J Clin Aesthet Dermatol 2020;13(11 Suppl 2):S8–S23
Innovations in Psoriasis Management

Based on Selected Presentations from
Symposium for Cosmetic Advances & Laser Education (SCALE) 2020 Virtual Congress
July 24—26, 2020, Nashville, Tennessee.
 by Jo Ann LeQuang
by Jo Ann LeQuang
Owner, LeQ Medical, Angleton, Texas; Director of Scientific Communications, NEMA Research, Inc., Naples, Florida; Founding Director, No Baby Blisters, Colorado Springs, Colorado
Based on Presentations By
 Linda Stein Gold, MD
Linda Stein Gold, MD
Director of Clinical Research, Henry Ford Health System; Detroit, Michigan
 Dario Kivelevitch, MD
Dario Kivelevitch, MD
Baylor, Scott & White; Dallas, Texas

Mark Lebwohl, MD
Waldman Professor and Chairman, Kimberley and Eric J. Waldman Department of Dermatology, Icahn School of Medicine; Mount Sinai, New York
 Mark S. Nestor, MD, PhD
Mark S. Nestor, MD, PhD
SCA Dermatology, Center for Clinical and Cosmetic Research, Aventura, Florida; Department of Dermatology and Cutaneous Surgery and Department of Surgery, Division of Plastic Surgery, University of Miami, Miller School of Medicine, Miami, Florida
 Adrian O. Rodriguez, MD, FAAD
Adrian O. Rodriguez, MD, FAAD
Nashville Skin: Comprehensive Dermatology Center, University of Tennessee Health Science Center, Department of Internal Medicine; St. Thomas, Midtown
FUNDING: This supplement was funded by Amgen.
DISCLOSURES: Ms. LeQuang has no conflicts of interest relative to the content of this article. Dr. Kivelevitch has been an investigator for Abbvie, Arcutis, Avillion, Boehringer-Ingelheim, Bristol Myers-Squibb, and has been a speaker for AbbVie and Eli Lilly. Dr. Gold has no conflicts of interest relevant to the content of this article. Dr. Lebwohl is an employee of Mount Sinai and receives research funds from: Abbvie, Boehringer-Ingelheim, Celgene, Eli Lilly, Incyte, Janssen/Johnson & Johnson, LEO Pharma, Medimmune/Astra Zeneca, Novartis, Pfizer, Sciderm, UCB, Valeant, and ViDac. Dr. Lebwohl is also a consultant for Allergan, Aqua, Arcutis, Boehringer-Ingelheim, Bristol-Meyers Squibb, LEO Pharma, Menlo, Mitsubishi, Promius, Theravance, and Verrica. Dr. Nestor is a member of the JCAD Editorial Advisory Board and a consultant and investigator for research study for Aerolase. Dr. Rodriguez is on the Speakers’ Bureau at Eli Lilly, Novartis, Janssen Pharmaceuticals, Sun Pharma, LEO Pharma, and UCB. Dr. Stein Gold has been a consultant, advisory board member, and/or has received research funding from Ortho Dermatologics, Dermavant, Arcutis, and LEO Pharma.
A Message from the SCALE Program Chairs
 Michael H. Gold, MD
Michael H. Gold, MD
Program Co-Organizer, Music City SCALE meeting; Medical Director, Gold Skin Care Center and Tennessee Clinical Research Center; Assistant Clinical Professor, Department of Medicine, Division of Dermatology, Vanderbilt Univerisity School of Medicine, Nashville, Tennessee
 Brian S. Biesman, MD, FACS
Brian S. Biesman, MD, FACS
Program Co-Organizer, Music City SCALE meeting; Clinical Assistant Professor, Ophthalmology, Dermatology, and Otolaryngology-Head and Neck Surgery, Vanderbilt University Medical Center, Nashville; Clinical Associate Professor, University of Tennessee Health Sciences Center, Memphis, Tennessee; Brian S. Biesman, MD, FACS private practice, Nashville, Tennessee
Dear Colleagues:
We are thrilled to be partnering with the Journal of Clinical and Aesthetic Dermatology once again to bring you this incredible supplement based on lectures from the 2020 Virtual SCALE Meeting. SCALE has truly become one of the most important educational events in the United States. Now that we have completed our 15th meeting, we are primed, even during this pandemic. We want to become even stronger as we head into 2021 for our 16th meeting. A meeting we hope will take place live in Music City—Nashville, Tennessee.
SCALE has become known for its cutting edge presentations, which have been delivered by some of the most renowned faculty members from all over the world. On the medical dermatology side of the meeting, we are proud to say that are faculty is second to none. We learn from the best, not only in regard to dermatologic diseases that are seen regularly in clinics, but about the latest therapeutics, including those that are new to the market and those that will be released soon.
This supplement from the 2020 Virtual SCALE meeting covers the latest trends in our understanding of and treatments for psoriasis and related psoriatic arthritis. We hope you enjoy this supplement and we look forward to seeing everyone in Nashville in 2021.
With regards,
Michael H. Gold, MD and Brian S. Biesman, MD, FACS
Introduction
The Symposium for Cosmetic Advances and Laser Education (SCALE) meeting was held as a virtual event on July 24 to 26, 2020. While the format was changed as a result of the COVID-19 pandemic, the meeting continued to bring cutting-edge research on psoriasis and other cutaneous conditions to the forefront and showcase the latest clinical research and newest innovations in pharmacological treatment from the top key opinion leaders in the field. This supplement focuses on some of the important psoriasis-related topics presented at the 2020 SCALE meeting. Psoriasis is a prevalent inflammatory skin condition, the underlying mechanisms of which are gradually being elucidated—resulting in exciting new breakthroughs and growing hope for better treatments. Today, it is not unusual for patients with psoriasis to get complete clearance, and good-to-excellent results are becoming more the norm than the exception in patients receiving the latest treatments. In this supplement, we provide an overview of six sessions by five presenters.
An Update on Psoriasis 2020
Based on a presentation by Mark Lebwohl, MD—Waldman Professor and Chairman, Kimberley and Eric J. Waldman Department of Dermatology, Icahn School of Medicine; Mount Sinai, New York.
Over the past decades, the mechanisms underlying psoriasis have become better understood and these insights have shifted the way dermatologists approach the disease. Dr. Mark Lebwohl presented the highlights of new breakthroughs and clinical studies in psoriasis care, grouped by pharmacological agents.
Tumor necrosis factor alpha blockers. The most recently approved tumor necrosis factor alpha (TNF-alpha) blocker for psoriasis is certolizumab pegol (CZP), which was the subject of the recent CIMPASI-1 and CIMPASI-2 clinical studies.1 Data from these studies show that CZP is highly effective, with 51.3 percent and 62.7 percent of patients achieving Psoriasis Area and Severity Index (PASI) scores of PASI90 at 48 weeks for CZP doses of 200mg or 400mg once every two weeks (Q2W), respectively. The open-label extension periods on these studies now provides 144 weeks of data, showing durable results with PASI90 scores of 48.7 percent and 42.7 percent, respectively for 200mg and 400mg Q2W dosing.2 The early 48-week data showed that CZP was certainly among the most effective TNF-alpha blockers for psoriasis treatment. Over 81 percent of patients in the higher-dose group achieved PASI75 at 48 weeks.
Interleukin-17 blockers. Secukinumab is an interleukin-17 (IL-17) blocker which was evaluated in the ECLIPSE study, the first head-to-head comparison study of secukinumab 300mg (n=514) versus the anti-IL-23 agent, guselkumab 100mg (n=534). The primary endpoint was PASI90 response at 48 weeks, and results show that this endpoint was achieved by 70.0 percent of patients taking secukinumab and 84 percent of patients taking guselkumab.3 However, it is important to note that secukinumab had better results in the first 12 weeks and it was only around Week 24 of the study that results diverged, with guselkumab showing better results. For example, had the study ended at 12 or 20 weeks, the conclusions drawn would have differed from the results at 48 weeks. In evaluating head-to-head clinical trial results, it is important to consider the unique attributes of each drug and that points of comparison can differ at specific time points. Thus, clinicians must evaluate studies carefully, as it is possible for investigators to tailor trial endpoints to showcase the specific attributes of the drug. In this case, it is clear that guselkumab is an effective drug, but it takes longer than secukinumab to achieve peak effectiveness. Note that guselkumab was also studied in a head-to-head comparison study with another IL-17 blocker, ixekizumab, in the IXORA-R study. This study set its endpoint at 12 weeks, and showed that ixekizumab was more effective.4
Dermatologists must be mindful that specific results from a clinical trial are captured for the study at one specific point in time, for instance, at 12 weeks or 44 weeks, and these values are of less interest to patients with psoriasis than good results over the long term. Psoriasis is a chronic condition that requires long-term therapy. The National Psoriasis Foundation has coined the term “happy days” in order to define the area under the curve (AUC) of study results where patients achieved good results from their psoriasis treatment. The “happy days” score is clinical metric that is very meaningful to patients and should be considered when evaluating clinical study data, which may offer a snapshot of results at a specific point in time that is not indicative of the overall “happy days” enjoyed by the patient. As an example using the “happy days” methodology, both secukinumab and guselkumab in the ECLIPSE trial offer similar “happy days” and very positive results for patients, although at certain specific points in time, one drug may be appear to be more effective than the other.3
A welcome new agent to the psoriasis armamentarium in 2020, ixekizumab has been approved for use in pediatric patients. The IXORA-PEDS study reported results in pediatric patients aged 6 to 17 with moderate to severe plaque psoriasis in a double-blind, placebo-controlled, Phase III study. Patients were randomized to receive weight-based dosing of ixekizumab every four weeks (n=115) or placebo (n=56) over 12 weeks, whereupon all patients entered an open-label period of ixekizumab every four weeks. Ixekizumab was significantly more effective than placebo for achievement of PASI75 (89% vs. 25%, p<0.001) and results as 12 weeks were sustained over 48 weeks. Adverse events were reported by 56 percent of patients who received ixekizumab and 42 percent of placebo patients.5
Bimekizumab is a novel agent that blocks both IL-17A and IL-17F and has been studied in the BE ACTIVE trial. IL-17A and IL-17F have similar structures and biological functions, but it had only recently been determined that IL-17F is also active in psoriatic skin disease.6 In psoriatic arthritis (PsA), bimekizumab has demonstrated effectiveness with ACR50 response rates at 24 weeks maintained to 48 weeks. In the BE ACTIVE trial, adults with PsA were randomized to one of five groups: bimekizumab 16mg; bimekizumab 160mg; bimekizumab 160mg with a one-off loading dose of 320mg; bimekizumab 320mg; or placebo. Bimekizumab was administered by subcutaneous injection every four weeks for 12 weeks. At 12 weeks, patients in the placebo or 16mg groups were randomly re-assigned to receive bimekizumab 160mg or 320mg, while other patients remained as assigned. The primary endpoint was ARC50 at 12 weeks which was achieved by significantly more patients in the 16mg, 160mg, 160mg plus loading dose groups than placebo patients.7 These results for PsA were paralleled by similarly effective results for cutaneous psoriasis. PASI90 scores at 12 weeks were achieved by 20.7 percent of 16mg patients, 46.4 percent of 160mg patients, 53.8 percent of 160mg plus loading dose patients, and 51.3 percent of 320mg patients compared to 7.1 percent of the placebo group.7
M1095 is a novel anti-IL-17A and anti-IL-17F nanobody evaluated in a Phase I study (N=44) at doses of 30mg, 60mg, 120mg, and 240mg over six weeks in ascending-dose cohorts. The terminal half-life of M1095 was 11 to 12 days. Treatment-emergent adverse events were pruritus (n=4) and headache (n=3). Marked decreases in inflammatory markers for skin psoriasis were observed. At 85 days, 100 percent of patients in the 240mg group for M1095 had achieved PASI90 and 56 percent reached PASI100.8 These dramatic results were limited by the trial’s small patient population. Nevertheless, M1095 appears to be a promising new agent and it is likely to be the subject of future studies.
IL-23 blockers. Guselkumab is an IL-23 blocker that binds specifically to the p19 subunit. The DISCOVER-2 Phase III clinical trial evaluated guselkumab in biologic-naïve patients with PsA (N=1,153). Patients were randomized to receive placebo or guselkumab 100mg every four or eight weeks. The primary endpoint was ACR20 scores at 24 weeks, which was achieved by 64 percent of both guselkumab groups (4- and 8-week dosing plans) and 33 percent of the placebo group.9 Although guselkumab is not approved to treat PsA, even the four-week dosing plan in this study was found to reduce radiographic disease progression. Thus, guselkumab has shown results in the treatment of PsA similar to those of TNF-alpha blocker therapy.
Tildrakizumab is an IL-23 blocker which has been approved for treating plaque psoriasis and has also been shown to be effective in treating PsA. When studied in a Phase II trial for PsA, interim results were promising. When dosed at 200mg every four weeks; 200mg every 12 weeks; 100mg every 12 weeks; and 20mg every 12 weeks, tildrakizumab was associated with ACR20 scores ranging from 71.4 percent to 79.5 percent. It must be noted that the placebo group in this study had 50.6-percent ACR20, which appears high.10
Based on previous study data from earlier clinical trials using tildrakizumab for psoriasis (P05495, reSURFACE-1 and reSURFACE-2), there were significantly more tildrakizumab patients than placebo patients who achieved PASI75 or PASI90 at 12 weeks. Furthermore, data analysis showed that younger patients (<65 years), leaner patients (90kg or less), and biologic-naïve patients had better response.11 The reSURFACE-1 trial provides good evidence for long-term effectiveness of tildrakizumab, with around 60 percent of patients achieving and maintaining PASI90 status at 144 weeks and over 20 percent of patients doing the same with PASI100.12 Among tildrakizumab responders, the median time to relapse (defined as loss of PASI75) was 20 or 25 weeks for 100 and 200mg doses, respectively.13 The recapture of response with tildrakizumab is good, but full recapture can be elusive, so “drug holidays” might be counterproductive. Starting patients on tildrakizumab early in the course of the disease appears to produce better outcomes.
Risankizumab targets IL-23p19 and has been approved for use in moderate to severe plaque psoriasis. Three earlier studies (UltiMMa-1, UltiMMa-2, IMMvent, and IMMHance) found that risankizumab was more effective than ustekinumab, adalimumab, and placebo in terms of the proportion of patients who achieved PASI90.14 The ongoing LIMMitless trial is an open-label extension study of UltiMMa and IMMvent studies aimed at investigating the long-term safety and efficacy of risankizumab. Data out to over 100 weeks are promising, but have not yet been published.
A new IL-23p19 inhibitor, mirikizumab, is currently in Phase III clinical studies.15 PASI90 was achieved by 67 percent of patients at 16 weeks and of those patients who did not meet this 16-week endpoint, many went on to achieve PASI90 by Week 52.16
Anti-IL-36 agents. The discovery of T-helper cells producing IL-17 helped to elucidate T-lymphocyte-driven inflammatory processes which, in turn, helped to better explain the etiology of psoriasis. The T-cells that produce IL-17 are regulated by IL-23, creating a self-perpetuating, ever-amplifying response that leads to keratinocyte proliferation, thickened skin lesions, and inflammation.17 IL-36 is an inflammatory cytokine and part of the IL-1 superfamily. IL-36 is known to be involved in the activation of immune cells, presentation of antigens, and the production of pro-inflammatory factors. Because it dysregulates and interrupts inflammatory processes, IL-36 has been of great interest as a drug target for pustular psoriasis and other polygenic inflammatory diseases.18 Pustular psoriasis is phenotypically and genetically different from psoriasis vulgaris, but both present with plaque psoriasis lesions.19
A novel anti-IL-36 agent (BI-655130) is currently in Phase I clinical trials and interim results support the safety and efficacy of this agent in treating generalized pustular psoriasis flares with no serious adverse events reported at 20 weeks. In preliminary findings, five of seven patients achieved clear or almost clear skin with at Week 1 and 100 percent of patients had clear skin by four weeks.20
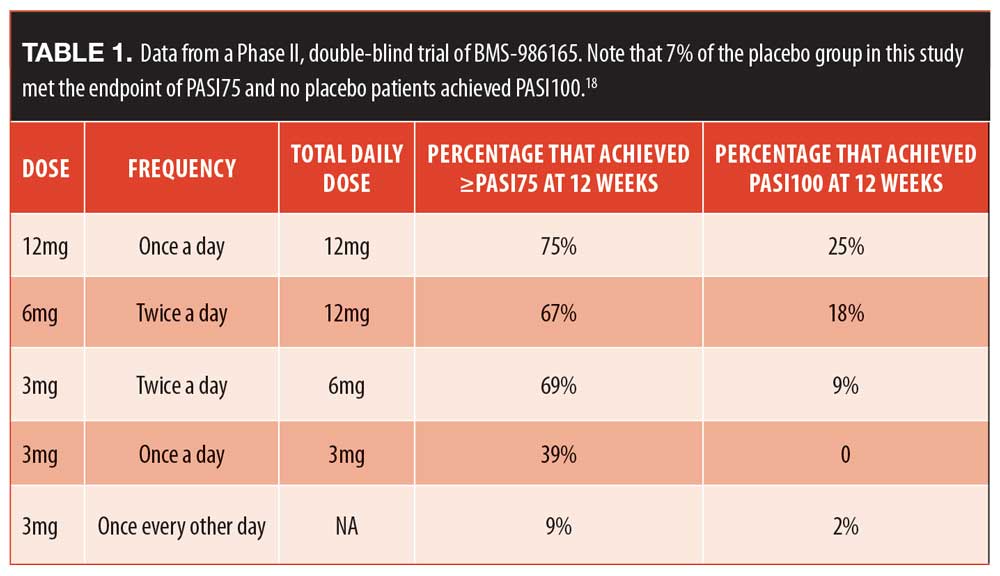
Oral agents. A new tyrosine kinase 2 inhibitor has been evaluated (BMS-986165) for the treatment of moderate to severe plaque psoriasis in adults. It targets the tyrosine kinase pathway to interrupt cytokine signaling. From a Phase II double-blind trial (N=267), patients were randomized to one of five groups and the percentage of patients in that group achieving PASI75 or better was recorded (Table 1).21 There were no deaths or serious adverse events in this study, but there were more instances of nasopharyngitis and upper respiratory tract infections in the active treatment group of 6mg twice daily than placebo. Regarding adverse events, nine percent of patients taking the active drug developed acne during the course of this study; which warrants further exploration.
Baricitinib is an oral Janus kinase (JAK) inhibitor, specifically a JAK1/JAK2 inhibitor, approved for the treatment of adults with moderate to severe plaque psoriasis. A study published in 2016 reported about 271 patients randomized to receive oral baricitinib once a day at doses of 2mg, 4mg, 8mg, or 10mg or placebo; at 12 weeks, 43 percent of the 8mg and 54 percent of the 10mg group had achieved PASI75 and more than 81 percent of responders maintained results through 24 weeks.22 However, blood-related adverse events were reported, including elevated serum lipids and creatinine, which suggests that patients taking this agent may require monitoring over the course of their treatment.
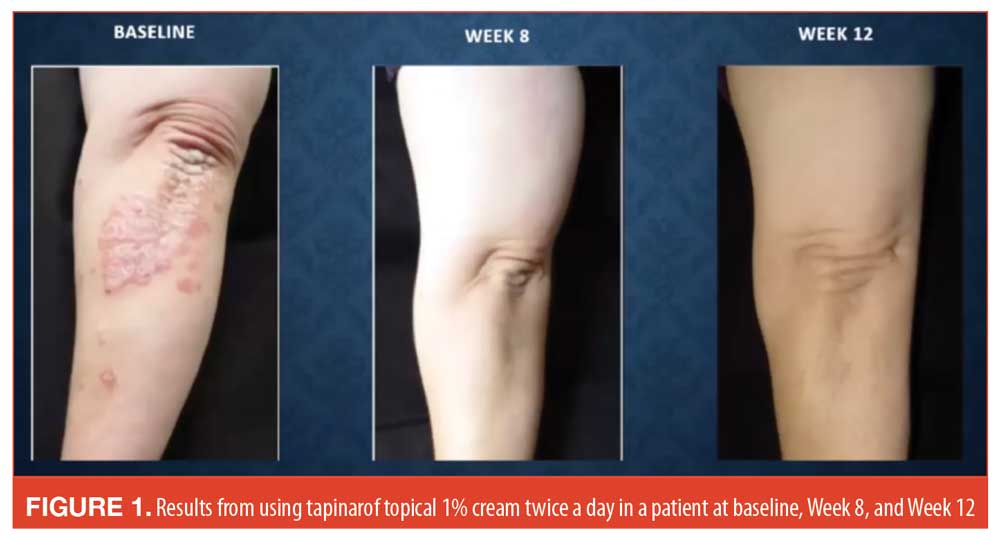
Topical therapies. The novel topical agent tapinarof cream is an aryl hydrocarbon (AhR) agonist with an anti-inflammatory effect. Creams have been studied at 1% and 2% concentration, with the lower potency cream preferred, as it has demonstrated comparable efficacy to the 2% cream with fewer side effects.23 In a double-blind, randomized, vehicle-controlled study with endpoints of PASI75 and a Physician’s Global Assessment (PGA) score of 0 or 1 at 12 weeks (and a two-grade improvement over baseline), 65 percent of patients who received 1% tapinarof daily, 46 percent of those who used 0.5% twice daily, and 36 percent of those who used 0.5% once daily met the endpoint at 12 weeks compared to 11 percent in the vehicle group. Responders maintained their results for four weeks after treatment stopped.24 Before-and-after images appear in Figure 1.
Device-based treatments. A new hand-held device tied to a smartphone application permits patients to self-administer dermatologist-prescribed ultraviolet-B (UV-B) phototherapy at home (Clarify Home Light Therapy System; Clarify Medical, San Diego, California).25 The novel device delivers specific wavelengths of UV-B light for therapy, controlled using a patient-friendly, phone-based interface so that patients may receive exactly the prescribed treatment in their home. The phone application also captures patient data, including optional photography. The patient’s information is stored in a cloud-based system, secure and adherent with the standards set by the Health Insurance Portability and Accountability Act (HIPAA) regulations. This system allows for the electronic delivery of progress reports to the clinic and permits the physician to monitor the patient remotely but in real time. This system can be used to treat psoriasis or vitiligo.25
In a single-blind multicenter study from Holland, device-based phototherapy treatment as described above was compared to conventional outpatient UV-B phototherapy in 196 patients with psoriasis (PLUTO study).25 Results were similar for the two groups (40.7% of the device group and 41.7% of the inpatient group achieved PASI75 or better) and adverse events, mainly erythema or burning sensations, were also similar. However, the patients who used the device to self-administer home-based phototherapy were happier with their treatment.25
What’s New in Topical Therapy for Psoriasis?
Based on a presentation by Linda Stein Gold, MD—Director of Clinical Research, Henry Ford Health System; Detroit, Michigan.
Topical treatments have been a mainstay in the treatment of patients with psoriasis, and while they are familiar to dermatologists and most patients, there is much that is new in the field of topicals. Dr. Linda Stein Gold presented practical clinical tips for using topical therapy in the treatment of psoriasis and discussed some of the latest innovations and findings in topical therapy.
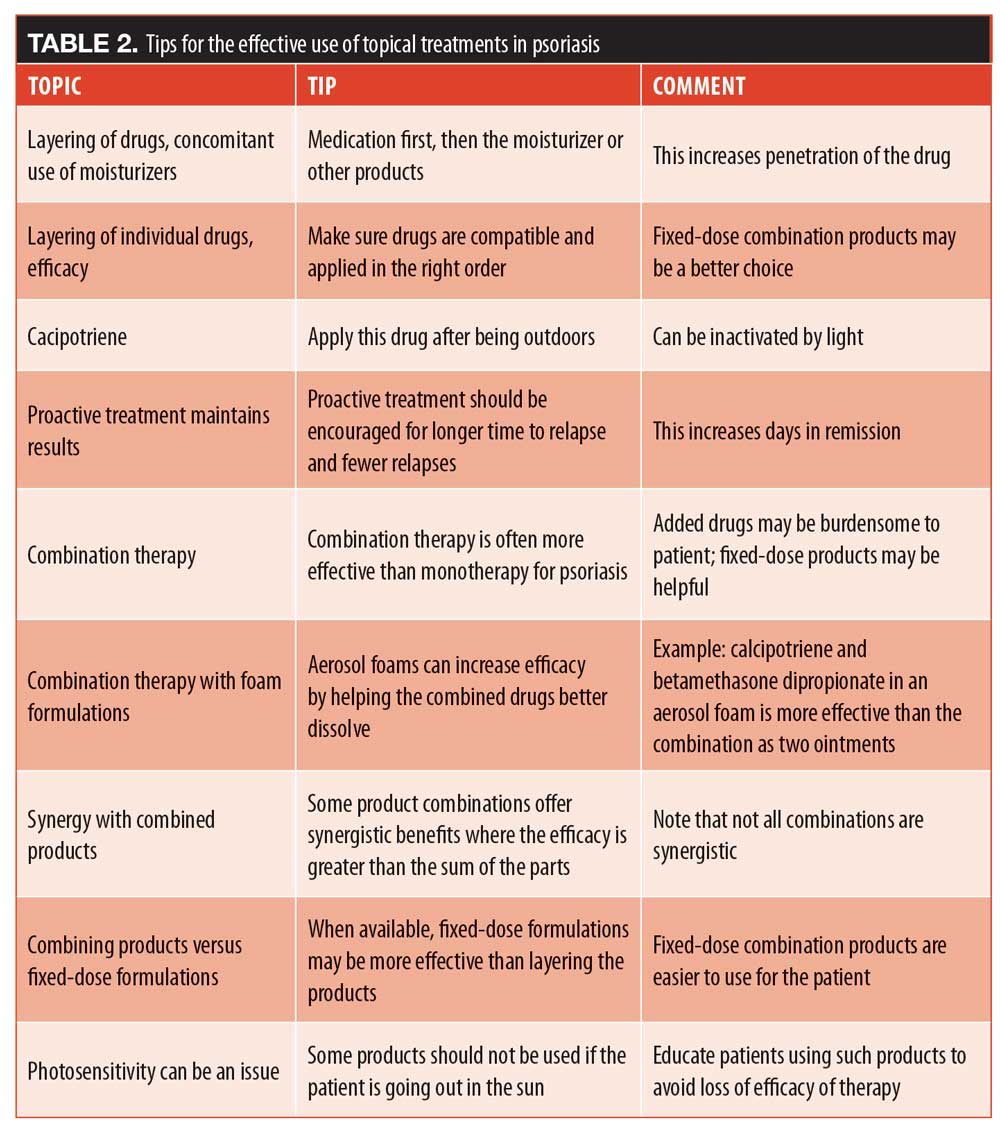
Tip: vehicles matter. The cumulative amount of the drug delivered and, by extension, the efficacy of the treatment, may be substantially affected by the vehicle used to deliver the agent. This was determined in a study by Huang et al,26 which evaluated three different formulations of betamethasone valerate (BMV). The cumulative amount of BMV delivered to the patient in 24 hours after topical application was greatest for the ointment, lowest for the cream, and midway between those two for the foam formulation, demonstrating that the ointment achieved best penetration.26 Thus, vehicles should be considered when prescribing topical treatments.
Tip: layering drugs can boost efficacy, but not always. Further, when tacrolimus ointment was applied on top of the BMV cream in the study by Huang et al,26 it significantly enhanced BMV penetration. It appears that the tacrolimus ointment created an occlusive barrier that allowed for greater penetration of the steroid beneath it. However if tacrolimus ointment was applied on top of the BMV ointment—the result is a dilution of the steroid, rather than an enhancement. The effect is not trivial; the dilution reduces the penetration of BMV by about half.26
Further, a study was conducted in which crisaborole was applied to ex-vivo healthy abdominal human skin with an over-the-counter (OTC) emollient applied on top 15 minutes later. Two OTC products were used—a healing ointment and a moisturizing cream. In both cases, no significant differences in crisaborole penetration were observed. However, when the OTC emollient was applied first and the crisaborole applied on top 15 minutes later, investigators observed a diluting effect and less crisaborole penetration.27
Tip: compatibility counts. In cases where products are incompatible, treatment efficacy may be drastically and negatively impacted. When it comes to layering, betamethasone dipropionate and calcipotriene may be incompatible, although both drugs are familiar and valued members of the topical armamentarium and are available in specially formulated fixed-dose combination products.28 Betamethasone dipropionate, a corticosteroid, has optimal stability at a different pH value than calcipotriene, a synthetic vitamin D derivative, which is unstable in aqueous environments.28 Thus, layering these drugs may not be as effective as using these drugs as monotherapy or using a specially formulated fixed-dose product. This is not to say these drugs may not be used concurrently, but they can be incompatible drugs for a layering approach.
In a study of a compatible combinations, calcipotriene foam 0.005% was used together with (layered) halobetasol propionate foam 0.05%, and the two active drugs did not reduce the activity of each other.29
Tip: watch out for photosensitivity. Calcipotriene can be inactivated by light, so it should be applied when the patient does not plan to spend time outdoors. Since patients tend to manage their own topical applications, it is important to educate patients about drugs and exposure to sun.
Tip: proactive treatment maintains results. Once a patient with psoriasis has achieved good results and is stabilized with topical therapy, the question naturally arises as to whether topical therapy should be maintained, tapered, or even discontinued. In a Phase III randomized clinical trial of patients with psoriasis vulgaris, patients were treated with a fixed-dose combination product of calcipotriene 0.005% and betamethasone dipropionate foam 0.064%. This fixed-dose combination was designed to allow these drugs to work together compatibly. All patients used the product daily for a four-week open-label study phase. Patients with a PGA of 0/1 were then randomized to use the foam product twice weekly or a vehicle for the next 52 weeks. The group that maintained treatment twice weekly had a 46-percent reduction in relapse rates (p<0.001) and 41 extra days of remission a year (p<0.001). The reactive (control) group had 4.8 relapses per year compared to 3.1 in the proactive group.30 The time to first relapse was longer in the proactive than the reactive group. This suggests that topical therapy should be maintained rather than tapered or discontinued even after the patient has achieved the desired results.
Tips on topicals: conclusion. Nearly all dermatologists prescribe topical therapy, and over the years, patients with psoriasis have derived great benefit from these many products. A short summary of the tips on topical appears in Table 2.
What’s new in combination topical therapy? Combination therapeutic approaches may be more effective than monotherapy for certain patients with psoriasis. Authors of the most recent American Academy of Dermatology guidelines gave combinations of corticosteroids plus a vitamin D analog or topical corticosteroids plus a vitamin A derivative its highest level of recommendations.31 For example, a fixed-dose calcipotriene and betamethasone dipropionate ointment has been shown to be more effective in combination than either drug used alone.32 When calcipotriene and betamethasone dipropionate are made into an aerosol foam, the two drugs combine better, penetration improves, and efficacy is enhanced.33
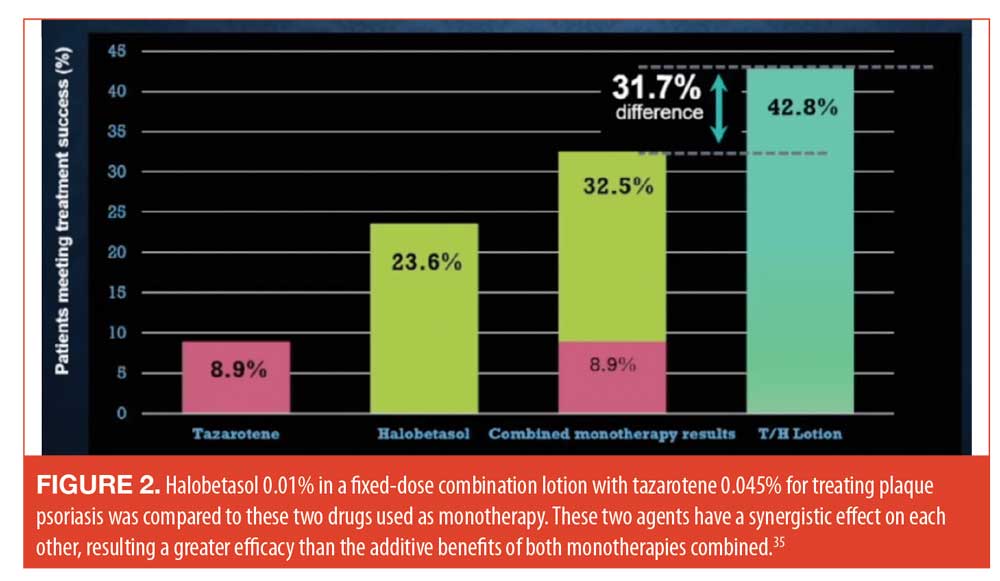
Tazarotene is an effective drug for treating psoriasis but may irritate the skin. By combining tazarotene with topical steroids, tazarotene tolerability improves along with efficacy.34 A new fixed-dose product which combines halobetasol 0.1% with tazarotene 0.045% in a lotion formulation to treat plaque psoriasis is currently in Phase II clinical trials. These two agents have demonstrated a synergistic interaction in terms of efficacy (Figure 2).35 When this same fixed-dose combination product was used once a day for eight weeks in a Phase III study, the results showed that the combination product demonstrated better efficacy than vehicle.36 An interesting aspect of this study was that during the eight weeks of treatment, a decrease in plaque thickness was observed, along with improvements redness, dryness, and sensations of burning and stinging.36 See Figure 3.
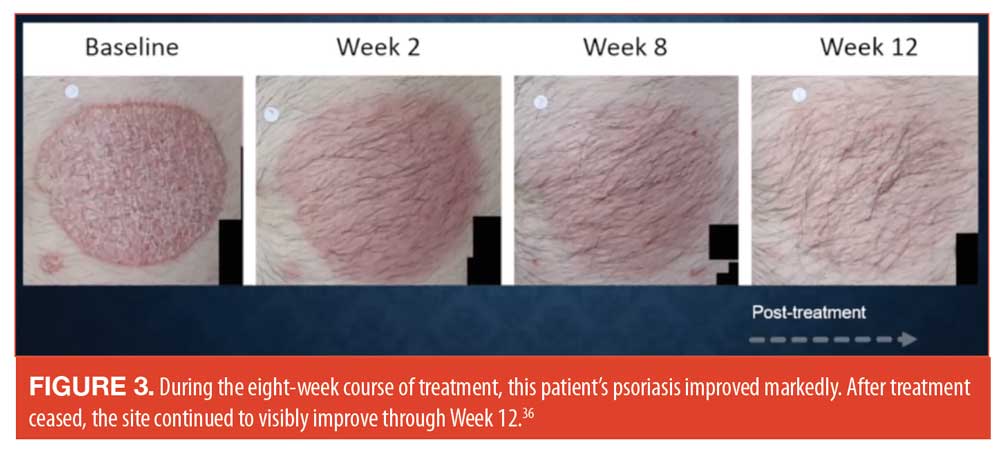
These positive results from the fixed-dose combination product of halobetasol plus tazarotene often leads to the logical question as to why dermatologists and their patients cannot simply layer one drug on top of another to achieve the same effect. Layering can be effective in some cases, but in this case, layering the two drugs on the skin can impede the penetration of tazarotene. This is one of the reasons why fixed-dose products may be superior than layering. Additionally, they can be less burdensome to the patient and encourage better patient adherence.
New topical treatments. New topical products in the pipeline show considerable promise. These include a topical JAK inhibitor (delgocitinib), a topical phosphodiesterase-4 (PDE-4) inhibitor (roflumilast), a tapinarof topical formulation, and new fixed-dose combination topical products. There is also a novel polyaphron dispersion (PAD) technology which improves penetration of cream products, thus boosting their efficacy.
Tapinarof, a therapeutic aryl hydrocarbon (AhR) modulating agent, is a small molecule that binds to and activates the AhR transcription factor. Although relatively new, tapinarof has already exhibited some intriguing and important effects. It downregulates Th17 cytokines, which in turn decreases the inflammation associated with psoriasis.37 Furthermore, it decreases Th2 cytokines, associated with atopic dermatosis, making it a versatile agent. It diminishes oxidative stress by enhancing antioxidant activity.37 The topical tapinarof product repairs and protects the skin barrier as well.37 Tapinarof was studied in Phase IIb studies among patients with psoriasis for 12 weeks. The study compared topical tapinarof 1% twice a day; tapinarof 1% once a day; tapinarof 0.5% twice a day; tapinarof 0.5% once a day; vehicle twice a day; and vehicle once daily.24 At 12 weeks, all of the active study arms were statistically superior to the vehicle groups and effects were maintained through 16 weeks.24
Conclusion. Insights into psoriasis pathophysiology and drug mechanisms has made for exciting developments in topical therapies for psoriasis. Topical products will likely remain a cornerstone of psoriasis care and, as always, dermatologists need to be able to provide patients with not only appropriate topical and other treatments, but also supportive and patient-friendly advice.
IL-23 Inhibition Strategies
Based on a presentation by Mark Lebwohl, MD—Waldman Professor and Chairman, Kimberley and Eric J. Waldman Department of Dermatology, Icahn School of Medicine; Mount Sinai, New York.
It may be that the addition of IL-23 inhibitors to the psoriasis treatment arsenal has fundamentally changed how dermatologists and other scientists view psoriasis. According to Dr. Mark Lebwohl’s presentation on IL-23 blockers, these agents have certainly changed for the better how psoriasis is treated. These IL-23 blockers include guselkumab, tildrakizumab, risankizumab, and mirikizumab. Note that mirikizumab will be included in this discussion although it is not yet cleared for market release in the United States. These drugs are promising, powerful new agents, but their use in patients with psoriasis must be guided by evidence and a certain measure of strategy. As more is learned about IL-23 inhibition, more is revealed about the pathophysiology of psoriasis.
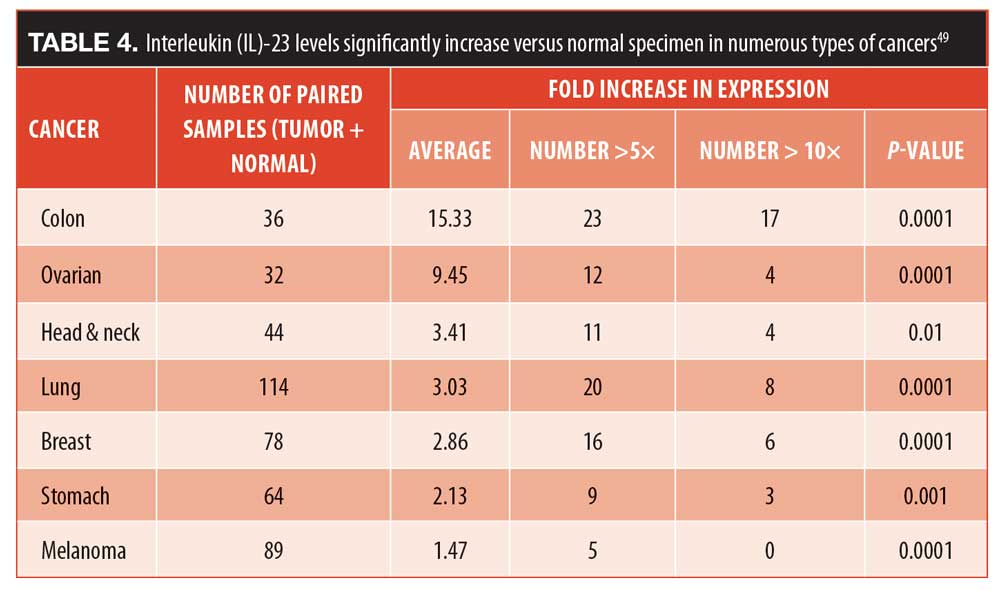
The mechanisms of action of IL-23 blockers. While the exact process is not known, it is now apparent that Th17 cells are differentiated from naïve T-cells in a complex series of steps.40 Blocking IL-23 prevents the Th17 cells from producing IL-17, putting the Th17 cells into a form of prolonged hibernation. It is this protracted “hibernation” that gives IL-23 blockers their long-lasting efficacy. For that reason, IL-23 blockers do not require frequent dosing, as they have a very prolonged effect on the Th17 cells. Dosing regimens appear in Table 3.
The efficacy of IL-23 blockers. Tildrakizumab data from the reSURFACE trials show good results at 12 weeks that improve markedly at 28 weeks, when over 30 percent of patients achieved PASI100 and over 50 percent achieved PASI90.13 Pooled long-term efficacy data go out to nearly three years with little change. The median time to relapse at the approved dose is 226 days.13
Guselkumab was studied in the VOYAGE trials, which demonstrated effectiveness, in that 47 percent of patients achieved PASI100 at 48 weeks. Guselkumab works relatively quickly, with 91 percent of patients achieving PASI75 at 16 weeks, maintaining these results with only a slight drop to Week 48.41 Responses were durable to 156 weeks, and half of patients who achieved PASI100 maintained those results at three years.41 It was noted that patients with moderate psoriasis showed excellent response to guselkumab,41 and dermatologists should consider biologics even for patients without severe disease. In addition to good efficacy, the infrequent dosing makes it convenient for patients.
Risankizumab resulted in PASI90 response from 75 percent of patients at 16 weeks, which was maintained, rising slightly, to 82 percent at one year (the highest results occurred around 34 weeks, in 85% of patients).42 At Week 16, PASI100 was achieved by 36 percent and 51 percent, in the ultiMMa-1 and -2 trials, respectively. PASI90 scores by 87 percent of patients were maintained for three years, PASI100 responses were around 61 percent.42
Mirikizumab is a new drug and not yet commercially available for a psoriasis indication. Trials are ongoing.43
Obesity is comorbid with psoriasis44 and concern has been raised about whether various psoriasis drugs work as well in patients with psoriasis and obesity versus patients with psoriasis without obesity.45 In a meta-analysis, obesity was associated with a 60-percent higher odds of failure for TNF-alpha blockers and each one-point increase in the body mass index (BMI) score of a patient increased the odds of failure of the TNF blockade by 6.5 percent.46
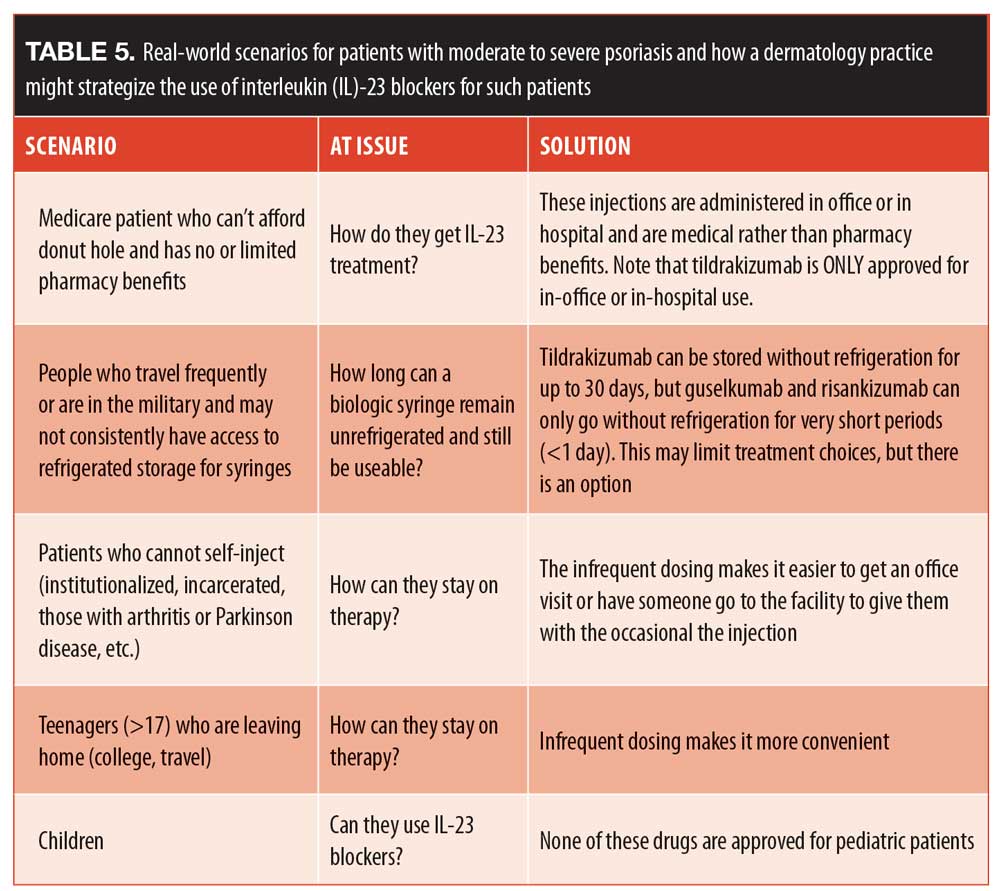
The safety of IL-23 blockers. An important safety consideration with any biologic agent is its potential to interfere with the complex immune response needed by the body to fight off pathogens. Th17 cells, for example, play a crucial role in mucosal immunity.40 A study of 141 patients from 30 different countries who were born IL-12Rbeta1-deficient revealed that the only infections they experienced were mycobacterial and salmonella infections. These individuals did not have higher rates of cancer, opportunistic infections, or other disorders.47 In a study of colon cancer, IL-23 levels were found to be extremely elevated in patients with colon adenocarcinoma but not in patients with a normal colon.48 In fact, IL-23 levels have been shown to increase in a variety of cancers (Table 4).49 In a murine model, IL-23 was shown to promote both the incidence and growth of squamous cell carcinoma tumors, but if IL-23 was blocked, it reduced the cancer in mice.50 In many models, it has been demonstrated that increased Il-23 and IL-17 are needed for tumor growth and proliferation.50 Although it cannot yet be stated definitively, this at least suggests that Il-23 blockers may be oncoprotective.
Pivotal trial data for the Il-23 blockers tildrakizumab, guselkumab, and risankizumab present low rates of severe infections and malignancies.3,13,42 Cardiovascular adverse events are also few and there is a low rate of hypersensitivity. In fact, these rates are comparable to placebo rates and rates achieved with etanercept.13
Long-term safety data for guselkumab from VOYAGE-1 and -2 studies shows that serious adverse events declines at Year 3 compared to Year 1, which is the opposite of cyclosporine and many other agents, where serious adverse events increase with cumulative use.51 TNF-alpha-blockers carry black-box warnings about cancer risks.52 Note that non-melanoma skin cancers decrease markedly year-over-year with guselkumab, but not with cyclosporine.53 Tildrakizumab evidences that same decrease in malignancy rates with years of use.13 That ratio of basal cell carcinoma to squamous cell carcinoma is higher than 2:1 for these two IL-23 blockers; however, the rate of lymphoma is not increased and remains at 1 per 900 patients.13 Literature searches show that risankizumab, tildrakizumab, and guselkumab are not associated with any reports of increased malignancies and there is no verbiage in package inserts to suggest that these drugs would be contraindicated in patients with cancer. In conclusion, IL-23 blockers offer many good solutions for patients with psoriasis confronting clinical challenges. See Table 5 for real-world scenarios that illustrate how a dermatologist might strategize the use of IL-23 blockers for patients with moderate to severe psoriasis.
Challenging Areas to Treat
Based on a presentation by Adrian O. Rodriguez, MD, FAAD—Nashville Skin: Comprehensive Dermatology Center, University of Tennessee Health Science Center, Department of Internal Medicine; St. Thomas, Midtown.
Dr. Adrian Rodriguez presented on managing psoriasis on difficult-to-treat areas of the body, a topic of tremendous concern for many patients with psoriasis. Treatment for psoriasis vulgaris can be relatively straightforward, but intertriginous or inverse psoriasis, genital psoriasis, scalp psoriasis, erythrodermic psoriasis, and palmoplantar psoriasis can be recalcitrant, pose important challenges to care, and be distressing to the patient. The key to effective treatment is prompt and accurate diagnosis, but these various conditions each pose unique challenges. Scalp psoriasis is relatively easy to diagnose but may resist treatment.54 Patients with genital psoriasis often feel embarrassed, impeding their desire to discuss the condition.54 Palmoplantar psoriasis can be treated, but treatments may limit the patient’s activities of daily living.56 Erythrodermic psoriasis can be frightening to the patient, as it can be severe enough to require hospitalization.57 In addition to types of psoriasis that are difficult to treat, some patients with psoriasis enter treatment with special circumstances that can complicate care or limit prescribing options, such as trauma patients or those with amputations.
Inverse or intertriginous psoriasis. Inverse or intertriginous psoriasis is “the great imitator.” Its typical presentation is smooth, demarcated inflammation with limited scaling that causes intense pruritus.58 The abdominal and genital regions are most often affected. Intertriginous psoriasis can occur in 21 to 30 percent of all patients with psoriasis.54
In one illustrative case, a 28-year-old Asian male patient presented with a three-year history of an inferior abdominal, suprapubic, inguinal skin eruption that had been treated as tinea and eczema (Figure 4). He had no significant improvement and had become resigned to his condition.
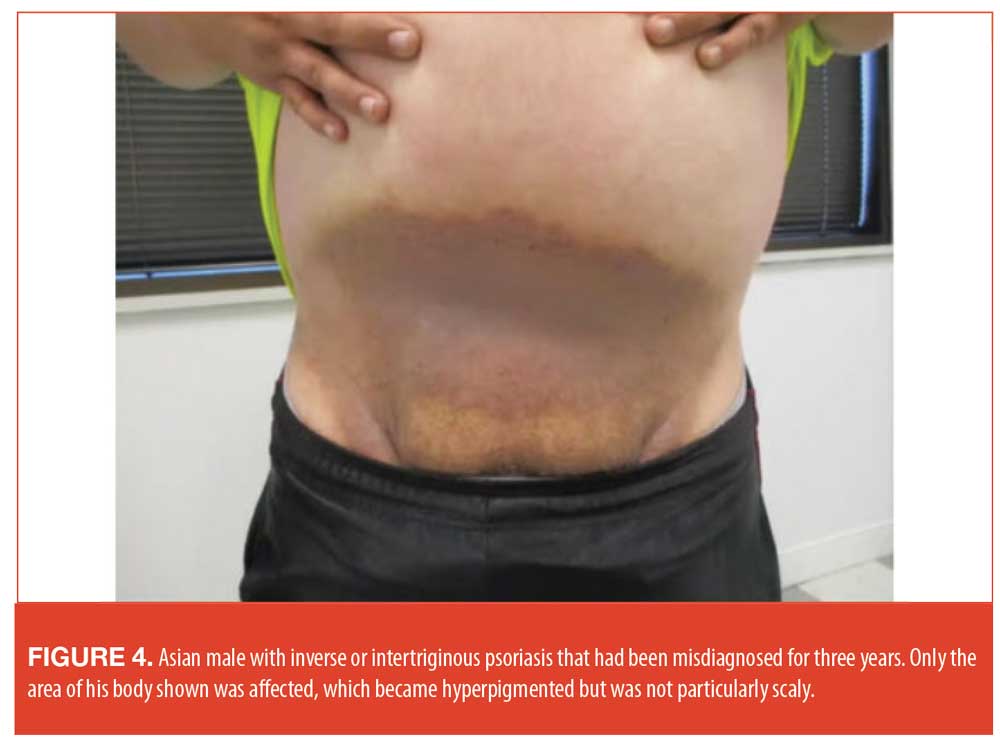
A diagnosis of inverse psoriasis was obtained and, due to the atypical presentation of the disease, a biopsy was taken to confirm the diagnosis. In making the diagnosis, it is important to rule out tinea, Candida, infections, and squamous cell carcinoma. The patient was prescribed clobetasol cream twice daily for one week and saw a notable improvement. This was then decreased to mometasone cream 0.1% and calcipotriene cream. At the time of Dr. Rodriguez’s presentation, the patient was intermittently using mometasone and calcipotriene, alternating them as needed.
Genital psoriasis. The emotional and psychosocial impact of genital psoriasis should not be underestimated, but patients who have this distressing condition are sometimes too embarrassed to discuss it with their dermatologists.59,60 On the other hand, a subset of patients with genital psoriasis may arrive at a clinic alarmed by the commonly painful condition, ready to discuss possible treatments.55 Genital psoriasis does not always respond well to topical therapy.59 In making the diagnosis, it is important to rule out squamous cell carcinoma, syphilis, Bowen’s disease, allergic contact dermatitis, and fixed drug eruptions. An effective treatment strategy involves a short course of a high-potency steroid, transitioning to a lower potency as the condition responds. This can be alternated with calcipotriene. In severe cases, systemic agents might be appropriate.
Scalp psoriasis. Unlike many other skin conditions, scalp psoriasis is straightforward to diagnose once the patient realizes it is not just a bad case of dandruff. Because scalp psoriasis is visible and can be difficult to conceal, many patients are extremely distressed by it.54 There are a few challenges to treating scalp psoriasis that must be mentioned, namely that it can be resistant to topical treatments, topical therapies may have to be varied by ethnic background, and treatment may be influenced by the presence of chemically treated or dyed hair.61
Ear involvement may also occur with scalp psoriasis.62 There are a number of topical products available which can be tried and rotated, as needed, including foam-based products, solutions, suspensions, and shampoos. Fluocinolone in peanut oil overnight for a week may be helpful to manage scaling, and for some cases it can be appropriate to alternate with high-potency steroids. However, there are side effects associated with intralesional steroids, such as lipoatrophy, and therefore, other modalities may be more appropriate.
Erythrodermic psoriasis. Erythrodermic psoriasis is a diffuse erythema that can occur over 75 percent of the patient’s body surface area.57 It is typically of acute onset and may progress so rapidly that the patient requires hospitalization.57 The challenge in accurate diagnosis of erythrodermic psoriasis is that there are multiple causes of erythroderma, including seborrhea, atopic dermatitis, erythrodermic cutaneous lymphoma or Sézary Syndrome, pityriasis rubra pilaris, and acute drug reactions. Patients should be monitored for electrolytes, especially for renal and hepatic function.57 While biopsies can be helpful and may be required for accurate diagnosis, the cutaneous histology may not reveal signs of classic psoriasis.
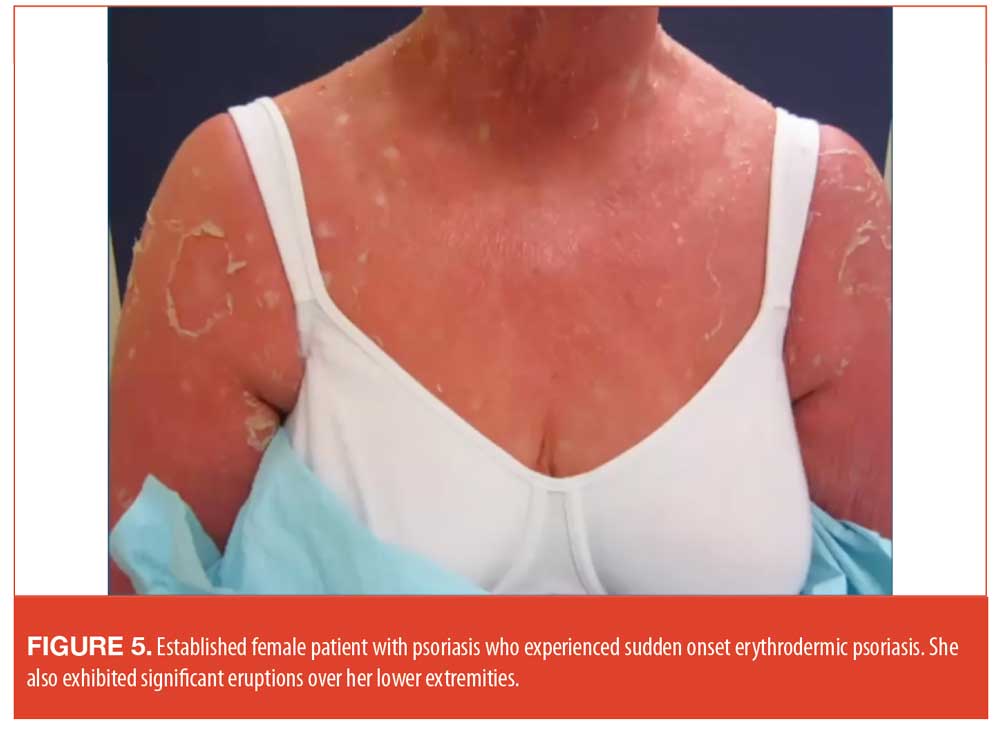
One illustrative case involved an established female patient with a history of psoriasis. She had received high-dose steroid therapy from her primary care physician to treat arthritis. When she discontinued the steroids, erythrodermic psoriasis occurred abruptly (Figure 5). It was decided that hospitalization was not necessary in her case, and she was started on a treatment of cyclosporine for four weeks, transitioning to acitretin. Once her condition had stabilized, she was started on adalimumab.
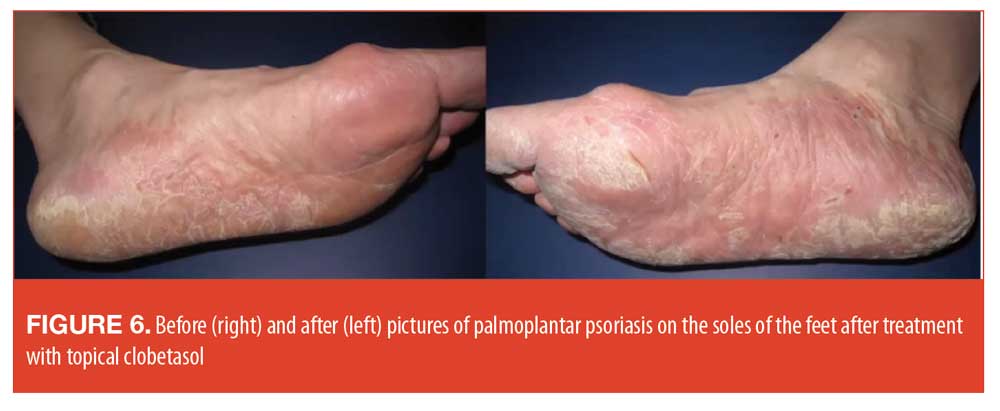
Palmoplantar psoriasis. Palmoplantar psoriasis may be one of the most debilitating forms of psoriasis in that it can interfere with daily life activities for most patients.64 Some patients with palmoplantar psoriasis suffer such functional deficits that they have to alter their lifestyle and sacrifice considerable quality of life.56 Palmoplantar psoriasis may resist treatment and topical products are often more effectively used under occlusion.65 Lesions may be intensely painful with deep fissures and hyperkartosis.65 In diagnosing palmoplantar psoriasis, differential diseases to consider are contact dermatitis, eczematous process, tinea infection, and pagetoid reticulosis. Biopsies are not always conclusive in palmoplantar psoriasis. See Figure 6 for before-and-after case photography.
Special circumstances. Dermatologists are often called to care for patients in challenging clinical circumstances that can make diagnosis and treatment more difficult. At Dr. Rodriguez’s clinic, a 58-year-old Caucasian man with an amputation of the right leg for thrombosis secondary to a genetic hypercoagulable state presented with a four-year history of plaque psoriasis on his stump that was steadily growing larger and more prominent. At first, he suspected he was allergic to the liner on his prosthetic leg and tried to make some changes using other materials. He then developed new-onset erythema with fissures on his hands and his left foot. His prosthetic leg limited the use of topicals, as they were impractical and interfered with the prosthetic liner. The stump lesion grew so enlarged that he could no longer wear his prosthesis. After laboratory tests, he was started on cyclosporine for rapid results and then transitioned to etanercept. His care was complicated by the fact that he had a coagulation disorder, so drug therapy for psoriasis could not interact with his anticoagulation medications. Over time, the psoriasis cleared. At the time of Dr. Rodriguez’s presentation, the patient was taking ustekinumab and was completely clear.
Psoriatic Arthritis for the Dermatologist
Based on a presentation by Dario Kivelevitch, MD—Baylor, Scott & White; Dallas, Texas.
Dermatologists are crucial healthcare professionals in early diagnosis and prompt care of PsA. Dr. Dario Kivelevitch talked about the important role of dermatologists in correctly diagnosing and managing patients with PsA. Since about 30 percent of patients with skin psoriasis will develop PsA, dermatology clinics regularly see patients with PsA.66 In about 80 percent of PsA patients, skin psoriasis preceded PsA by about 5 to 10 years, and sometimes even longer, with a typical age of onset between 30 and 50 years.66 The simultaneous onset of both skin psoriasis and PsA is less frequent and occurs in 11 to 15 percent of patients.66 Thus, dermatologists are on the front lines of diagnosis of PsA; depending on the patient, they may treat PsA or refer PsA patients to a rheumatic disease specialists.
Diagnosis. It can be challenging to diagnose PsA because it must be differentiated from enthesitis, dactylitis, skin and joint pain, arthritis mutilans, spondylitis, and other forms of rheumatic disease, all of which are common in patients with psoriasis and PsA but which may also occur outside of psoriasis. These conditions may mimic PsA, be harbingers of PsA, or suggest worse outcomes in PsA patients.67
Dactylitis, nicknamed “sausage digit,” is the diffuse and often acute swelling of a finger or toe accompanied by painful inflammation. In some cases, the swelling persists after acute inflammation subsides.68 Dactylitis is one of the cardinal features of PsA and is observed in about 40 percent of all patients with PsA.69 In a study of 537 patients registered at a PsA clinic from 1979 to 1999, 48 percent had dactylitis, of which 65 percent affected only their feet, 24 percent only their hands, and 12 percent had it in both hands and feet. Dactylitis recurred in 44 percent of patients and was significantly more associated with radiological disease progression than those without dactylitis (50% vs. 38%, respectively, p<0.0001).70
Enthesitis can suggest or mimic PsA. The entheses are the regions of the body where a tendon, ligament or joint capsule attaches to bone.71 It is believed that the overexpression of TNF-alpha can lead to osteitis and entheseal inflammation.72 In a study from 2008 to 2014 of 803 patients with PsA, the prevalence of enthesitis was 35 percent with an annual incidence of one percent. About half (48%) of patients had one tender entheseal site and 32 percent had two sites. The mean number of tender sites per clinic visit was 2.03±1.6.73 The most frequently reported sites for enthesitis are the insertions of the Achilles tendon, the plantar fascia on the calcaneus, and the lateral epicondyles (24%, 21%, and 17%, respectively).73 In patients with PsA, the presence of enthesitis could be associated with more active disease, greater pain intensity, and less clinical damage.73 Risk factors for enthesitis were found to be a high BMI, more inflamed joints, and younger age.73 In many cases, enthesitis resolves spontaneously without treatment modifications.
When dactylitis or enthesitis occur concurrently with PsA, they are associated with a greater disease burden of PsA.74 Dactylitis and enthesitis can also be early indicators of approaching PsA.68,73 When treating patients with skin psoriasis, screening for dactylitis and enthesitis (particularly checking around the Achilles tendon for tenderness) along with patient history may help determine if they have PsA or if they may be susceptible to worse PsA outcomes.
Asymmetric oligoarthritis, as the name describes, is an asymmetric distribution of arthritic joints that is considered a subset of PsA, although it can occur independently of PsA. It can affect large and small joints and has a reported prevalence of 43 percent among patients with PsA.75 Symmetric arthritis occurs more often in women and typically affects the joints of hands and feet and may include larger joints.75 The prevalence of symmetric arthritis in PsA has been reported at 33 percent, and these patients have a higher rate of disability.75 A systematic literature review found that symmetric polyarthritis was the most frequently reported subtype of PsA.76 Clinically, symmetric arthritis can mimic rheumatoid arthritis (RA).77
The distal interphalangeal (DIP) joint is the most frequent location for osteoarthritis. The incidence of hand arthritis increases with age, and finger involvement is common in people with osteoarthritis.77 DIP joint inflammation occurs more frequently in patients with PsA than any other forms of inflammatory arthritis.79 For diagnosis, it must be differentiated from dactylitis and nail dystrophy, both of which are also common in patients with PsA.
The global prevalence of spondylitis is approximately 0.2 to 1.6 percent, varying by geographical region.80 Its occurrence is associated, but not limited to PsA, and it is more common in females.80 The inflammation of the bones of the spinal column associated with spondylitis, on the other hand, is more common in men and may be associated with intense pain and compromised posture.80 Over time, spondylitis can result in the natural fusing together of small vertebral bones and may even extend into the ribs. Symptoms of spondylitis include hunched-over posture, pain in the upper spine, and low back pain. A genetic component to spondylitis is suspected (gene HLA-B27).81
In this connection, it is important to mention a rare variant, arthritis mutilans. It occurs in about five percent of patients with PsA, typically those with long-term PsA, and is more frequent in women.66,82,83 It involves telescoping digits and flail joints, osteolysis, and joint instability.
Accurate diagnosis is crucial but may be challenging in light of the fact that these subtypes may occur concomitantly as well as individually. Note that these conditions are associated with PsA but can occur independently of it.
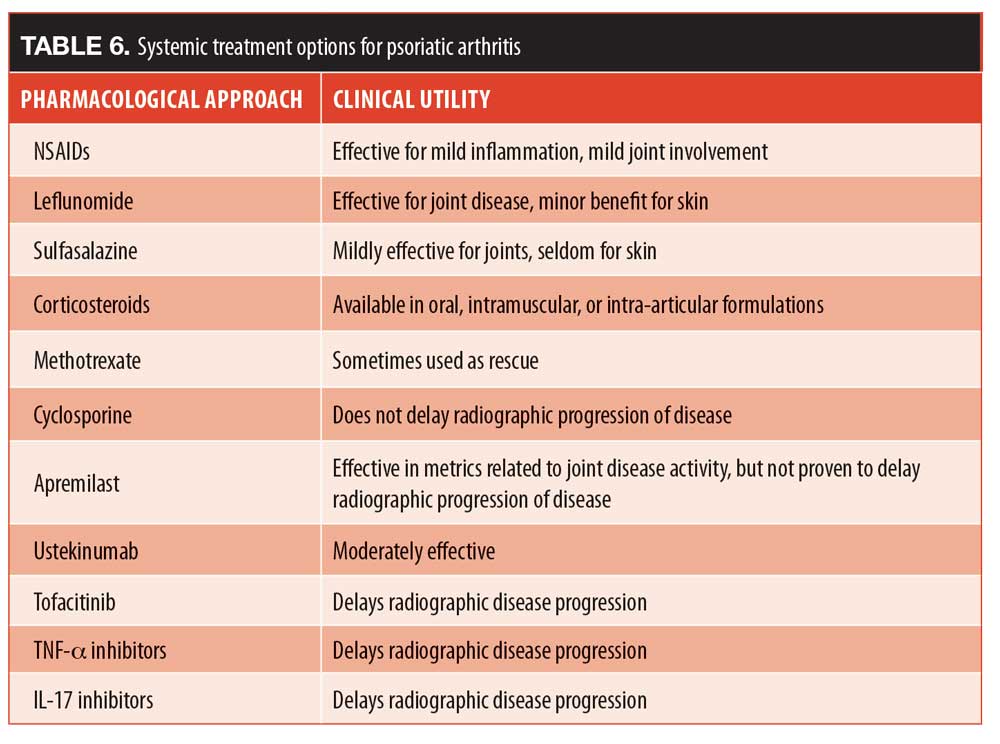
Treatment. There are multiple approaches to PsA with specific uses in the clinical setting (Table 6). Unfortunately, the same number of highly effective agents that we have in the toolkit for cutaneous psoriasis does not exist for PsA. In fact, care for PsA lags noticeably behind the treatments available for cutaneous psoriasis. For example, early skin psoriasis trials often set study endpoints of PASI50 or PASI75, but today, endpoints of PASI90 and even PASI100 (complete clearance) are frequently used. On the other hand studies on PsA still use the American College of Rheumatology (ACR)-20 endpoint, suggesting how far PsA treatments lag behind. When making prescribing choices for patients with skin psoriasis and PsA, it can be beneficial to the patient if both skin manifestations as well as PsA are considered.
Tips for front-line dermatologists. Dermatologists are typically the first healthcare professionals that people with PsA encounter, affording dermatologists the opportunity to take the lead in diagnosis and treatment of these individuals. Skin psoriasis typically presents first, and about a third of patients with skin psoriasis go on to develop PsA, which can be debilitating, disabling, and associated with severe pain.72 Two key characteristics of PsA are dactylitis and enthesitis; every patient with skin psoriasis should be checked for these symptoms at every visit.69
Patients with PsA should be screened at every visit for joint pain. Morning stiffness lasting up to half an hour is common in patients with PsA.84 All patients with psoriasis and PsA should also be evaluated for nail involvement, which has been statistically associated with DIP involvement. There is a misconception that nail involvement is associated with PsA, but evidence suggests nail dystrophy is associated with DIP.67 Nevertheless, it is important to evaluate patients with psoriasis at each clinical encounter.
Differential diagnosis can be challenging, as rheumatic disease has multiple manifestations and is very common, particularly in older patients. Dermatologists who are unsure about a clinical presentation should refer the patient to a rheumatologist. While much remains to be done for better treatment options for PsA, three biologic agents, TNF-alpha blockers, IL-17 inhibitors, and tofacitinib, have been demonstrated to slow radiographic progression of the disease, and these should be considered for appropriate patients.85,86
The Future of Phototherapy Using 650Ms YAG Lasers
Based on a presentation by Mark S. Nestor, MD, PhD—SCA Dermatology, Center for Clinical and Cosmetic Research, Aventura, Florida; Department of Dermatology and Cutaneous Surgery and Department of Surgery, Division of Plastic Surgery, University of Miami, Miller School of Medicine, Miami, Florida
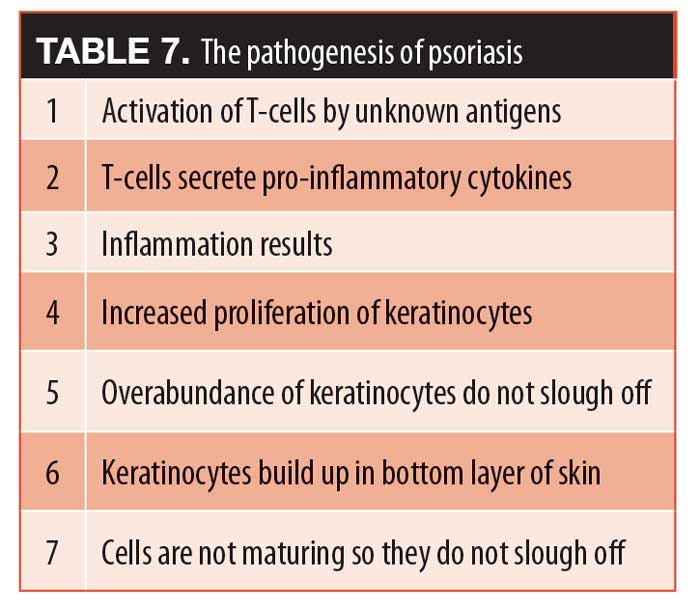
Energy-based devices (EBDs) are often used to deliver phototherapy for the treatment of skin psoriasis. The pathogenesis of psoriasis, as an autoimmune disorder, is associated with increased vascularity, making phototherapy one of the pillars of care (Table 7). Dr. Mark Nestor presented on the use of phototherapy to treat psoriasis with emphasis on different types and applications of these systems. Phototherapy is one of the pillars of psoriasis treatment and has been effectively used to treat psoriatic lesions on many parts of the body, such as arms, legs, and trunk.87 The main types of phototherapy that had been used for decades were ultraviolet-B (UVB), narrowband ultraviolet-B (NB-UVB), and psoralen ultraviolet A (PUVA). A recent development in phototherapy is the technological advancement to more specifically targeted laser light sources, such as the excimer laser, the pulsed-dye laser, and the short-pulse YAG laser (Aerolase Neo).
Phototherapy mechanisms of action. It appears that phototherapy, either with light or laser, has the ability to modulate the local immune environment of psoriasis plaques.87 Note that NB-UVB phototherapy can trigger systemic immune modulation.88 Vascular lasers have an additional mechanism of action, in that they reduce the network of small blood vessels associated with the psoriasis.89 This results in a number of measurable immune changes: the local Th-17 pathway is downregulated, regulatory T-cells (Treg cells) increase, Treg function is restored by way of the upregulation of forkhead box P3 (FOXP3), vascular endothelial growth factor receptor (VEGFR) 2 and 3 and E-selectin proteins are reduced, activated and memory-effector Th cells in the dermis diminish, and, likewise, activated cytotoxic T-cells in the epidermis are reduced.87
Practical considerations when using phototherapy. Light boxes can deliver UVB, NB-UVB, and PUVA and are typically used to treat extensive plaques. These systems are effective but require substantial space in the office. There can be issues with patient adherence and patient satisfaction with light-box treatments. Reimbursement for phototherapy can sometimes be problematic. PUVA has been associated with burns and an increased rate of non-melanoma skin cancers (NMSC).87
Laser treatment, on the other hand, is used on specific and more limited areas of the body, but laser therapy offers fewer practical drawbacks because, in general, the equipment has a small footprint, relatively few treatments are needed, and reimbursement is more straightforward.87 Patient satisfaction may be improved when using laser treatments versus light-box therapy.90
Laser 308nm NB-UVB. This first well-known type of laser treatment provides a therapeutic narrowband phototherapeutic wavelength in a very precise delivery system.91 Overall, 308nm lasers have a good safety record.91 Psoriasis has been shown to be very responsive to this type of NB-UVB laser phototherapy.92 The spot size is about four square centimeters (2×2 cm) and only the targeted area is exposed to light therapy; healthy tissue around the area remains largely unaffected.91 Lasers are able to deliver very high doses of UVB, which must be used with caution, but they have the advantage that they can significantly shorten treatment durations.91
Patient response to laser 308nm NB-UVB depends, in part, on the nature of the plaque.93 Location of the plaque can affect treatment duration, with plaques on the scalp, hands, or feet necessitating multiple treatments (>20 in some cases).94 Clearance can also be affected by plaque severity. Thin and mild plaques are more effectively treated in fewer sessions than thick lesions. For particularly thick plaques, 20 or more treatments may be needed to obtain the desired clinical outcome.92 Side effects can occur, including burning and hyperpigmentation. Hyperpigmentation is usually reversible and will likely start to fade once treatment concludes. Some patients develop blisters, which can be painful, but may have a beneficial effect on the underlying plaque.92
The medical literature contains many case reports detailing the use of laser 308nm NB-UVB, and a recent meta-analysis found it to be generally safe and well tolerated, with a range of efficacies depending on the protocols used. The meta-analysis concluded that although this laser therapy is not a first-line approach to psoriasis, it is safe, effective, and a good option for certain patients.92
Pulsed dye laser. The pulsed dye laser (PDL) is the preferred laser phototherapy option for cutaneous vascular lesions and for nonvascular indications, such as psoriasis, acne vulgaris, papulopustular rosacea, and hypertrophic scars.95 A study to find the optimal parameters of PDL therapy in psoriasis recommended 585nm, 0.45ms, 7mm, overlapping 20% at 5.5 to 6.5 Joules/cm2 for four treatments every three weeks.87 It is important to note here that PDL was not as effective as the excimer laser. In a prospective within-patient controlled study of patients with localized plaque psoriasis (N=22) with a mean Psoriasis Symptom Inventory (PSI) of 7.1, two plaques were treated every week with excimer and V-beam PDL. The mean improvement in PSI was 4.7±2,1 with excimer treatment and 2.7±2.4 with PDL (p=0.003). This study found that on the whole, excimer was significantly more effective for treating psoriasis than PDL, although there may be a subset of patients who respond better to PDL. Long-term remission is possible with both PDL and excimer treatments.95
650ms 1064 YAG. Long-pulse 1064 YAG laser phototherapy has been suggested for a number of inflammatory conditions, but its use is limited, as it causes patient pain and discomfort.96 A new 650 ms “short-burst” 1064 YAG laser therapy offers split-second bursts of energy just below the “burning threshold.”87 This decreases patient pain and discomfort and can modulate the immune system. The new 650 ms 1064 YAG laser therapy has been used to effectively treat acne, rosacea, psoriasis, and nail psoriasis.97
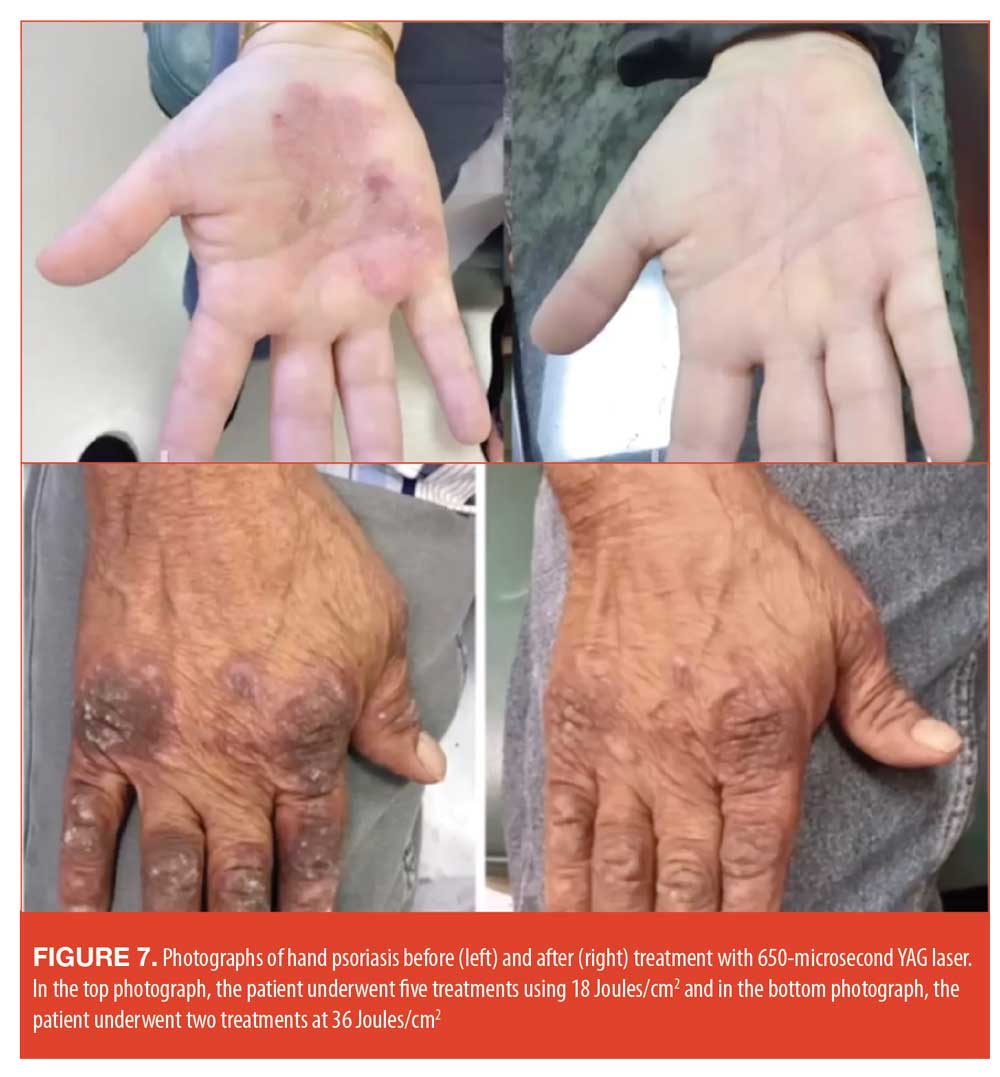
The mechanism of action is twofold. First, the laser light suppresses the inflammation, and, second, deep-heating laser light penetrates the psoriatic plaque and causes the feeder blood vessels below the plaque to coagulate.87 This allows for significant depth of phototherapy; the laser is able to target deep structures that were previously all but inaccessible to phototherapy and can do it without collateral damage. A case where a 650 ms 1064 YAG laser was used on challenging-to-treat hand psoriasis appears in Figure 7.
Clinical effectiveness. A randomized clinical trial has provided a head-to-head comparison of the 650 ms 1064 YAG laser versus the 308-nm excimer laser for treating psoriatic plaques on the limbs and trunks of patients with mild to moderate psoriasis.98 The investigator-blinded study included 15 subjects with mild to moderate psoriasis vulgaris. They were randomized so that they received each treatment on one side (right or left) of the body; thus, patients served as their own controls. Patients were administered a maximum of 15 treatments twice a week ± two days for a maximum of eight weeks or until full clearance occurred.98 The modified PASI score was used along with local skin reaction (LSR) and visual analog scale at treatments 2, 5, 8, 11, 14, 16, and 17. At the initial screening visit, the Physician’s Global Assessment (PGA), body surface area (BSA) involvement of psoriasis vulgaris assessment were conducted. The study included 11 men (73.3%) and the mean age of the patients was 55.6 years. Ten of the 15 subjects were Caucasian and five of the 15 subjects were Black or African-American. A variety of skin types were represented: seven percent had Skin Type II, 27 percent had Type III, 33 percent had Type IV; 20 percent had Type V; and 13 percent had Type VI. Results showed that the Aerolase (650 ms 1064 YAG laser) and the excimer laser were similarly effective.
Tolerability was similar for the two treatments, with the most commonly reported adverse effect being minor scaling. Two patients experienced some burning with the excimer treatments, which was addressed with a dose adjustment. Over the eight-week course of the study, both the 640 ms YAG laser and the excimer laser significantly improved the appearance of plaque psoriasis with no significant differences between effectiveness and clearance between groups. No adverse events or serious adverse events were reported over the course of the study.98
Final Considerations. Even in our age of biologics and pharmacological breakthroughs, phototherapy holds a key position in psoriasis care. UVB, NB-UVB, and PUVA phototherapy options all have many benefits and some limitations for use in patients with psoriasis. It should be noted that laser phototherapy is reimbursable and the reimbursement may be independent of the type of laser used in the treatment.
Conclusion
Advancements in the understanding of psoriasis pathophysiology has resulted in a plethora of biologic options and other pharmacological breakthroughs, which, in turn, has led to greater understanding of cytokines and the inflammatory process. At the same time, psoriasis has also fallen back on mainstay treatments, such as topicals and phototherapy, but infusing them with new innovations. Combination topical products and greater appreciation for the vehicle in topicals has vastly improved their efficacy and adherence rates. Phototherapy remains an important treatment option and a new short-duration laser has demonstrated efficacy similar to that of excimer laser therapy, a safe and effective treatment for plaque psoriasis. Emphasis is given to the role of the dermatologists on the front lines of patient care, as they often see PsA first and can help the patient get an early diagnosis and rapid treatment, which may confer better outcomes. The virtual SCALE meeting was a different experience for both presenters and participants, but provided high-quality information on psoriasis care from key thought leaders.
References
- Gottlieb AB, Blauvelt A, Thaci D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302-314.e306.
- Gordon KB, Warren RB, Gottlieb AB. Long-term efficacy of certolizumab pegol for the treatment of plaque psoriasis: 3-year results from two randomized phase III trials (CIMPASI-1 and CIMPASI-2). Br J Dermatol. 2020 Jul 11. Online ahead of print.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839.
- Blauvelt A, Papp K, Gottlieb A, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2019.
- Paller AS, Seyger MMB, Alejandro Magariños G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183(2):231–241.
- Reis J, Vender R, Torres T. Bimekizumab: The First Dual Inhibitor of Interleukin (IL)-17A and IL-17F for the Treatment of Psoriatic Disease and Ankylosing Spondylitis. BioDrugs. 2019;33(4):391-399.
- Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395(10222):427–440.
- Svecova D, Lubell MW, Casset-Semanaz F, Mackenzie H, Grenningloh R, Krueger JG. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin 17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81(1):196–203.
- Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230): 1126–1136.
- Mease PJ, Chohan S, Fructuoso FJG, et al. LB0002 Randomised, double-blind, placebo-controlled, multiple-dose, phase 2b study to demonstrate the safety and efficacy of tildrakizumab, a high-affinity anti-interleukin-23p19 monoclonal antibody, in patients with active psoriatic arthritis. Annals of the Rheumatic Diseases. 2019;78(Suppl 2):78–79.
- Poulin Y, Ramon M, Rosoph L, et al. Efficacy of tildrakizumab by patient demographic and disease characteristics across a phase 2b and 2 phase 3 trials in patients with moderate-to-severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2020;34(7):1500–1509.
- Crowley JJ, Warren RB, Cather JC. Safety of selective IL-23p19 inhibitors for the treatment of psoriasis. J Eur Acad Dermatol Venereol. 2019;33(9):1676-1684.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288.
- Blair HA. Risankizumab: A Review in Moderate to Severe Plaque Psoriasis. Drugs. 2020;80(12): 1235–1245.
- Salimi S, Yamauchi PS, Thakur R, et al. Interleukin 23p19 inhibitors in chronic plaque psoriasis with focus on mirikizumab: A narrative review. Dermatol Ther. 2020:e13800.
- Reich K, Rich P, Maari C et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019 Jul;181(1):88–95.
- Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol. 2018;201(6):1605–1613.
- Yuan Z-C, Xu W-D, Liu X-Y, et al. Biology of IL-36 Signaling and Its Role in Systemic Inflammatory Diseases. Frontiers in Immunology. 2019;10(2532).
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178(3):614–618.
- Bachelez, H. Efficacy and safety of BI 655130, an anti-interleukin-36 receptor antibody, in patients with acute generalized pustular psoriasis. Presented at: European Academy of Dermatology and Venereology Congress 2018; September 12–16, 2018.
- Papp K, Gordon K, Thaci D, et al. Phase 2 Trial of Selective Tyrosine Kinase 2 Inhibition in Psoriasis. N Engl J Med. 2018;379(14):1313–1321.
- Papp KA, Menter MA, Raman M, et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2016;174(6):1266–1276.
- Bissonnette R, Vasist LS, Bullman JN, Collingwood T, Chen G, Maeda-Chubachi T. Systemic Pharmacokinetics, Safety, and Preliminary Efficacy of Topical AhR Agonist Tapinarof: Results of a Phase 1 Study. Clin Pharmacol Drug Dev. 2018;7(5):524–531.
- Robbins K, Bissonnette R, Maeda-Chubachi T, et al. Phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J Am Acad Dermatol. 2019;80(3):714–721.
- Koek MB, Buskens E, Steegmans PH, van Weelden H, Bruijnzeel-Koomen CA, Sigurdsson V. UVB phototherapy in an outpatient setting or at home: a pragmatic randomised single-blind trial designed to settle the discussion. The PLUTO study. BMC Med Res Methodol. 2006;6:39.
- Huang X, Tanojo H, Lenn J, Deng CH, Krochmal L. A novel foam vehicle for delivery of topical corticosteroids. J Am Acad Dermatol. 2005;53(1 Suppl 1):S26–38.
- Draelos ZD, Ports WC , Vlahos B, et al. Skin permeation and penetration of crisaborole when coapplied with emollients. J Am Acad Dermatol. 2020 Oct 14;S0190-9622(20)30541–30547.
- Simonsen L, Hoy G, Didriksen E, Persson J, Melchior N, Hansen J. Development of a new formulation combining calcipotriol and betamethasone dipropionate in an ointment vehicle. Drug Dev Ind Pharm. 2004;30(10):1095–1102.
- Stein Gold L, Horgan A, Schreiber R, Ye R. (2019). Determination of the In Vitro Chemical Compatibility of Calcipotriene Foam, 0.005%, in Combination with Halobetasol Proprionate Foam, 0.05%. Winter Clinical Dermatology Conference. Kawaii, HI.
- Lebwohl M. Comment on “PGA×BSA composite versus PASI: Comparison across disease severities and as therapeutic response measure for Cal/BD foam in plaque psoriasis”. J Am Acad Dermatol. 2020.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659.
- Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205(4): 389–393.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris–A randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127.
- Lebwohl MG, Breneman DL, Goffe BS, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39(4 Pt 1):590–596.
- Strom MA, Mohan GC, Lio PA. Therapeutic Synergy, Neutrality, and Antagonism: Combination Treatment in Dermatology. J Drugs Dermatol. 2016;15(10): 1203–1207.
- Stein Gold L, Bagel J, Lebwohl M, Lin T, Martin G, Pillai R. Halobetasol and Tazarotene: Further Defining the Role of a Unique Fixed Combination Topical Lotion in Moderate-to-Severe Plaque Psoriasis. J Drugs Dermatol. 2018;17(12):1290–1296.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J Invest Dermatol. 2017;137(10):2110–2119.
- Papp KA, Gooderham M, Droege M, et al. Roflumilast Cream Improves Signs and Symptoms of Plaque Psoriasis: Results from a Phase 1/2a Randomized, Controlled Study. J Drugs Dermatol. 2020;19(8): 734–740.
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of Roflumilast Cream for Chronic Plaque Psoriasis. N Engl J Med. 2020;383(3):229–239.
- McDonald DR. TH17 deficiency in human disease. J Allergy Clin Immunol. 2012;129(6):1429–1437.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Ibler E, Gordon KB. IL-23 inhibitors for moderate-to-severe psoriasis. Semin Cutan Med Surg. 2018;37(3):158–162.
- Love TJ, Qureshi AA, Karlson EW, Gelfand JM, Choi HK. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003–2006. Arch Dermatol. 2011;147(4):419–424.
- Costa L, Ramonda R, Ortolan A, et al. Psoriatic arthritis and obesity: the role of anti-IL-12/IL-23 treatment. Clin Rheumatol. 2019;38(9):2355–2362.
- Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS One. 2018;13(5):e0195123.
- de Beaucoudrey L, Samarina A, Bustamante J, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine. 2010;89(6):381–402.
- Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019;45:1–8.
- Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465.
- He D, Li H, Yusuf N, et al. IL-17 mediated inflammation promotes tumor growth and progression in the skin. PLoS One. 2012;7(2):e32126.
- Puig L. Guselkumab for the treatment of adults with moderate to severe plaque psoriasis. Expert Rev Clin Immunol. 2019;15(6):589–597.
- Cron RQ, Beukelman T. Guilt by association – what is the true risk of malignancy in children treated with etanercept for JIA? Pediatr Rheumatol Online J. 2010;8:23.
- Geller S, Xu H, Lebwohl M, Nardone B, Lacouture ME, Kheterpal M. Malignancy Risk and Recurrence with Psoriasis and its Treatments: A Concise Update. Am J Clin Dermatol. 2018;19(3):363–375.
- Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: Nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589–e12589.
- Yang EJ, Beck KM, Sanchez IM, Koo J, Liao W. The impact of genital psoriasis on quality of life: a systematic review. Psoriasis (Auckland, NZ). 2018;8:41–47.
- Chung J, Callis Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623–632.
- Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckland, NZ). 2016;6: 93–104.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse Psoriasis: From Diagnosis to Current Treatment Options. Clin Cosmet Investig Dermatol. 2019;12:953–959.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72(6):978–983.
- Czuczwar P, Stepniak A, Goren A, et al. Genital psoriasis: a hidden multidisciplinary problem – a review of literature. Ginekol Pol. 2016;87(10): 717–721.
- Papp K, Berth-Jones J, Kragballe K, Wozel G, de la Brassinne M. Scalp psoriasis: a review of current topical options. J Eur Acad Dermatol Venereol. 2007;21:1151–1160.
- Dopytalska K, Sobolewski P, Blaszczak A, Szymanska E, Walecka I. Psoriasis in special localizations. Reumatologia. 2018;56(6):392–398.
- Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14(1):19–23.
- Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60(6):1024–1031.
- Raposo I, Torres T. Palmoplantar Psoriasis and Palmoplantar Pustulosis: Current Treatment and Future Prospects. Am J Clin Dermatol. 2016;17(4):349–358.
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64 Suppl 2:ii14–17.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61(2):233–239.
- Yamamoto T. Optimal management of dactylitis in patients with psoriatic arthritis. Open Access Rheumatol. 2015;7:55–62.
- Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284.
- Brockbank JE, Stein M, Schentag CT, Gladman DD. Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheum Dis. 2005;64(2):188–190.
- Benjamin M, McGonagle D. Basic concepts of enthesis biology and immunology. J Rheumatol Suppl. 2009;83:12–13.
- Anandarajah AP, Ritchlin CT. Pathogenesis of psoriatic arthritis. Curr Opin Rheumatol. 2004;16(4):338–343.
- Polachek A, Li S, Chandran V, Gladman DD. Clinical Enthesitis in a Prospective Longitudinal Psoriatic Arthritis Cohort: Incidence, Prevalence, Characteristics, and Outcome. Arthritis Care Res. 2017;69(11):1685–1691.
- Mease PJ, Karki C, Palmer JB, et al. Clinical Characteristics, Disease Activity, and Patient-Reported Outcomes in Psoriatic Arthritis Patients With Dactylitis or Enthesitis: Results From the Corrona Psoriatic Arthritis/Spondyloarthritis Registry.Arthritis Care Res. 2017;69(11):1692–1699.
- Veale D, Rogers S, Fitzgerald O. Classification of clinical subsets in psoriatic arthritis. Br J Rheumatol. 1994;33(2):133–138.
- Bakirci Ureyen S, Ivory C, Kalyoncu U, Karsh J, Aydin SZ. What does evidence-based medicine tell us about treatments for different subtypes of psoriatic arthritis? A systematic literature review on randomized controlled trials. Rheumatol Adv Pract. 2018;2(1):rkx019.
- Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)–an analysis of 220 patients. Q J Med. 1987;62(238):127–141.
- Haugen IK, Englund M, Aliabadi P, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70(9):1581–1586.
- Spies C, Langer M, Hahn P, Muller L, Unglaub F. The treatment of primary arhtritis of the finger and thumb joint. Dtsch Arztebl Int. 2018;115(16):269–275.
- Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res. 2016;68(9):1320–1331.
- Parameswaran P, Lucke M. HLA B27 Syndromes. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright ©2020, StatPearls Publishing LLC.; 2020.
- Helliwell P. Established psoriatic arthritis: clinical aspects. J Rheumatol. 2009;36:21–23.
- Bell L, Murphy C-L, Wynne B, Cunnane G. Acute Presentation of Arthritis Mutilans. J Rheumatol. 2011;38(1):174.
- Brockbank J, Gladman D. Diagnosis and management of psoriatic arthritis. Drugs. 2002;62(17):2447–2457.
- Miyagawa I, Nakayamada S, Tanaka Y. Optimal Biologic Selection for Treatment of Psoriatic Arthritis: the Approach to Precision Medicine. Curr Rheumatol Rep. 2019;21(5):21.
- Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: Early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21–37.
- Zhang P, Wu MX. A clinical review of phototherapy for psoriasis. Lasers Med Sci. 2018;33(1):173–180.
- Vieyra-Garcia PA, Wolf P. From Early Immunomodulatory Triggers to Immunosuppressive Outcome: Therapeutic Implications of the Complex Interplay Between the Wavebands of Sunlight and the Skin. Front Med. 2018;5:232–232.
- Qin J, Jiang J, An L, Gareau D, Wang RK. In vivo volumetric imaging of microcirculation within human skin under psoriatic conditions using optical microangiography. Lasers Surg Med. 2011;43(2): 122–129.
- Huang A, Austin E, Jagdeo J. Patient-reported outcomes in lasers and light therapy. G Ital Dermatol Venereol. 2019;154(2):120–126.
- Salah Eldin MM, Sami NA, Aly DG, Hanafy NS. Comparison Between (311-312 nm) Narrow Band Ultraviolet-B Phototherapy and (308 nm) Monochromatic Excimer Light Phototherapy in Treatment of Vitiligo: A Histopathological Study. J Lasers Med Sci. 2017;8(3):123–127.
- Abrouk M, Levin E, Brodsky M, et al. Excimer laser for the treatment of psoriasis: safety, efficacy, and patient acceptability. Psoriasis (Auckl). 2016;6:165–173.
- Asawanonda P, Anderson RR, Chang Y, Taylor CR. 308-nm excimer laser for the treatment of psoriasis: a dose-response study. Arch Dermatol. 2000;136(5):619–624.
- Gattu S, Rashid RM, Wu JJ. 308-nm excimer laser in psoriasis vulgaris, scalp psoriasis, and palmoplantar psoriasis. J Eur Acad Dermatol Venereol. 2009;23(1):36–41.
- Taibjee SM, Cheung ST, Laube S, Lanigan SW. Controlled study of excimer and pulsed dye lasers in the treatment of psoriasis. Br J Dermatol. 2005;153(5):960–966.
- Zhang R-n, Zhuo F-l, Wang D-k, et al. Different Numbers of Long-Pulse 1064-nm Nd-YAG Laser Treatments for Onychomycosis: A Pilot Study. Biomed Res Int. 2020;2020:1216907.
- Gold MH, Goldberg DJ, Nestor MS. Current treatments of acne: Medications, lights, lasers, and a novel 650-us 1064-nm Nd: YAG laser. J Cosmet Dermatol. 2017;16(3):303–318.
- Nestor MS, Fischer D, Arnold D. Randomized, Investigator-Blinded Study to Compare the Efficacy and Tolerance of a 650-microsecond, 1064-nm YAG Laser to a 308-nm Excimer Laser for the Treatment of Mild to Moderate Psoriasis Vulgaris. J Drugs Dermatol. 2020;19(2):176–183.

