 J Clin Aesthet Dermatol. 2020;13(10):18–22
J Clin Aesthet Dermatol. 2020;13(10):18–22
by Luis Puig, MD, PhD; Baojin Zhu, PhD; Russel Burge, PhD, MA; David Shrom, PhD; Yan Dong, PhD; Wei Shen, PhD; Lotus Mallbris, MD, PhD; and Kristian Reich, PhD
Dr. Puig is with Hospital de la Santa Creu i Sant Pau and Universitat Autonoma de Barcelona in Spain. Drs. Zhu, Burge, Shrom, Dong, Shen, and Mallbris are with Eli Lilly and Company in Indianapolis, Indiana. Dr. Burge is also with the University of Cincinnati in Cincinnati, Ohio. Dr. Reich is with the Institute for Health Services Research in Dermatology and Nursing at the University Medical Center Hamburg-Eppendorf and Skinflammation® Center in Hamburg, Germany.
FUNDING: The study was sponsored by Eli Lilly and Company.
DISCLOSURES: Professor Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Covagen, Eli Lilly and Company, Forward Pharma, Fresenius Medical Care, Galapagos NV, GlaxoSmithKline, Janssen-Cilag, Kyowa Kirin, Leo, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and Xenoport. Dr. Puig has received grants/research supports or participation in clinical trials (paid to Institution). Drs. Zhu, Burge, Shrom, Dong, Shen, and Mallbris are employees and minor stockholders of Eli Lilly and Company.
ABSTRACT: Background: Rapid improvements in health-related quality of life (HRQoL) and psoriasis severity have been reported in patients treated with ixekizumab (IXE), an interleukin (IL)-17A antibody.
Objective: We assessed the relationship between early Psoriasis Area and Severity Index (PASI) response and long-term Dermatology Life Quality Index (DLQI) improvement in patients in the randomized clinical trial IXORA-S (NCT0256186) treated with IXE or IL-12/23 (ustekinumab [UST]).
Methods: The proportion of patients achieving DLQI (0,1), an outcome equivalent to the patient’s skin condition having no impact on HRQoL after 52 weeks of IXE or UST by PASI response at Weeks 4, 12, and 24 was quantified. Optimal thresholds for PASI response by treatment to predict Week 52 DLQI (0,1) were calculated based on Youden’s Index.
Results: Early and higher levels of skin clearance were associated with improved patient outcomes regardless of treatment. Patients treated with IXE achieved faster and more pronounced PASI response than patients treated with UST. The optimal thresholds at Weeks 4, 12, and 24 for predicting DLQI (0,1) at Week 52 were ~PASI 75 for IXE versus ~PASI 50 for UST at Week 4, PASI 90 for IXE versus PASI 75 for UST at Week 12, and ~PASI 100 for IXE versus ~PASI 90 for UST at Week 24. Among patients achieving these thresholds, the probability of achieving a DLQI (0,1) was significantly higher.
Conclusion: Earlier and higher levels of skin clearance are associated with improved patient outcomes over the long term, regardless of treatment.
Keywords: Dermatology Life Quality Index, psoriasis, ixekizumab, Psoriasis Area and Severity Index
Psoriasis is a chronic condition which poses a significant negative impact on patients’ physical and mental status as well as their health-related quality of life (HRQoL).1,2 Approximately 60 percent of patients consider psoriasis a large problem, adversely impacting sleep, sexual activity, and manual tasks.1,3
Consequently, improvement in psoriasis signs, as measured by Psoriasis Area and Severity Index (PASI), has a significant correlation with improved HRQoL outcomes.2,4,5 Until recently, improvement of at least 75 percent from baseline in PASI score (PASI 75) has been the treatment goal for patients with psoriasis and is the primary endpoint in many Phase III studies. However, achieving at least a 90-percent improvement from baseline in PASI score (PASI 90) with biologics has become the new treatment goal for patients with psoriasis.6,7 It has been shown that achieving PASI 90 has a significantly higher impact on improvement in Dermatology Life Quality Index (DLQI), with significantly greater proportions of patients reporting no impact on HRQoL (DLQI [0,1]).7
Although it is understood that higher levels of skin clearance is important for patients, it is unknown how fast psoriasis should be improved for optimal outcomes. Several patient surveys have indicated rapid onset is a highly valued treatment attribute, as patients with moderate-to-severe psoriasis desire at least a 50-percent improvement in disease at two weeks.8–10 While patient preference is an important aspect of shared decision making, understanding the long-term benefits associated with rapid improvements in symptoms with treatment may help clinicians better understand the value of this treatment aspect.
Rapid improvements in HRQoL and disease severity have been reported in patients treated with ixekizumab (IXE), an interleukin (IL)-17A antibody.11–13 IXE has demonstrated robust PASI 90 and PASI 100 (complete clearance) response in clinical studies as well as early significant PASI response at Week 1 and Week 2.12,14 Previous reports suggest an early clinical response (achieving PASI 50 at Week 4 or 6) is predictive of future response and symptom control in patients treated with IXE or methotrexate.15,16 No studies have yet reported the impact of achieving early skin clearance on predicting long-term HRQoL in patients with psoriasis.
The objective of this post-hoc analysis was to assess the association between early clinical response and long-term quality of life (QoL) impact in patients treated with IXE or ustekinumab (UST).17
Methods
Study design and participants. As previously reported, IXORA-S (NCT0256186) was a Phase IIIB, 52-week, double-blind, parallel-group trial comparing IXE to UST. Patients were randomized to either IXE 80mg every two weeks (Q2W; N=136) until Week 12 after a 160-mg loading dose (Week 0) or UST 45/90 mg at Week 0 and Week 4 and then every 12 weeks (N=166).18 Key eligibility criteria have been described previously. In summary, adults 18 years of age or older with chronic plaque psoriasis for at least six months and a PASI score of at least 10 who had previously failed or had a contraindication/intolerability to at least one systemic therapy were included.18 The study protocol was approved by an Ethical Review Board and the trial was compliant with applicable guidelines.
Statistical analysis. Proportions of PASI responders at Weeks 4, 12, and 24 were compared between treatments using logistic regression models and included terms for treatment group, weight group, and geographical region, after imputation of missing values using nonresponse imputation (NRI).18 Modified baseline observation carried forward (mBOCF) calculations were used to impute missing data on continuous outcome measures. The proportion of patients achieving DLQI (0,1) at Week 52 was reported among patients who achieved different levels of PASI response (PASI 50 , PASI 75, PASI 90, and PASI 100) at Weeks 4, 12, and 24, separately.
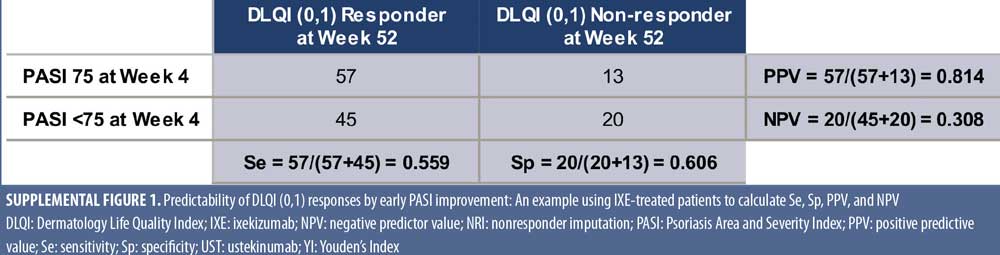
For the predictor analysis, the optimum thresholds of actual PASI and PASI improvement at Weeks 4 and 12 for predicting DLQI (0,1) at Week 52 were calculated. Sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) for PASI improvement or PASI scores at Weeks 4 and 12 versus DLQI (0,1) at Week 52 were calculated (Supplemental Figure 1). Receiver operating characteristic (ROC) analyses were performed using one percent increments of PASI percent improvement or one incremental in actual PASI score to assess the predictability of DLQI (0,1) at Week 52 by different levels of PASI response at Weeks 4 and 12. Optimal PASI response thresholds were chosen based on Youden’s Index (YI), YI = Sensitivity + Specificity – 1, where the peak YI reflects optimal predictability. In order to assess the predictability of PASI improvement or PASI actual scores, sensitivity analysis was conducted on the ROC curve using PASI improvement or PASI actual scores at Week 24 to predict DLQI (0,1) responses at Week 52.
Results
Demographics. Patient demographics and clinical characteristics have been published previously.18 Baseline PASI scores were mean (SD) 19.9 (8.2) and 19.8 (9.0) for IXE and UST, respectively; DLQI values for IXE versus UST were 11.1 (7.2) and 12.0 (7.3), respectively.18
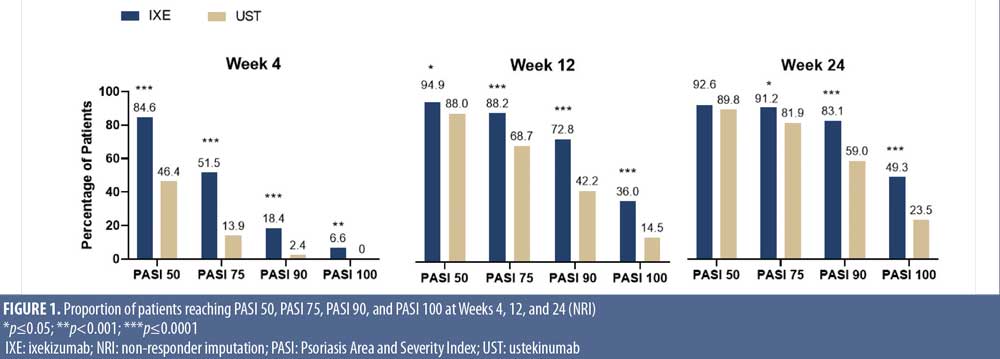
Onset of response for IXE and UST. The proportion of patients reaching PASI 50, PASI 75, PASI 90, and PASI 100 response at Weeks 4, 12, and 24 was consistently higher in patients treated with IXE versus UST (Figure 1) with more IXE-treated patients achieving PASI 75 (51.5%) and PASI 90 (18.4%) and even complete skin clearance (6.6%) as early as Week 4 versus UST-treated patients: 13.9 percent, 2.4 percent, and 0 percent, respectively (p<0.001).
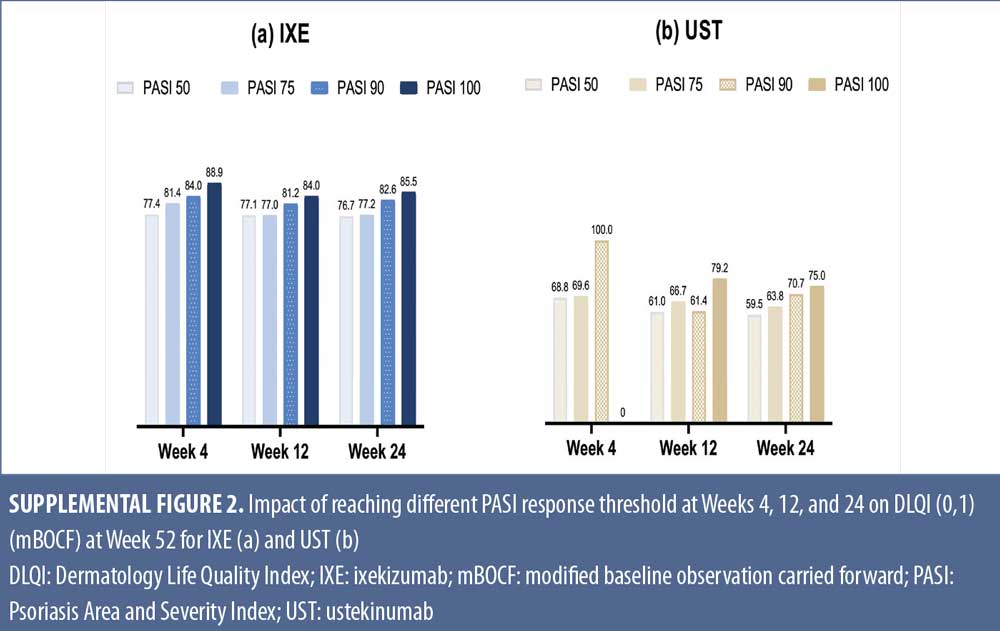
Relationship between levels of early PASI response and long-term DLQI. Early and high levels of PASI response were associated with greater improvements in QoL. The percentage of patients with DLQI (0,1) at Week 52 increased across PASI response categories at Weeks 4, 12, or 24 (Supplemental Figure 2). Similar trends were observed for IXE and UST even though, on average, IXE patients achieved higher DLQI (0,1) at the same PASI response level and time point as UST. In an additional analysis, PASI improvement at Weeks 4, 12, and 24 significantly correlated with DLQI improvement at Week 52 with correlation coefficients (p-values) for IXE of 0.30 (p<0.001), 0.28 (p<0.01), and 0.46 (p<0.001), respectively, and for UST, of 0.25 (p<0.01), 0.35 (p<0.001), and 0.41 (p<0.001), respectively.
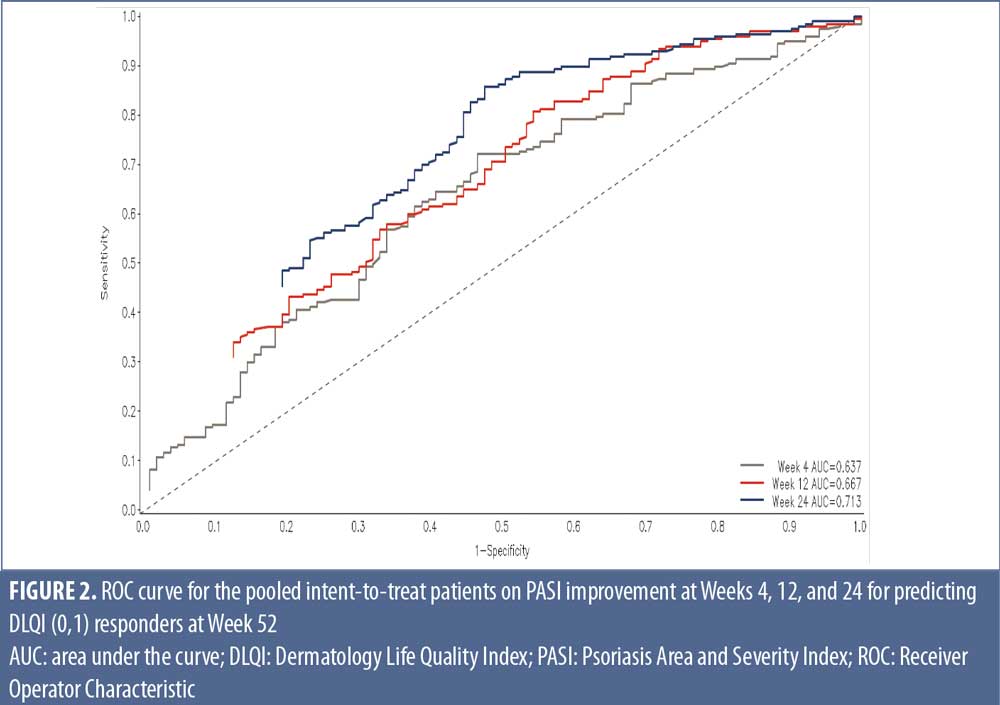
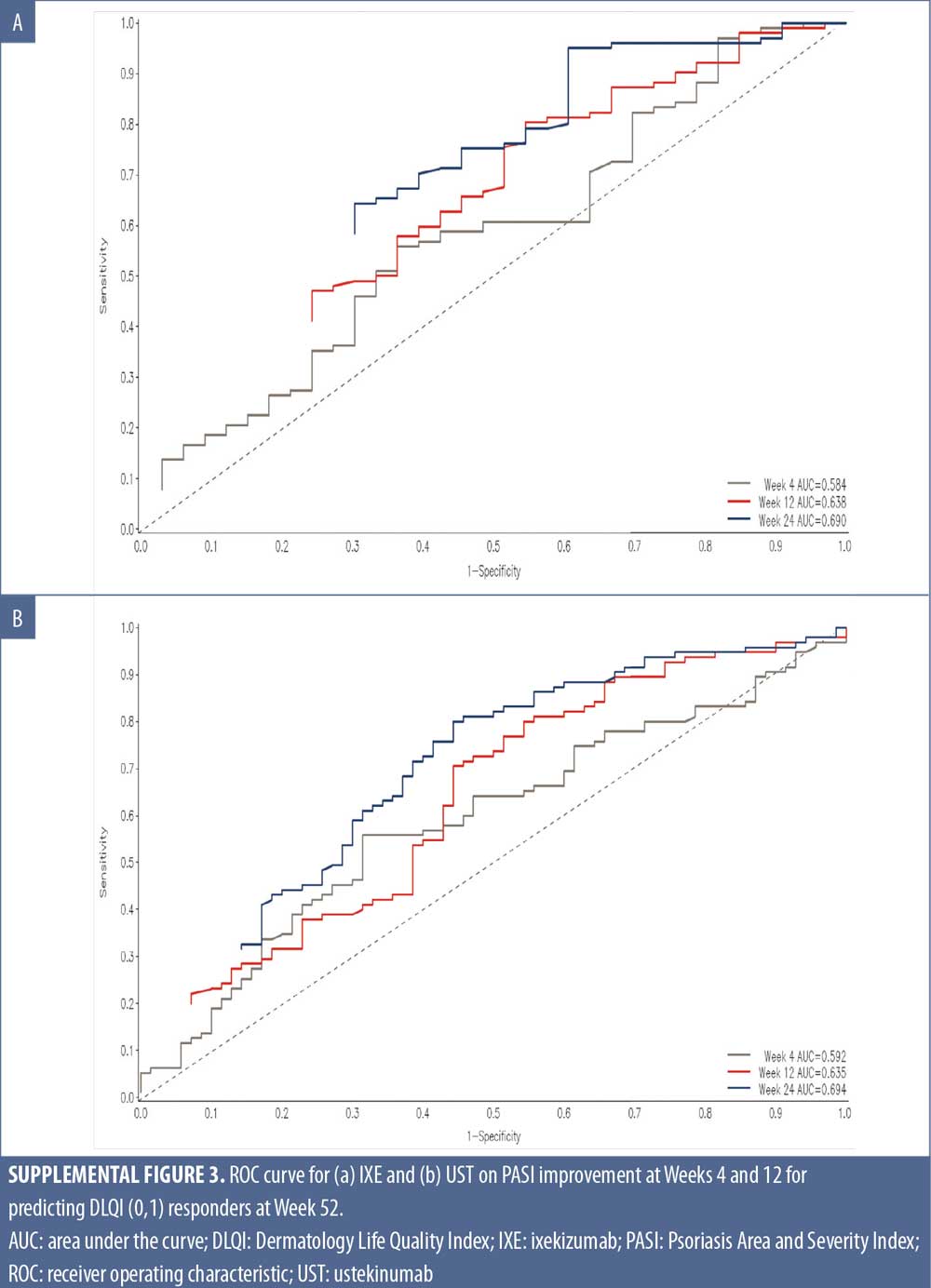
ROC curves further demonstrate an association between early response and DLQI (0,1) rates at Week 52. The predictability of PASI response for DLQI (0,1) increased from Weeks 4 to 24, regardless of treatment with Area under the ROC curves (AUC-ROC) of 0.637-0.713 where greater AUC-ROC means greater predictability of PASI response at each time point (Max AUC-ROC=1) (Figure 2; Supplemental Figure 3).
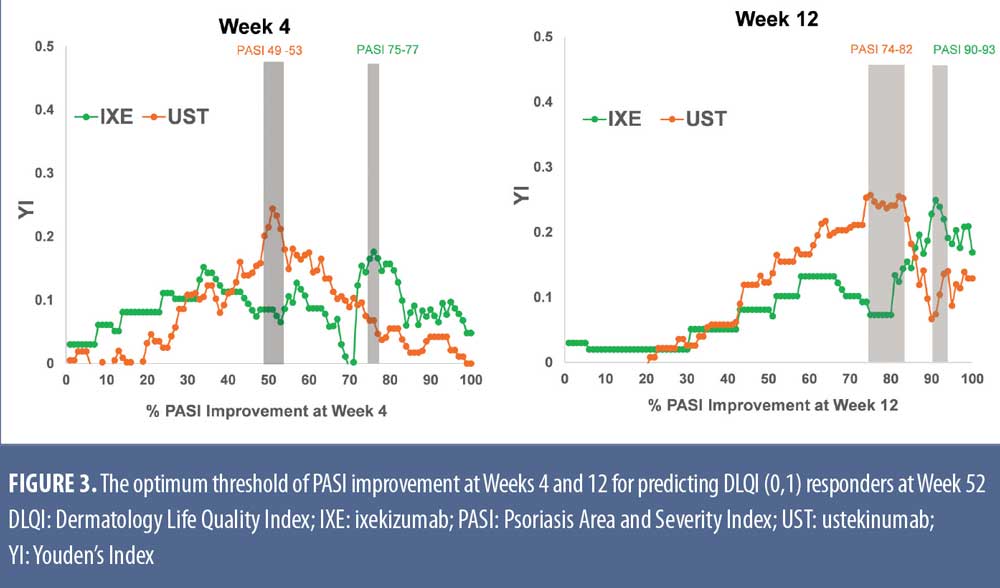
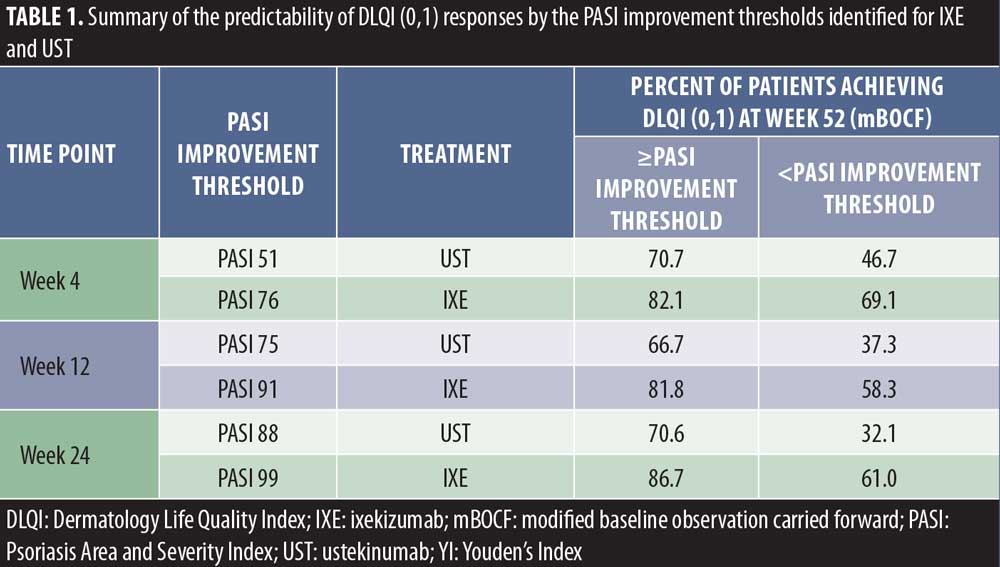
Optimal PASI improvement targets at Weeks 4, 12, and 24 for predicting DLQI (0,1) at Week 52 were calculated using YI based on the ROC data. The peak YI, indicating the optimal PASI response thresholds, was higher for IXE versus UST (76% for IXE vs. 51% for UST at Week 4, 91% for IXE vs. 78% for UST at Week 12, and 99% for IXE vs. 88% for UST at Week 24) (Figure 3). For patients achieving these PASI response targets, significantly more patients achieved DLQI (0,1) at Week 52 (Table 1). Achieving these early response thresholds resulted in DLQI (0,1) in 82 to 87 percent of IXE-treated patients at Week 52. For patients treated with UST, 67 to 71 percent were predicted to achieve these response thresholds associated with DLQI (0,1).
Discussion
In patients with moderate-to-severe psoriasis, PASI and HRQoL assessments are currently receiving significant attention as outcome measures for effective treatment in clinical practice.5,7,19 The definition of long-term treatment goals for patients with psoriasis has evolved from PASI 50 with methotrexate and other treatments, to PASI 75 with tumor necrosis factor therapies and, more recently, to PASI 90 since IL-17 therapeutic options entered the market.4,7,19,20 These incremental increases in treatment response are positively correlated with significant improvements in QoL. With new biologics, such as IXE, which demonstrate a quick onset of action, higher treatment goals are achieved more quickly.12,14 While patients prefer treatments which clear skin quickly, the association of rapid and high levels of improvement with longer term patient outcomes is not well studied.
It has been established that early clinical symptom improvement is predictive of long-term response in patients treated with IXE15 and methotrexate.16 Patients who maintain high levels of clinical response report improvements in QoL.4 The purpose of this study was to assess the association of early improvements in PASI with long-term QoL. Significant correlations of PASI response at Weeks 4, 12, and 24 with Week 52 DLQI (0,1) were identified. ROC curve analyses illustrated meaningful AUC-ROC for early PASI improvements, regardless of treatment, and later QoL improvements. Improvements in PASI with both IXE and UST showed similar overall predictive ability, even though PASI thresholds differed between the two treatments.
Optimum PASI improvement thresholds estimated from either IXE or UST treatments using YI were approximately equal to PASI 75, PASI 90, and PASI 100 for IXE-treated patients at Weeks 4, 12, and 24, respectively. For UST-treated patients the optimum early thresholds were PASI 50, PASI 75, and PASI 90 at those early time points. Achieving these early thresholds resulted in up to 80 percent of patients treated with IXE reporting DLQI (0,1) at Week 52 or up to 70 percent of UST-treated patients reporting DLQI (0,1) at Week 52.
Many patients prefer to reach higher skin clearance for immediate symptom relief.8–10 Data from this study provide further evidence that early improvements in psoriasis are valuable for patients. Furthermore, the rapid and significant PASI response with IXE may have an even greater impact on QoL compared with UST.
Limitations. This analysis has limitations. Firstly, a single measure on PASI improvement was used to predict long-term outcomes. Even though this approach is intuitive and could be relevant to clinical practice, other approaches which employ multiple factors and time-dependent prediction in analyses, such as deep machine learning, could be used to confirm the findings from this analysis. Secondly, predictability may also depend on the distribution of the data for the predicting variable. For example, the PASI scores at Week 4 ranged from 0 to 42 for UST and from 0 to 22 for IXE. Even though IXE showed more efficacy and less dispersed PASI data than UST, the narrow range of data used to develop the ROC curve impacts the PASI predictability. Future studies might examine if other therapies with different levels of efficacy could show similar predictability. Finally, these results are based on patients with moderate-to-severe psoriasis and may not be generalizable to less severe patients.
Conclusion
Earlier and higher levels of skin clearance are associated with improved patient outcomes over the long term, regardless of treatment. Patients treated with IXE achieved faster and more pronounced PASI response and, subsequently, greater long-term HRQoL improvements compared to UST. Data from this study support the concept that drugs providing rapid onset and high levels of skin clearance offer optimal long-term outcomes for patients.
Acknowledgment
The authors would like to acknowledge Gina Moore of Syneos Health for assistance in the development of this manuscript.
References
- Kimble AB. Unmet needs in the management of plaque psoriasis. Managed Care. 2009;18(Suppl 1):1–7.
- Lee YW, Park EJ, Kwon IH, et al. Impact of psoriasis on quality of life: relationship between clinical response to therapy and change in health-related quality of life. Ann Dermatol. 2010;22:389–397.
- Stern RS, Nijstan T, Feldman SR, et al. Psoriasis is common, carries a substantial burden event when non extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–139.
- Revicki DA, Willian MK, Menter A, et al. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate-to-severe psoriasis. Dermatology. 2008;216:260–270.
- Mattei P, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients with biological therapies. J Eur Acad Dermatol Venereol. 2014;28:333–337.
- European Medicines Agency. Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis. 2004. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-rheumatoid-arthritis_en.pdf. Accessed April 9, 2019.
- Puig L. PASI 90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29:645–648.
- Gorelick J, Shrom D, Sikand K, et al. Understanding Treatment Preferences in Patients with Moderate to Severe Plaque Psoriasis in the US: Results from a Cross-Sectional Patient Survey. Dermatol Ther (Heidelb). 2019;9:785–797.
- Maul J-T, Navarini AA, Sommer R, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis-a lesson for practice. JEADV. 2018;33:700–708.
- Blome C, Gosau R, Radtke MA, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308:69–78.
- Leonardi CL, Blauvelt A, Sofen JL, et al. Rapid improvements in health-related quality of life and itch with ixekizumab treatment in randomized phase 3 trials: results from UNCOVER-2 and UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:1483–1490.
- Khattri S, Goldblum O, Solotkin K, et al. Early onset of clinical improvement with ixekizumab in a randomized, open-label study of patients with moderate-to-severe plaque psoriasis. J Clin Aesthet Dermatol. 2018;11(5):33–37.
- Taltz [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2017. http://uspl.lilly.com/taltz/taltz.html#pi. Accessed April 9, 2019.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanerept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–551.
- Zhu B, Edson-Heredia E, Cameron GS, et al. Early clinical response as a predictor of subsequent response to ixekizumab treatment: results from a phase II study of patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2013;169(6): 1337–1341.
- Gordon KB, Betts KA, Sundaram M, et al. Poor and early response to methotrexate portends inadequate long-term outcomes in patients with moderate-to-severe psoriasis: Evidence from 2 phase 3 clinical trials. J Am Acad Dermatol. 2017;77(6):1031-1039.
- Stelara [prescribing information]. Horsham, PA; Janssen; 2018. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf. Accessed April 9, 2019.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4): 1014–1023.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate-to-severe psoriasis: a European Consensus. Arch Dermatol Res. 2011;303:1–10.
- Pongparit K, Chularojanamontri L, Limphoka P, et al. Effectiveness of factors associated with clinical response to methotrexate under daily life conditions in Asian patients with psoriasis: A retrospective cohort study. J Dermatol. 2018;45:540–545.

