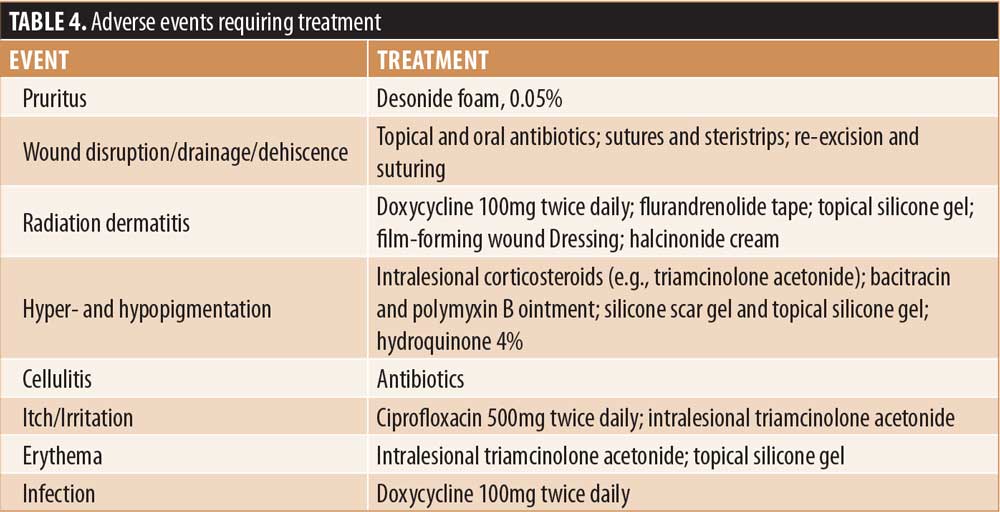
Surgery-related complications. Complications associated with surgery included wound disruption (n=2, 3%); infection; wound disruption and infection; itching, pain, tenderness, irritation; cellulitis; erythema; hematoma; wound drainage, dryness; postoperative bleeding and alopecia (for each, n=1, 1%). AEs requiring treatment are summarized in Table 4.
Discussion
Although keloid scars are relatively benign, they have an unsightly appearance, cause significant pain and pruritis, and have a considerable negative impact on quality of life.7–9 Post-keloidectomy radiotherapy has been shown to be superior to excision alone, and the use of different modalities and treatment schemes has resulted in recurrence rates ranging from 3 to 60 percent.21 The purpose of this study was to assess the recurrence rate, defined as presence of any new tissue growth on the surgical scar, following keloidectomy using SRT with a BED value of 30Gy.
The overall rate for both clinically and nonclinically significant recurrences of 12.7 percent in the present study using a BED value of 30Gy immediately following surgical excision is consistent with previous studies using a similar dose and schedule.21,23 These results underline the importance of appropriate dose and treatment schedule of SRT to obtain optimal outcomes. An interesting observation was recurrence of one keloid scar in patients with multiple keloid scars treated by keloidectomy and SRT, suggesting recurrence is not determined systemically but by local factors.
Although regression analyses did not determine any independent risk factors for lesion recurrence, it was noted that the greatest incidence of keloid scar recurrence was on the chest which is consistent with previous findings with recurrences observed at sites of high stretch-tension.22,24
Treatment-related hyperpigmentation has previously been reported following keloid scar excision and SRT and may reflect the greater susceptibility of postinflammatory hyperpigmentation among dark-skinned individuals in this study population.24,26 Fractionation of the SRT dose reduces the risk of hyperpigmentation and long-term sequelae are improved with three doses.21,22 Although hyperpigmentation and hypopigmentation in SRT-treated keloidectomy patients are generally considered transient, additional fractionation may be worth investigating in dark-skinned individuals in future studies.27
Although subjects were followed for a limited time period, there was no evidence of SRT treatment-related malignancies at the treatment site. A systematic review of 72 studies concluded that the risk of neoplasm from radiotherapy for the treatment of keloid scars is low, and most radiation oncologists consider the technique acceptable.22 Furthermore, recent consensus guidelines suggest that there is little evidence that exposing keloids or surrounding healthy skin to a BED of 30Gy causes skin cancer.28
Limitations. Several limitations to this study include the retrospective nature and missing data from some patient charts. Also, the definition of keloid scar recurrence was not well-defined. Other studies of keloid scar recurrence do not define keloid scar recurrence or use a variety of objective and subjective measures.15,29–31 Although 11 keloid scars recurred in the present study, five were reported as “clinically significant” by the treating physician and required additional treatment but without further description.
These limitations could be addressed in a prospective study of keloid scar recurrence. The use of a three-dimensional stereoscopic optical system appears to be an accurate and objective means for measuring changes in keloid scar volume.32.33 Similarly, three-dimensional impressions of keloid scars also appear to be a precise and reliable method of assessing changes in keloid scar volume.34,35 Furthermore, a prospective study with longer follow-up would help establish the efficacy of SRT following surgical excision of keloid scars.
Conclusion
We found a fractionated, biologically effective dose value of 30Gy, obtained with three 6Gy SRT treatments on Days 1, 2, and 3 following surgery was well-tolerated and resulted in fewer long-term recurrences than reported following keloidectomy alone. The vast majority of keloid scar recurrences occurred within one year. No malignancies were detected during follow-up evaluations. These findings are consistent with recent consensus recommendations on the treatment of keloidectomy sites with superficial radiation.28 A prospective study of keloid scar recurrence rate following keloidectomy and SRT is planned.
Ackowledgements
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript and the statistical analyses support of Elvira Cawthon, Reg Insight. This study was sponsored by Sensus Healthcare, Boca Raton, FL USA.
References
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43:S3–S18.
- Atiyeh BS, Costagliola M, Hayek SN. Keloid or hypertrophic scar: the controversy: review of the literature. Ann Plast Surg. 2005;54:676–680.
- Brown JJ, Bayat A. Genetic susceptibility to raised dermal scarring. Br J Dermatol. 2009;161:8–18.
- Glass DA 2nd. Current understanding of the genetic causes of keloid formation. J Investig Dermatol Symp Proc. 2017;18:S50–53.
- Jones ME, McLane J, Adenegan R, et al. Advancing keloid treatment: a novel multimodal approach to ear keloids. Dermatol Surg. 2017;43:1164–1169.
- Young WG, Worsham MJ, Joseph CL, et al. Incidence of keloid and risk factors following head and neck surgery. JAMA Facial Plast Surg. 2014;16:379–380.
- Mari W, Alsabri SG, Tabal N, et al. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec. 2016;7:1–7.
- Bijlard E, Kouwenberg CA, Timman R, et al. Burden of keloid disease: a cross-sectional health-related quality of life assessment. Acta Derm Venereol. 2017;97:225–229.
- Walliczek U, Engel S, Weiss C, et al. Clinical outcome and quality of life after a multimodal therapy approach to ear keloids. Arc Facial Plastic Surg. 2015;17:333–339.
- Jfri A, Rajeh N, Karkashan E. A case of multiple spontaneous keloid scars. Case Rep Dermatol. 2015;7:156–160.
- Sclafani AP, Gordon L, Chadha M, et al. Prevention of earlobe keloid recurrence with postoperative corticosteroid injections versus radiation therapy: a randomized, prospective study and review of the literature. Dermatol Surg. 1996;22:569–574.
- Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571.
- Tosa M, Ghazizadeh M, Shimizu H, et al. Global gene expression analysis of keloid fibroblasts in response to electron beam irradiation reveals the involvement of interleukin-6 pathway. J Invest Dermatol. 2005;124:704–713.
- Ji J, Tian Y, Zhu YQ, et al. Ionizing irradiation inhibits keloid fibroblast cell proliferation and induces premature cellular senescence. J Dermatol. 2015;42:56–63.
- Norris JE. Superficial x-ray therapy in keloid management: a retrospective study of 24 cases and literature review. Plast Reconstr Surg. 1995;95:1051–1055.
- Xu J, Yang E, Yu NZ, et al. Radiation therapy in keloids treatment: history, strategy, effectiveness, and complication. Chin Med J (Engl). 2017;130:1715–1721.
- Pozzi M, Zoccali G, Drago MC, et al. Radiotherapy following surgery in keloid treatment: our protocol. G Ital Dermatol Venereol. 2016;151: 492–498.
- Maarouf M, Schleicher U, Schmachtenberg A, et al. Radiotherapy in the management of keloids. Clinical experience with electron beam irradiation and comparison with X-ray therapy. Strahlenther Onkol. 2002;178:330–335.
- Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–1201.
- McKeown SR, Hatfield P, Prestwich RJ, et al. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol. 2015;88:20150405.
- Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Dose-effect relationship. Strahlenther Onkol. 2005;181:717–723.
- Mankowski P, Kanevsky J, Tomlinson J, et al. Optimizing radiotherapy for keloids: a meta-analysis systematic review comparing recurrence rates between different radiation modalities. Ann Plast Surg. 2017;78:403–411.
- Duan Q, Liu J, Luo Z, et al. Postoperative brachytherapy and electron beam irradiation for keloids: A single institution retrospective analysis. Mol Clin Oncol. 2015;3:550–554.
- Bischof M, Krempien R, Debus J, et al. Postoperative electron beam radiotherapy for keloids: objective findings and patient satisfaction in self-assessment. Int J Dermatol. 2007;46:971–975.
- Jones ME, Hardy C, Ridgway J. Keloid management: a retrospective case review on a new approach using surgical excision, platelet-rich plasma, and in-office superficial photon X-ray radiation therapy. Adv Skin Wound Care. 2016;29:303–307.
- Borok TL, Bray M, Sinclair I, et al. Role of ionizing irradiation for 393 keloids. Int J Radiat Oncol Biol Phys. 1988;15:865–870.
- Cheraghi N, Cognetta A Jr, Goldberg D. Radiation therapy for the adjunctive treatment of surgically excised keloids: a review. J Clin Aesthet Dermatol. 2017;10:12–15.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12: 12–18.
- Recalcati S, Caccialanza M, Piccinno R. Postoperative radiotherapy of auricular keloids: a 26-year experience. J Dermatolog Treat. 2011;22:38–42.
- Jones ME, Hardy CJ, Ridgway JM. Head and neck keloid management: A retrospective early review on a new approach using surgical excision, platelet rich plasma and in-office superficial photon X-ray radiation. Edorium J Otolaryngol. 2015;2:14–19.
- Song C, Wu HG, Chang H, et al. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J Radiat Res. 2014;55: 912–916.
- Salameh F, Koren A, Sprecher E, et al. Novel stereoscopic optical system for objectively measuring above-surface scar volume-first-time quantification of responses to various treatment modalities. Dermatol Surg. 2018;44:848–854.
- Verhiel SH, Piatkowski de Grzymala AA, Van den Kerckhove E, et al. Three-dimensional imaging for volume measurement of hypertrophic and keloid scars, reliability of a previously validated simplified technique in clinical setting. Skin Res Technol. 2016;22:513–518.
- Berman B, Young VL, McAndrews J. Objective assessment of the precision, accuracy, and reliability of a measurement method for keloid scar volume (PARKS Study). Dermatol Surg. 2015;41:1274–1282.
- van der Aa T, Verhiel SH, Erends M, et al. A simplified three-dimensional volume measurement technique in keloid scars: Validity and reliability. J Plast Reconstr Aesthet Surg. 2015;68:1574–1580.

