 J Clin Aesthet Dermatol. 2021;15(6):53–58.
J Clin Aesthet Dermatol. 2021;15(6):53–58.
by Jane Han, BS; Angelina Palomino, BS; Blanca Estupinan, MD; Amy Wozniak, MS; and James Swan, MD
Ms. Han, Ms. Palomino and Dr. Swan are with the Stritch School of Medicine at Loyola University in Maywood, Illinois. Drs. Estupinan and Swan are with the Division of Dermatology at Loyola University Medical Center in Maywood, Illinois. Ms. Wozniak is with the Department of Biostatistics at Loyola University in Maywood, Illinois.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. We sought to investigate the relationship between psychiatric comorbidity, socioeconomic status (SES), and mental health utilization among patients with prurigo nodularis (PN).
Methods. We conducted a retrospective cohort study of patients with PN from 2007 to 2019. SES was approximated using zip codes; per capita income data was compared to the Livable Income Threshold.
Results. 288 patients were included. Patients were predominantly female (57%) and significantly more likely to have a psychiatric disorder than men (p=0.001). 44.1 percent of patients had at least one psychiatric comorbidity, with mood (74.8%) and anxiety (63.0%) disorders being most common. Patients with PN in lower SES groups had a higher incidence of psychiatric disorder (p=0.566) and utilization of mental health services (p=0.617). 40.9 percent of patients with a diagnosed psychiatric disorder had no record of seeing a psychiatrist or psychologist.
Limitations. Patient records were retrospectively reviewed for encounters with a psychiatrist or psychologist, but did not account for other forms of mental health services. Per capita income used to determine SES may not be an accurate representation of an individual’s income, nor did it account for the number of people within a household.
Conclusion. Psychiatric comorbidity was common among patients with PN and many went without receiving mental health services. Further studies with larger sample sizes are needed to better understand the impact of SES on these factors.
KEYWORDS: Prurigo nodularis, prurigo, psychiatric disorder, socioeconomic status
Prurigo nodularis (PN) is an uncommon chronic disease of intense pruritus, characterized by multiple erythematous, hyperkeratotic papules and nodules of symmetric distribution that spares regions where the patient cannot reach.1 The severe and chronic nature of PN has significant impacts on quality of life, with patients having the third worst self-reported health among other dermatological diseases.2, 3
Limited understanding of PN’s pathophysiology is compounded by lack of efficacious treatments, making PN a challenging condition to manage.1,4 High quality evidence regarding treatment options is sparse, with most treatments only supported by case studies, anecdotal evidence, or few randomized control trials of small sample sizes.4
Psychiatric disorders might be implicated in PN’s disease process, although the exact relationship is unclear.5 Patients have been reported to suffer from a variety of psychiatric conditions, which are often severe enough to warrant pharmacologic therapy and have been associated with longer inpatient psychiatric stays compared to matched controls.6–9
Population-level determinants, such as lower socioeconomic status (SES) have been associated with higher morbidity and mortality as well as a higher prevalence of psychiatric comorbidity.10–13 Lower SES may have implications on accessibility of mental health services due to factors such as location of residence and availability of mental health providers.13,14 However, the impact of SES on PN has not been well established. Furthermore, current data on SES and mental health utilization is conflicting, and no studies have examined these concepts in the PN patient population.15,16 Understanding the influence of environmental factors on PN can enhance the care of this patient population beyond medical treatment and provide alternative ways to address PN’s burden.
In this study, we aimed to assess the relationship between SES, the presence of psychiatric comorbidity, and mental health service utilization among patients with PN.
Methods
After receiving institutional review board approval, a retrospective chart review from January 1, 2007, to December 31, 2019, was conducted for patients diagnosed with PN at Loyola University Medical Center in Maywood, Illinois. Patients were identified using the diagnosis codes ICD-9 698.2 (prurigo) and ICD-10 L28.1 (prurigo nodularis). All psychiatric diagnoses were included for analysis, regardless of specialty type of the healthcare provider that entered the diagnosis into the electronic health record. Collected data included demographic information, zip codes, psychiatric diagnoses, and history of a documented encounter with a psychiatrist or psychologist, including those conducted at outside facilities. Psychiatric disorders were grouped into the following categories: anxiety, mood, psychotic, or other.
SES was approximated using zip codes. The per capita income (U.S. Census Bureau 2019) associated with each zip code was compared to the livable income threshold (LIT) for a household of one adult and zero children for the zip code’s respective county.17,18 Using a comparison model adapted from Silvernale et al,12 patients were then stratified into four socioeconomic groups: 1) <100% above the LIT 2) >100% above the LIT 3) >200% above the LIT 4) >300% above the LIT. For zip codes with populations of less than 5,000 or specific townships without data reported by the U.S. Census Bureau, per capita income was determined using the Census Reporter, an organization that reports statistics from the American Community Survey.19
LIT was used to approximate SES because it is a more accurate measurement of the minimum income required to meet basic needs, such as food, healthcare, housing, and transportation, than the federal poverty level. Assessment of need using federal poverty level underestimates the income required to meet basic needs and does not take into account geographical cost fluctuations.
Median and quartiles were used to summarize continuous variables; counts and percentages were used to summarize categorical variables for the entire population. Wilcoxon tests were used to test the associations of continuous variables. Chi-square test or Fisher’s exact test were used to test the associations of categorical variables. Univariable and multivariable logistic regression models were used to estimate odds ratios of psychiatric disorders between SES categories. Due to sparse data, some categories were collapsed into smaller categories for statistical modeling. All analyses were performed with SAS 9.4 (Cary, NC) and two-sided p<0.05 were deemed statistically significant.
Results
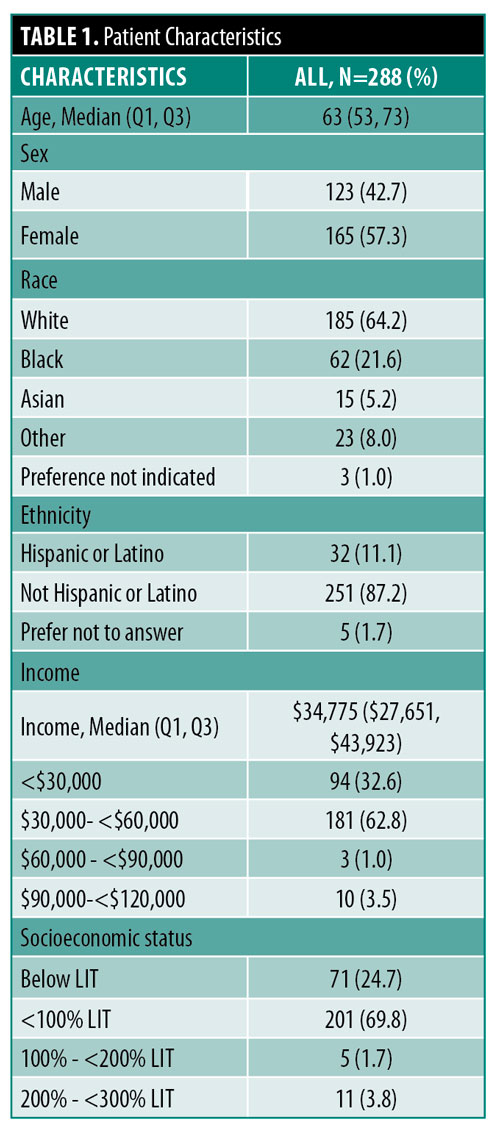
288 patients with a diagnosis of PN were included in the study. The median age was 63 years (Q1, Q3: 53, 73) and the majority of patients were female (n=165, 57.3%) and White (n=185, 64.2%). Median income was $34,775 and 24.6 percent of patient incomes fell below the LIT (Table 1).
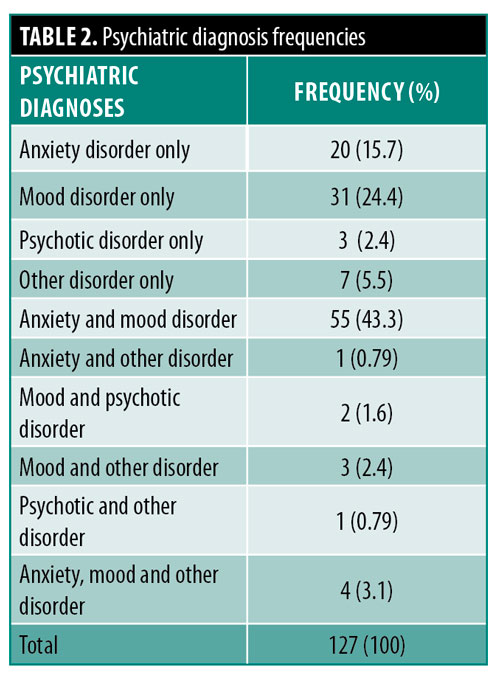
A total of 44.1 percent of patients had at least one psychiatric disorder. Of these patients, 48.0 percent had one psychiatric diagnosis, 48.8 percent had two psychiatric diagnoses, and 3.1 percent had three psychiatric diagnoses (Table 2). 74.8 percent of patients had a mood disorder, with the most common diagnosis being depression (unspecified) (n=61, 64.2%) followed by major depressive disorder (n=24, 25.3%). 63.0 percent of patients had an anxiety disorder, with the most common diagnosis being anxiety (unspecified) (n=53, 66.2%), followed by generalized anxiety disorder (n=22, 27.5%). 4.7 percent and 12.6 percent of patients had a psychotic disorder or other disorder, respectively. The most common disorder in the other category was adjustment disorder (n=14, 63.6%).
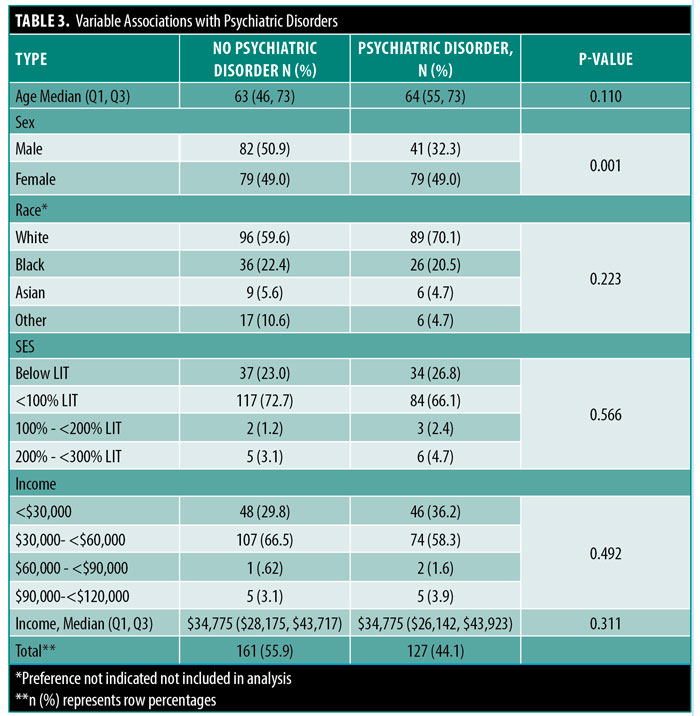
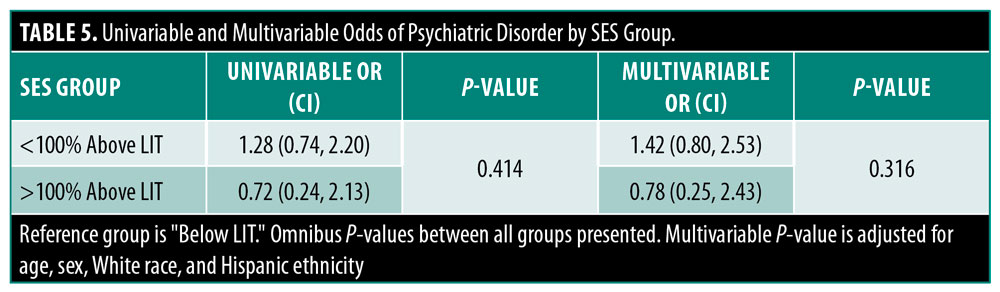
Female patients were significantly more likely to have a diagnosed psychiatric disorder (67.7% vs. 32.3%, p=0.001) than male counterparts. There were no other patient characteristics significantly associated with having a psychiatric disorder (Table 3). Those in the lowest SES groups had a higher incidence of psychiatric disorders than other SES groups, but this difference did not reach statistical significance (47.9% vs. 42.9%, p=0.459) (Table 4). There was no significant difference in the likelihood of having a diagnosed psychiatric disorder with changes in SES (<100% Below LIT: OR=1.42, CI=0.80–2.53; >100% Above LIT: OR=0.78, CI=0.25–2.43; p=0.316) even after adjustment for age, sex, White race, and ethnicity (Table 5).
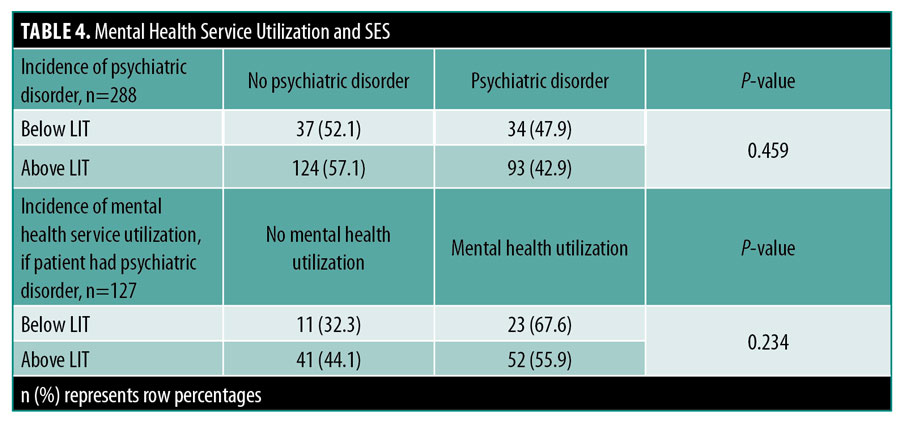
Among patients with a diagnosed psychiatric disorder, 40.9 percent had no record of an encounter with a psychiatrist or psychologist. The lowest SES group had a higher incidence of utilizing psychiatric services compared to those in a higher SES group, although this difference did not reach statistical significance (67.6% vs 55.9%, p=0.234) (Table 4).
Discussion
Our results demonstrate that a significant proportion of patients with PN had psychiatric comorbidities. Several studies exploring depression and anxiety in the PN population reported a significantly greater chance of having a psychiatric disorder compared to age-matched controls.6–7 Other psychiatric diagnoses in PN have rarely been explored, though one study found increased risks of self-harm, attention deficit hyperactivity disorder, eating disorders, and schizophrenia.7 In the current study, 17.3 percent of patients with PN carried a diagnosis other than mood or anxiety disorders, suggesting the overall prevalence of psychiatric disorders might be higher than previously thought.
We did not find an increased risk of psychiatric disease in relation to SES, which might be due to small sample size. Lower SES has been associated with an increased risk of psychiatric disorders, including chronic depression and anxiety, likely attributable to higher rates of environmental stressors in this population.10–13 However, this lack of association among patients with PN might support the implication of psychiatric symptoms in the pathogenesis of, or at least perpetuation of PN symptoms, regardless of SES. The stimulus of PN is itch, which can be a primary somatoform dissociative symptom.20 A cross-sectional study conducted in an outpatient dermatology facility found that patients with lichen simplex chronicus, a pruritic disorder similar to PN, had significantly higher dissociative experiences and general psychopathology scores compared to tinea patients.21 Underlying emotional dysregulation has also been linked to self-induced dermatoses and might help explain the relationship between psychiatric disorders and PN.22 Physiologic consequences of emotional dysregulation might result in increased histamine and glucocorticoid release, resulting in pruritus, impaired wound healing, and an increased predisposition to psychiatric issues.22,23 Increasing severity of depression has also been associated with more intense pruritus.24 Accordingly, patients with PN frequently report highly intense itch and are more likely to be diagnosed with depression compared to other chronic itch disorders.25,26
In concordance with prior studies, female patients were significantly more likely to have a psychiatric diagnosis compared to male patients.27-29 Although the exact influence of sex on psychiatric disorders is poorly understood, female patients with pruritus are more likely to be diagnosed with a psychiatric disorder compared to male patients.27 In the context of PN, this association might be explained by differences in disease burden. In an investigation of a large database of patients with chronic itch, female patients experienced more lesions and greater negative effects on quality of life compared to male patients.30 Given the potential for greater burden on physical and emotional well-being, physicians should remain vigilant to identify and address psychiatric disease in this at-risk population.
Regardless of sex differences, PN has a detrimental effect on quality of life due to the intensity and chronicity of pruritus.31, 32 Patients might be motivated to seek care for their cutaneous symptoms, thus dermatologists might have a unique opportunity to provide comprehensive care. Medical management is often difficult as there are no FDA-approved treatments and off-label therapies vary in efficacy and tolerability.4 Typically, initial treatment involves topical medications such as corticosteroids, calcineurin inhibitors, calcipotriol, or capsaicin. Alternatively, narrow-band ultraviolet B phototherapy may be recommended for persistent or widespread disease, though multiple weekly office-based treatments are not feasible for many working adults or those with limited transportation. Systemic therapies such as N-acetyl cysteine, neuromodulating agents (i.e. gabapentin, pregabalin, antidepressants, and naltrexone), immunomodulators (i.e., dupilumab), or immunosuppressants (i.e. methotrexate, cyclosporine, and mycophenolate mofetil) can be used in refractory cases. Lack of adequate medical treatment warrants alternative methods to address the burden of disease, such as psychosocial interventions when appropriate.33 However, a survey study of dermatologists found that 81 percent of dermatologists had limited or no education in psychodermatology and 90 percent were unaware of any patient resources for psychocutaneous disorders.34
Familiarity of psychodermatology principles is useful in the treatment of PN as it encompasses both understanding dermatologic manifestations of psychiatric disorders and psychiatric aspects of cutaneous disease. Despite appropriate psychiatry referrals, patients may be reluctant to undergo formal psychiatric evaluation due to societal stigma surrounding mental illness. Therefore, dermatologists may often assume the role of the sole provider to address patients’ psychocutaneous symptoms. A 2012 survey of U.S. dermatologists revealed that psychotropic medications were rarely prescribed and less than 20 percent felt comfortable prescribing antidepressants or antipsychotics.35 Interestingly, this trend maintained after stratification by year of board certification, suggesting that the lack of psychodermatologic education continues throughout a dermatologist’s career despite increased awareness of psychiatric disorder prevalence among dermatologic patients.
Our study also demonstrated a severe lack of psychotherapy among PN patients with psychiatric comorbidities, despite the overlap between the negative impact of PN and psychiatric disorders across multiple domains of biopsychosocial health.36, 37 Studies have also shown that psychotherapy in conjunction with medication is more effective than monotherapy for psychiatric disorders.36, 38 Psychotherapy can be useful in the treatment of PN not only by improving comorbid psychiatric disease, but also by addressing habitual scratching via behavioral habit reversal therapy.39, 40 Such therapy begins with awareness training including identifying times, locations, and positions of upper limbs while scratching, which can be logged in a scratch diary. Behavioral assessment can identify triggers for pruritus, such as clothing, temperature, and medications as well as defining goals such as improving skin healing and cessation or reduction of scratching. A competing response, such as outstretched arms, can also help reduce scratching. Typically this treatment plan is designed with a therapist and psychiatrist, but can be integrated into dermatologic practice. Compared to topical corticosteroids alone, combination therapy with behavioral training resulted in significantly decreased disease burden in atopic dermatitis.41, 42 Anecdotal clinical evidence has also demonstrated successful reduction of scratching using combination therapy in PN.43, 44
Our results showed that patients with PN of lower SES had a slightly higher incidence of psychiatric comorbidities than higher SES counterparts, supporting previous reports of the inverse relationship between SES and psychiatric disorders.36, 45 Although there have been inconsistent reports of the association between SES and mental health resource utilization, our results found that patients of low SES had encounters with a psychiatrist or psychologist more often than patients of higher SES. A previous study also reported that patients of lower SES had more frequent initial contact with mental health providers, but less regular visits, suggesting socioeconomic inequalities hinder patients’ ability to consistently follow up.46 Stressors beyond cutaneous symptoms may also contribute to the high burden of disease, and patients may benefit from a multidisciplinary approach. Prompt referrals to specialists such as psychiatry or social work may mitigate stressors, such as financial and transportation challenges and untreated psychiatric conditions.
Socioeconomic disparities may prevent patients from accessing mental health services due to systemic and individual-level barriers including cost, inadequate availability of mental health providers, stigma of seeking mental health treatment, and limited education.47,48 Addressing these barriers in conjunction with innovative, patient-centered solutions can help patients receive effective treatment. Telemedicine mental health visits may provide flexibility by addressing challenges with transportation or taking time off work, especially by offering appointments after normal working hours. Societal stigma and the fear of repercussions associated with having mental health disorders may also prevent adequate treatment, which emphasizes the importance of education and dispelling false beliefs.36, 49 Careful guidance of the conversation around psychiatric evaluation by healthcare providers using methods such as motivational interviewing and mental health workshops available both in-person or virtually may be pragmatic evidence-based solutions to reduce stigma around mental health.50
In summary, PN is a complex psychocutaneous disorder that often requires a multidisciplinary approach to care. Psychodermatology clinics also present an opportunity to maximize long-term patient outcomes. Although there is a demand from patients and it has been proven to be more cost-effective than individual specialty clinics, psychodermatology services are sporadic globally.51, 52 There is confusion among clinicians about choosing an appropriate model and the development of a psychodermatology multidisciplinary team.53 The European Society for Dermatology and Psychiatry has led the way in clinical and academic advances of psychodermatology including published reports of successful psychodermatology clinic models.52, 53 These models have the potential to provide quicker, more effective symptomatic relief and comprehensive care.54 Dermatology and psychiatry attending and resident physicians could also benefit from this clinic design by learning from one another and becoming more comfortable with managing patients with psychocutaneous disorders. If a multidisciplinary clinic design is not feasible, dermatologists can swiftly assess for psychiatric symptoms by using standardized questionnaires such as the PHQ-9 for depression and GAD-7 for anxiety disorders.55, 56 To identify patients who may benefit from social work referral, an 11-question screening tool created by the American Academy of Family Physicians assessing for social determinants of health can be distributed by either clinical or non-clinical staff.57 In addition, dermatology residency training programs can enhance standard curriculum with the inclusion of didactic sessions focused on psychodermatology and treatment algorithms including practical psychotropic medication regimens and monitoring practices to increase comfort with prescribing. For practicing dermatologists, Continuing Medical Education (CME) on psychodermatology concepts and social determinants of health can improve the management of PN and similar psychocutaneous conditions.
Limitations. The relatively small sample size likely contributed to the lack of statistical significance when considering the relationship between SES and psychiatric comorbidity. Included psychiatric diagnoses were not limited to those by a board-certified psychiatrist or psychologist. Although the gold-standard of psychiatric diagnoses is assessment by a psychiatrist or psychologist using DSM5 criteria, many patients with psychiatric diagnoses are initially assessed and treated by healthcare providers in other specialties.58 We used records of encounters with psychiatrists or psychologists as a measure to assess mental health service utilization, which does not take into account other forms of mental health services used, including mental health providers outside of our electronic medical record system, which may have resulted in an underestimation of psychiatric diagnoses in our cohort. Additionally, our study did not account for other potential confounders of mental health utilization such as insurance coverage or highest level of education achieved. LIT nor per capita income accounted for the number of children in a household. Per capita income also may not be an accurate representation of the income generated by an individual, as it is the average measure of income rather than accounting for the wide range of incomes possible for each individual. Future studies could include a prospective, cross-sectional design to collect patient income and number of dependents for more accurate SES stratification.
Conclusion
PN is a severely pruritic disease frequently encountered with concomitant psychiatric diagnoses and has significant negative impacts on patients’ quality of life. A significant proportion of patients did not receive formal psychiatric evaluation, regardless of SES. Socioeconomic disparities may contribute to barriers in accessing mental health providers in this patient population. Future studies with larger sample sizes are needed to further characterize the relationship between SES and mental health utilization among patients with PN.
References
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36(3):189–197.
- Janmohamed SR, Gwillim EC, Yousaf M, et al. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2020; Epub ahead of print.
- Brenaut E, Halvorsen JA, Dalgard FJ, et al. The self-assessed psychological comorbidities of prurigo in European patients: a multicentre study in 13 countries. J Eur Acad Dermatol Venereol. 2019;33(1):157–162.
- Qureshi AA, Abate LE, Yosipovitch G, et al. A systematic review of evidence-based treatments for prurigo nodularis. J Am Acad Dermatol. 2019;80(3):756–764.
- Zeidler C, Tsianakas A, Pereira M, et al. Chronic Prurigo of Nodular Type: A Review. Acta Derm Venereol. 2018;98(2):173–179.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31(2):e106–e107.
- Huang AH, Canner JK, Khanna R,et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140(2):480–483.e4.
- Rowland Payne CM. Nodular prurigo–a clinicopathological study of 46 patients. Br J Dermatol. 1985;113(4):431–439.
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: Epidemiology and clinical features. J Am Acad Dermatol. 2020;83(6):1559–1565.
- Kivimäki M, Batty GD, Pentti J, et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5(3):e140–e149.
- Lantz PM, House JS, Lepkowski JM, et al. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279(21):1703–1708.
- Silvernale C, Kuo B, Staller K. Lower socioeconomic status is associated with an increased prevalence of comorbid anxiety and depression among patients with irritable bowel syndrome: Results from a multicenter cohort. Scand J Gastroenterol. 2019;54(9):1070–1074.
- Diez Roux AV, Mair C. Neighborhoods and health. An NY Acad Sci. 2010;1186:125–145.
- Cummings J. Contextual socioeconomic status and mental health counseling use among US adolescents with depression. J Youth Adolescence. 2014;43(7):1151–1162.
- Roberts T, Esponda GM, Krupchanka D, et al. Factors associated with health service utilisation for common mental disorders: A systematic review. BMC Psychiatry. 2018;18(1):262.
- Epping J, Muschik D, Geyer S. Social inequalities in the utilization of outpatient psychotherapy: Analyses of registry data from german statutory health insurance. Int J Equity Health. 2017;16(1):147.
- U.S. Census Bureau. “U.S. Census Bureau QuickFacts: United States.” Census Bureau QuickFacts. Accessed December 01, 2020. www.census.gov/quickfacts/fact/table/US/PST045219.
- Massachusetts Institute of Technology. “Living Wage Calculator.” Living Wage Calculator. Accessed December 01, 2020. www.livingwage.mit.edu/.
- Census Reporter. “Grid View: Table B19301.” Accessed December 01, 2020. www. censusreporter.org/data/table/?table=B19301&geo_ids=86000US61317&primary_geo_id=86000US61317.
- Gupta MA, Gupta AK. Medically unexplained cutaneous sensory symptoms may represent somatoform dissociation: an empirical study. J Psychosom Res. 2006;60(2):131–136.
- Konuk N, Koca R, Atik L, et al. Psychopathology, depression and dissociative experiences in patients with lichen simplex chronicus. Gen Hosp Psychiatry. 2007;29(3):232–235.
- Gupta MA. Emotional regulation, dissociation, and the self-induced dermatoses: clinical features and implications for treatment with mood stabilizers. Clin Dermatol. 2013;31(1):110–117.
- Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10(2):213–219.
- Gupta MA, Gupta AK. Depression modulates pruritus perception. A study of pruritus in psoriasis, atopic dermatitis and chronic idiopathic urticaria. Ann N Y Acad Sci. 1999;885:394–395.
- Pereira MP, Hoffmann V, Weisshaar E, et al. Chronic nodular prurigo: clinical profile and burden: A European cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34(10):2373–2383.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79(4):714–719.e3.
- Whang KA, Khanna R, Thomas J, et al. Racial and Gender Differences in the Presentation of Pruritus. Medicines (Basel). 2019;6(4):98.
- Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74–81.
- Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67(2):247–257.
- Ständer S, Stumpf A, Osada N, et al. Gender differences in chronic pruritus: women present different morbidity, more scratch lesions and higher burden. Br J Dermatol. 2013;168(6):1273-1280.
- Kaaz K, Szepietowski JC, Matusiak Ł. Sleep quality among adult patients with chronic dermatoses. Postepy Dermatol Alergol. 2019;36(6):659–666.
- Todberg T, Zachariae C, Skov L. Treatment and Burden of Disease in a Cohort of Patients with Prurigo Nodularis: A Survey-based Study. Acta Derm Venereol. 2020;100(8):adv00119.
- Saarni S, Suvisaari J, Sintonen H, et al. Impact of psychiatric disorders on health-related quality of life: General population survey. Br J Psychiatry. 2007;190:326–332.
- Jafferany M, Vander Stoep A, Dumitrescu A, et al. The knowledge, awareness, and practice patterns of dermatologists toward psychocutaneous disorders: results of a survey study. Int J Dermatol. 2010;49(7):784–789.
- Gee SN, Zakhary L, Keuthen N, et al. A survey assessment of the recognition and treatment of psychocutaneous disorders in the outpatient dermatology setting: How prepared are we? J Am Acad Dermatol. 2013;68(1):47–52.
- Friedman MA, Detweiler-Bedell JB, Leventhal HE, et al. Combined psychotherapy and pharmacotherapy for the treatment of major depressive disorder. In: Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews. 1st ed. York, UK, Centre for Reviews and Dissemination; 1995.
- Oh CC, Li H, Lee W, et al. Biopsychosocial Factors Associated with Prurigo Nodularis in Endogenous Eczema. Indian J Dermatol. 2015;60(5):525.
- Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61(7):714–719.
- Abramowitz JS, Jacoby RJ. Pickers, Pokers, and Pullers: Obsessive-Compulsive and Related Disorders in Dermatology. In: Bewley A, Taylor RE, Reichenberg J, Magid M, eds. Practical Psychodermatology. 1st ed. Oxford, UK, Wiley-Blackwell; 2014:134–141.
- Long D, Long RA, Grillo MP, et al. Development of a psychological treatment service for pruritic skin conditions. Australas J Dermatol. 2006;47(4):237–241.
- Melin L, Frederiksen T, Noren P, et al. Behavioural treatment of scratching in patients with atopic dermatitis. Br J Dermatol. 1986;115(4):467–474.
- Norén P, Melin L. The effect of combined topical steroids and habit-reversal treatment in patients with atopic dermatitis. Br J Dermatol. 1989;121(3):359–366.
- Capoore HS, Rowland Payne CM, Goldin D. Does psychological intervention help chronic skin conditions? Postgrad Med J. 1998;74(877):662–664.
- Grillo M, Long R, Long D. Habit reversal training for the itch-scratch cycle associated with pruritic skin conditions. Dermatol Nurs. 2007;19(3):243–248.
- Hudson CG. Socioeconomic status and mental illness: tests of the social causation and selection hypotheses. Am J Orthopsychiatry. 2005;75(1):3–18.
- Packness A, Waldorff FB, Christensen RD, et al. Impact of socioeconomic position and distance on mental health care utilization: a nationwide Danish follow-up study. Soc Psychiatry Psychiatr Epidemiol. 2017;52(11):1405–1413.
- Andrade LH, Alonso J, Mneimneh Z, et al. Barriers to mental health treatment: results from the WHO World Mental Health surveys. Psychol Med. 2014;44(6):1303–1317.
- Steele L, Dewa C, Lee K. Socioeconomic status and self-reported barriers to mental health service use. Can J Psychiatry. 2007;52(3):201–206.
- Clement S, Schauman O, Graham T, et al. What is the impact of mental health-related stigma on help-seeking? A systematic review of quantitative and qualitative studies. Psychol Med. 2015;45(1):11–27.
- Mehta N, Clement S, Marcus E, et al. Evidence for effective interventions to reduce mental health-related stigma and discrimination in the medium and long term: Systematic review. Br J Psychiatry. 2015;207(5):377–384.
- Magid M, Reichenberg JS. An Evidence-Based Approach to Starting a Psychodermatology Clinic. JAMA Dermatol. 2020;156(6):617–618.
- Marshall C, Taylor R, Bewley A. Psychodermatology in Clinical Practice: Main Principles. Acta Derm Venereol. 2016;96(217):30–34.
- Aguilar-Duran S, Ahmed A, Taylor R, et al. How to set up a psychodermatology clinic. Clin Exp Dermatol. 2014;39(5):577–582.
- Patel A, Jafferany M. Multidisciplinary and Holistic Models of Care for Patients With Dermatologic Disease and Psychosocial Comorbidity: A Systematic Review. JAMA Dermatol. 2020;156(6):686–694.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097.
- Billioux AK, Verlander S, Anthony D. Alley. Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspectives. 2017.
- Schurman RA, Kramer PD, Mitchell JB. The hidden mental health network. Treatment of mental illness by nonpsychiatrist physicians. Arch Gen Psychiatry. 1985;42(1):89–94.

