 J Clin Aesthet Dermatol. 2019;12(4):32–36
J Clin Aesthet Dermatol. 2019;12(4):32–36
by Conrad Benedetto, DO; Dave Crasto, MS; Leila Ettefagh, MD; Navid Nami, DO
Drs. Benedetto, Ettefagh, and Nami are with the Western University of Health Sciences in Pomona, California; Mr. Crasto is with William Carey University College of Osteopathic Medicine in Hattiesburg, Mississippi.
FUNDING: No funding was received for this study.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: The development of periungual pyogenic granulomas while taking the oral acne drug isotretinoin is a known yet uncommon and potentially severe side effect of the oral vitamin A derivative. Previous reports have detailed the development of pyogenic granulomas most commonly arising at sites of previous acne lesions as well as both subungual and periungual locations, with associated paronychia, bleeding, and discomfort. This is thought to arise as a result of the nail bed’s fragility and propensity toward spicule formation brought on by the proliferative action of isotretinoin. Here, we report a case of periungual pyogenic granuloma with associated paronychia in a patient taking oral isotretinoin. A review of the pathogenesis and available treatment modalities based on the current literature is provided.
KEYWORDS: Isotretinoin, pyogenic granuloma, drug-induced, periungual, paronychia
Isotretinoin is a systemic retinoid that is effective in the treatment of nodulocystic acne in its most severe forms. In addition to its teratogenicity, there are a number of well-documented side effects related to the use of isotretinoin, including hypercholesterolemia, hyperlipidemia, hypertriglyceridemia, elevated liver transaminases, arthralgias and myalgias.1 Separately, cutaneous side effects include cheilitis, pruritus, and xerosis.2 The formation of a pyogenic granuloma (PG) is a known yet poorly understood reaction to the oral retinoid. A PG is a benign vascular tumor that typically presents as a smooth papule with excess granulation tissue at the site of trauma or infection, usually on the digits or in previous acne locations.
Exner et al3 and Valentic et al4 first reported the formation of PG-like lesions as a side effect of isotretinoin therapy in 1983, followed by a report of eight cases by Campbell et al5 in the same year and two cases each from Blumental et al6 and Gomezet al7 in 1984. A case series from Bigby et al8 in 1988 on the adverse effects of isotretinoin reported four cases of granulation tissue formation and paronychia of the lateral and distal nail folds following the initiation of oral treatment with isotretinoin. This was observed again in a trial of 16 total patients of which three patients were reported to have developed proliferative granulation tissue at the sites of previous acne lesions.9 Armstrong and Weinstein2 reported four cases of paronychia and periungual PGs that resolved upon discontinuation of isotretinoin. Puig et al10 reported the development of granulation tissue at previous acne sites. Rivard and Hurt11 again documented the onset of crusted lesions with a base consisting of granulation tissue at the site of previous acne lesions in 2013. Most recently, Cauas et al12 reported this phenomenon occurring in the bilateral greater toes.
Topical retinoids have also been reported to cause excessive granulation tissue formation. Dawkins et al13 first documented PG arising at the site of scalp psoriasis in a single patient being treated with 0.1% topical tazarotene gel in the same location.
Other reported causes of PG include the antiretrovirals indinavir and lamivudine; tumor necrosis factor alpha inhibitors; erythropoietin; chemotherapeutic agents 5-fluorouracil, capecitabine, mitoxantrone, docetaxel; epidermal growth factor receptor inhibitors (EGFRs); rituximab; and mammalian target of rapamycin inhibitors.14–18 With the growing pharmacologic industry and increased use of retinoids, it becomes essential for physicians to be able to recognize and distinguish an adverse reaction to a drug versus some other event. The following case not only depicts a rare manifestation of an adverse drug reaction but also represents an avoidable outcome of a common event that might have been prevented through increased awareness of the reaction.
Case Presentation
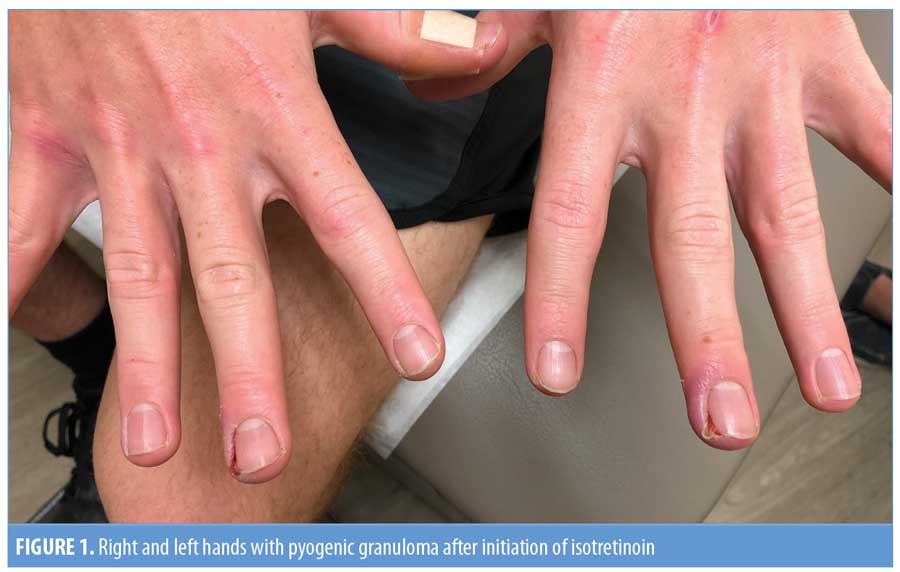
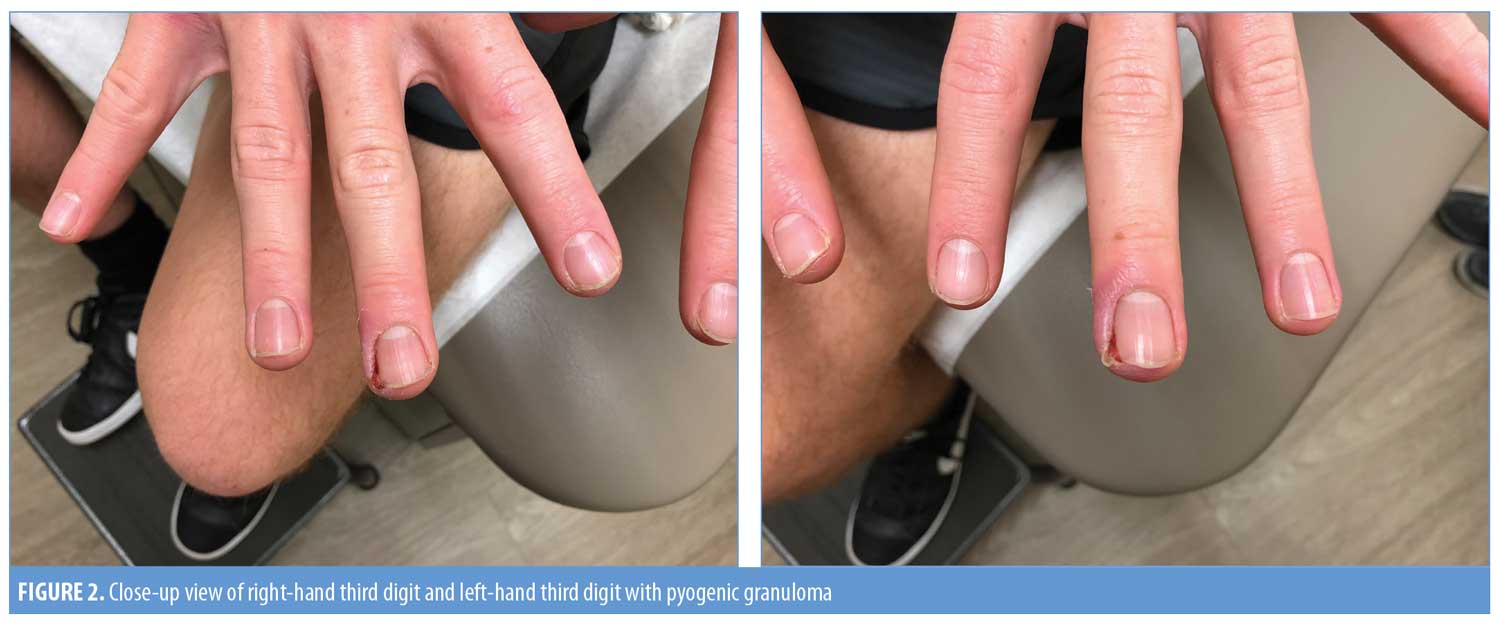
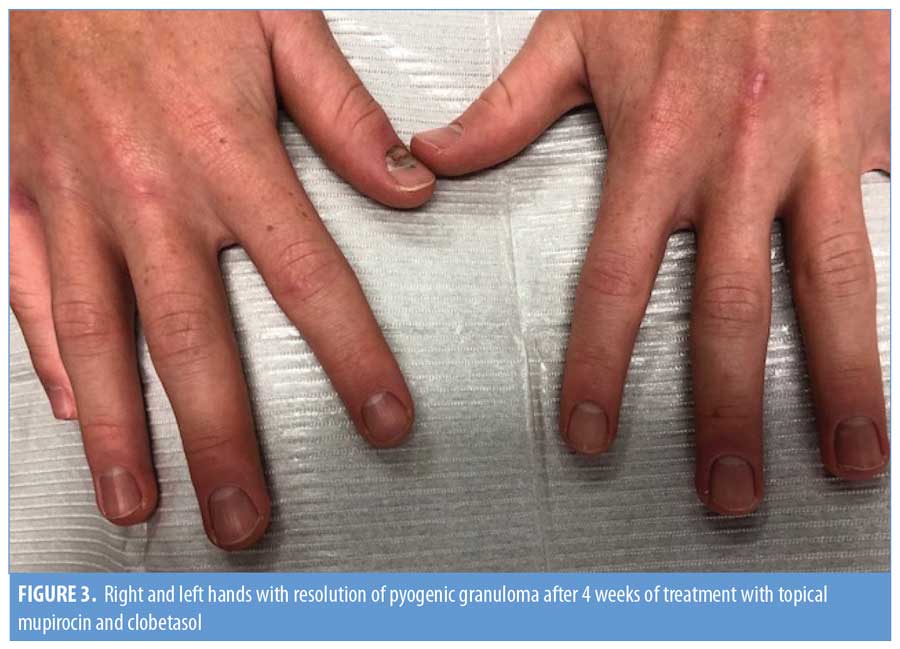
A 18-year-old man with severe nodulocystic acne on the face and neck presented to our clinic after completing his second month of isotretinoin therapy. He had no other medical problems and was not taking any other medications. After completing his first month of isotretinoin at 40mg daily without any side effects, his dose was increased to 60mg daily. When he returned after completing the second month at the higher dose, he reported painful “infections” on his left and right third digits of his hands (Figure 1). He reported that the infection began appearing one or two weeks after the recent dosage increase of isotretinoin. He was given antibiotics by his primary care provider, which provided no relief. He denied any trauma to his hands or fingers. Upon physical examination, he had a pink-to-red, friable-appearing papule at the lateral nail fold of the right third finger with surrounding erythema, swelling, and tenderness (Figure 2a). He also had a small amount of excoriated, pink, friable tissue at the lateral nail fold of the left third finger with surrounding erythema, swelling, and tenderness (Figure 2b). He denied purulent drainage of either digit. The patient was started on mupirocin 2% ointment, applied in the morning, and clobetasol 0.05% ointment, applied at night. Isotretinoin therapy was continued without further dose increase. At his next follow-up visit four weeks later, there was complete resolution of PG in both digits (Figure 3). He completed a six-month course of isotretinoin without recurrence of these lesions.
Discussion
Pathogenesis. Baran19 first proposed that an excess of retinoids can cause a local exfoliative dermatitis in the nail matrix that leads to an accumulation of scale and debris in the nail folds. The body then reacts as it would to the introduction of a foreign body by inducing an inflammatory reaction that results in the formation of granulation tissue. Baran postulated that the action of retinoids leads to increased skin fragility of the periungual tissue, increasing the likelihood that local trauma or infection will induce the formation of granulation tissue.
Piraccini et al1 hypothesized that retinoids cause onycholysis with subsequent desquamation in the proximal nail folds, leading to a localized foreign body reaction. This theory is the most widely accepted explanation for the development of PG during retinoid therapy. Frictional PG, first identified in 2001, highlighted the discovery that trauma can increase susceptibility to penetration, through an epidermal wound, by foreign bodies, which cause a localized inflammatory reaction that then can lead to the formation of PG.20 In a recent report, scratching was hypothesized to be the method by which a foreign body was introduced into the skin of a patient with psoriatic lesions, which eventually led to a localized overgrowth of pyogenic-like granulation material.21
Retinoids have also demonstrated angiogenic properties. The development of PG might stem from the effects of angiogenic growth factors, particularly vascular endothelial growth factor (VEGF).22 The link between the incidence of psoriasis and VEGF serum levels has previously been reported.23 VEGF is recognized as an indicator of psoriatic disease severity,24 and the VEGF receptor signaling system has been investigated as a potential therapeutic strategy for the treatment of psoriasis.25 Activated T-cells, inflammatory cells, and keratinocytes that are capable of eliciting VEGF secretion are present within psoriatic lesions.15 Moreover, an overexpression of the transcription factors STAT-3 and phosphorylated-ATF-2 have been implicated in the formation of PG.26 Therefore, it is reasonable to argue that the overproduction of VEGF and transcription factors could perpetuate the growth of PG in patients undergoing isotretinoin therapy.
In addition to the severity and location, the underlying cause of PG must be taken into account when considering treatment options. Because subungual and periungual PG is thought to occur via separate mechanisms, it should be considered separately from a classical PG. Piraccini et al1 described four methods by which subungual and periungual PG are thought to develop: drug side effect, mechanical trauma, systemic inflammatory disease, or peripheral nerve injury. Cutaneous sarcoidosis, psoriasis, and seronegative spondyloarthritis have reportedly been linked to the development of multiple periungual PGs of the toenails and fingernails, and were thought to have arisen secondary to chronic paronychia due to the systemic inflammatory condition.19 Moreover, a link between peripheral nerve injury due to cast immobilization and the development of PG has been reported.27
A plausible explanation for our case of isotretinoin-induced PG could be the synergistic relationship between the increased risk of mechanical trauma (in our case, due to the location of PG on the hands, which might be considered more prone to injury than other body parts) and the proliferative actions of a vitamin A derivative, which can cause keratinocytes to detach from one another and become lodged in the nail folds. Because the pathogenesis of PG caused by retinoid derivatives differs from that of idiopathic PG, it is unlikely that VEGF expression was increased. Further studies are needed to confirm this hypothesis, and additional discussion is warranted regarding the possible link between PG, antiretrovirals, and EGFR inhibitors.
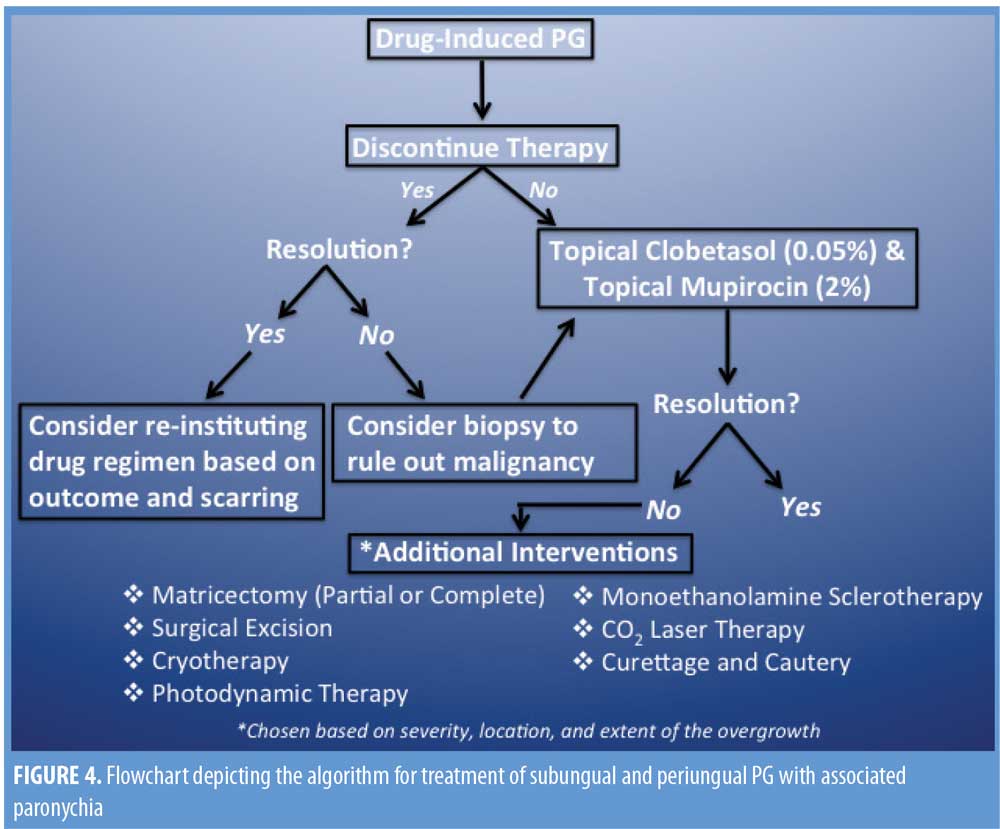
Treatment. There are several adequate treatment options for PG, and to ensure the selected treatment is both clinically effective and cost-efficient for the patient, there are many factors that must be taken into consideration by the treating physician. First, determination of the triggering event (e.g., local trauma, foreign body) should be given priority attention.2 Next, the lesion’s location and size, as well as the discomfort level of the patient, should be taken into consideration. In cases of drug-induced PG, discontinuation of the drug itself is effective and should be considered a first line of treatment. However, the severity of the primary condition and any comorbid conditions for which the offending drug(s) is being used to treat (e.g., isotretinoin for severe acne, gefitinib for small-cell lung cancer) must also be carefully considered, as discontinuation of the drug might not be the best option.28
Current guidelines suggest a 2- to 3-week course of topical antibiotics, applied to the lesion in the morning, and a high-potency topical steroid, applied to the lesion under occlusion in the evenings. Topical clobetasol propionate ointment 0.05% and topical mupirocin 2% are recommended as a first-line treatment regimen for subungual and periungual PG.2 It should be noted that a combination of oral steroids and topical antibiotics has been reported to be ineffective, which suggests the superior efficacy of topical steroids in cream or ointment form when treating PG.6
In a case report by Rivard and Hurt,11 a regimen of oral minocycline and steroids was reportedly effective in resolving granulation tissue, but resulted in local scarring of previous acne lesion sites.11 In such cases, further interventions and alternatives might be necessary.
In the advent of severe disruption to the nail matrix, reparations using noninvasive surgical techniques should be considered. In a case study by Figueriras et al,12 the authors reported that paronychia and granulation tissue were resolved following a course of oral antibiotics and oral prednisone while maintaining the dose of isotretinoin; however, onychocryptosis persisted after completion of the isotretinoin therapy. To treat the onychocryptosis, matricectomy was performed on the patient, which resulted in complete resolution.12 Blumental6 reported the persistence of paronychia and PG-like granulation overgrowth, which required partial matricectomy and cauterization. In a case report by Dika et al,28 a patient developed paronychia and periungual PG while taking gefitinib. A two-week course of topical clobetasol and mupirocin ointment was initiated, resulting in complete resolution of paronychia, but there was no resolution of the periungual PG. An eventual partial matricectomy with phenolization was performed with complete resolution of the PG, and gefitinib therapy was continued as planned.
A case series conducted by Raulin et al29 showed complete resolution of PG in 98 out of 100 patients using a single treatment session with a CO2 laser. Among those patients, 10 percent reported slight changes in skin texture and 12 percent saw some visible scarring.
The use of monoethanolamine as a sclerosing agent has been shown to be effective, without lesion recurrence and with minimal scarring, in nine patients following a single intralesional injection.30 In a report of 22 pediatric patients with small PGs of the face (measuring an average of 4mm), a pulsed dye laser was shown to be effective in 20 cases in 1 to 6 sessions, with an average of 2.25 treatment sessions.31 Due to lesion size, two children required shave excision with cautery.
In a comparison of curettage and cautery versus cryotherapy, Ghodsi et al32 reported that 97 percent of 36 patients with PG responded to a single treatment of curettage and cautery, whereas only 63 percent of 40 patients responded to cryotherapy in a single session and some required as many as three sessions. No significant difference was noted cosmetically between the two outcomes.32 On the other hand, a case series by Mirshams et al33 including 135 patients with PG treated with cryotherapy reported complete resolution in 58 percent following one treatment, 30 percent with two treatments, and the remaining 12 percent requiring three or more treatments. Of these 135 patients, 94 percent reported great satisfaction with the cosmetic outcome and five percent were left with residual hypopigmentation. It is therefore reasonable to conclude that cryotherapy is an effective treatment option for the removal of isolated PGs; however, in cases of trauma-induced granulation tissue overgrowth, destructive modalities should be used with caution. Additionally, care must be taken to avoid further damage to the surrounding tissue stroma, which could perpetuate the underlying condition.
Conclusion
In cases of isotretinoin-induced periungual and subungual PG with associated paronychia and proliferation of granulation tissue, we recommend maintaining the current dose of isotretinoin without any further increase and initiating a 2- to 3-week regimen of topical 2% mupirocin cream, applied in the morning, and 0.05% clobetasol propionate ointment, applied in the evenings. For lesions that continue to persist despite removal of the offending agent or that show no response to topical treatment, consider biopsy to rule out malignancy prior to utilizing excision or destruction treatment modalities. See Figure 4 for treatment algorithm. Of note, previous reports have found oral forms of antibiotic and steroidal therapies to be ineffective in the treatment of PG, and this was seen in our patient’s case as well after undergoing a course of oral antibiotics with no resolution of symptoms; this highlights the importance of utilizing topical therapies over oral therapies. In the advent of irreversible damage to the nail bed, partial or total matricectomy should be considered.
References
- Piraccini B, Bellavista S, Misciali C, et al. Periungual and subungual pyogenic granuloma. Br J Dermatol. 2010;163(5):941–953.
- Armstrong K, Weinstein M. Pyogenic granulomas during isotretinoin therapy. J Dermatol Case Rep. 2011;5(1):5–7.
- Exner J, Dahod S, Pochi P. Pyogenic granuloma-like acne lesions during isotretinoin therapy. Arch Dermatol. 1983;119(10):808–811.
- Valentic J, Barr R, Weinstein G. Inflammatory neovascular nodules associated with oral isotretinoin treatment of severe acne. Arch Dermatol. 1983;119(10):871–872.
- Campbell J, Grekin R, Ellis C, et al. Retinoid therapy is associated with excess granulation tissue responses. J Am Acad Dermatol. 1983;9(5): 708–713.
- Blumental G. Paronychia and pyogenic granuloma-like lesions with isotretinoin. J Am Aca Dermatol. 1984;10(4):677–678.
- Robertson D, Kubiak E, Gomez E. Excess granulation tissue responses associated with isotretinoin therapy. Br J Dermatol. 1984;111(6):689–694.
- Bigby M, Stern R. Adverse reactions to isotretinoin. A report from the Adverse Drug Reaction Reporting System. J Am Acad Dermatol. 1988;18(3):543–552.
- Azulay R, Abulafia L, Costa J, Sodré C. Tecido de granulação exuberante: efeito colateral da terapêutica com isotretinoína. An Bras Dermatol. 1985;60:179–182.
- Puig L, Moreno A, Llistosella E, et al. Granulation tissue proliferation during isotretinoin treatment. Int J Dermatol. 2007;25(3):191–193.
- Rivard S, Hurt M. Granulation tissue as a rare side effect of isotretinoin treatment: a case report. Pract Dermatol. 2013;43–44.
- Figueiras D, Ramos T, Ayana F, et al. Paronychia and granulation tissue formation during treatment with isotretinoin. An Bras Dermatol. 2016;91(2):223–225.
- Dawkins M, Clark A, Feldman S. Pyogenic granuloma-like lesion associated with topical tazarotene therapy. J Am Acad Dermatol. 2000;43(1):154.
- Gaudiello F, Scalvenzi M, Gallo L, Balato N. Excess granulation tissue and hair loss following acitretin. Dermatol Reports. 2011;3(1):e2.
- Patruno C, Balato N, Cirillo T, et al. Periungual and subungual pyogenic granuloma following anti-TNF-a therapy: is it the first case?. Dermatol Ther. 2013;26(6):493–495.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(9): 1425–1433.
- Wollina U. Multiple eruptive periungual pyogenic granulomas during anti-CD20 monoclonal antibody therapy for rheumatoid arthritis. J Dermatol Case Rep. 2010;4(3):44–46.
- Sibaud V, Dalenc F, Mourey L, Chevreau C. Paronychia and pyogenic granuloma induced by new anticancer mTOR inhibitors. Acta Derm Venereol. 2011;91(5):584–585.
- Baran R. Etretinate and the nails (study of 130 cases) possible mechanisms of some side-effects. Clin Exp Dermatol. 1986;11(2):148–152.
- Richert B. Frictional pyogenic granuloma of the nail bed. Dermatol. 2001;202(1);80–81.
- Liu J, Zhou B, Yi F, et al. Pyogenic granuloma in a patient with psoriasis successfully treated by 5-aminolevulinic acid photodynamic therapy: a case report. Expt Ther Med. 2016;11(1):345–347.
- Das R, Jain A, Ramesh V. Current concepts in the pathogenesis of psoriasis. Indian J Dermatol. 2009;54(1):7–12.
- Hugh J, Van Voorhees A, Nijhawan R, et al. From the medical board of the national psoriasis foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70(1):168–177.
- Flisiak I, Zaniewski P, Rogalska, et al. Effect of psoriasis therapy on VEGF and its soluble receptors serum concentrations. J Eur Acad Dermatol Venereol. 2012;26(3):302–307.
- Li W, Man X, Chen J, et al. Targeting VEGF/VEGFR in the treatment of psoriasis. Discov Med. 2014;18(98):97–104.
- Chen S, Takeuchi S, Urabe K, et al. Overexpression of phosphorylated-ATF2 and STAT3 in cutaneous angiosarcoma and pyogenic granuloma. J Cutan Pathol. 2008;35(8):722–730.
- Tosti A, Piraccini B, Camacho-Martinez F. Onychomadesis and pyogenic granuloma following cast immobilization. Arch Dermatol. 2001;137(2):231–232.
- Dika E, Balestri R, Vaccari S, et al. Successful treatment of pyogenic granulomas following gefitinib therapy with partial matricectomy and phenolization. J Dermatol Treat. 2009;20(6): 374–375.
- Raulin C, Greve B, Hammes S. The combined continuous-wave/pulsed carbon dioxide laser for treatment of pyogenic granuloma. Arch Dermatol. 2002;138(1):33–37.
- Matsumoto K, Nakanishi H, Seike T, et al. Treatment of pyogenic granuloma with a sclerosing agent. Dermatol Surg. 2001;27(6): 521–523.
- Tay Y, Weston W, Morelly J. Treatment of pyogenic granuloma in children with the flashlamp-pumped pulsed dye laser. Pediatr. 1997;99(3):368–370.
- Ghodsi S, Raziei M, Taheri A, et al. Comparison of cryotherpay and curettage for the treatment of pyogenic granuloma: a randomized trial. BMJ Paediatr Open. 2005;154(4):671–675.
- Mirshams M, Daneshpazhooh M, Mirshekari A, et al. Cryotherapy in the treatment of pyogenic granuloma. J Eur Acad Dermatol Venereol. 2006;20(7):788–790.

