 J Clin Aesthet Dermatol. 2021;14(8):24–32.
J Clin Aesthet Dermatol. 2021;14(8):24–32.
by Kunal Angra, MD; Michael B. Lipp, DO; Sahil Sekhon, MD; Douglas C. Wu, MD, PhD; and Mitchel P. Goldman, MD
Drs. Angra, Lipp, Wu, and Goldman are with Cosmetic Laser Dermatology in San Diego, California. Dr. Sekhon is with the Department of Dermatology at Howard University Hospital in Washington DC.
FUNDING: Cosmetic Laser Dermatology has received funding support to perform clinical trials from Pomega, Alastin, Skin Medica, Calecim, TR Therapeutics, and Pierre Fabre.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Laser resurfacing produces a controlled skin injury, resulting in a wound healing response. This wound healing response allows for collagen remodeling, which improves skin texture and tone. Topical agents are often employed following laser treatments to facilitate recovery. The introduction of newer small-molecule technologies allow for improved recovery and cosmesis.
Objective. We sought to perform a critical review of the safety and efficacy of newer small-molecule technologies employed following laser resurfacing.
Methods. We performed a PubMed search of the generic name of the following topicals and included literature relevant to laser procedures, with an emphasis on laser resurfacing: thermal spring water, conjugated linolenic acid, vitamin C/vitamin E/ferulic acid serum, tripeptide/hexapeptide technology-containing products, growth factor serum and gel, recombinant human epidermal growth factor ointment and gel, red deer umbilical cord lining mesenchymal stem cell extract cream and serum, silicone-based gel, and microparticulate (1-3, 1-6 beta-glucan) gel.
Results. Our search of the PubMed database yielded 62 results, out of which 17 clinical studies were included in this publication. The majority of aforementioned topicals show promise in terms of improving post-resurfacing recovery or cosmesis.
Conclusion. Clinical data regarding these agents is limited by the number and quality of studies. It is therefore challenging to propose a recommendation supporting any particular topical. We provide our own provider-specific post-laser resurfacing protocols to offer insight regarding new small-molecule technologies.
Keywords: Laser resurfacing, healing, topical agents, wound care, small-molecule
Ablative laser resurfacing utilizing carbon dioxide (CO2) and erbium-doped yttrium aluminum garnet (Er:YAG) lasers targets water in tissue, causing vaporization of the entire epidermis and varying levels of the dermis. The subsequent healing response results in structural rejuvenation, improving texture and tone. Depending on the degree of controlled injury, the healing process usually lasts several days to several weeks.1–5
There are three broad stages of the wound healing process: inflammatory, proliferative, and remodeling. The inflammatory stage involves the influx of leukocytes to the wound, leading to erythema, edema, and coagulation.6 Additionally, macrophages migrate to the wound to initiate the formation of granulation tissue.7 The proliferative stage starts at 48 hours and is characterized by vascular remodeling and angiogenesis. The remodeling phase, which begins two to three weeks after the initial wound, consists of resynthesis of the granulation tissue and the conversion of type III collagen to type I collagen, leading to increased strength of the forming scar.7
In order to maximize the safety and efficacy of laser-resurfacing procedures, careful attention must be paid to postoperative wound care. Standard of care generally includes strategies to control microbial overgrowth, optimize moisture balance, and protect the redeveloping skin barrier. In the past, closed dressings were often employed to achieve this goal.8
The advent of newer small-molecule technologies has enabled laser physicians to develop targeted approaches to facilitate wound healing. Typically formulated in topical creams, ointments, serums, or gels, the aim is to supply the skin with important factors that stimulate cell proliferation, migration, and protein synthesis, thus enhancing the wound-healing process.5,9,10 However, although many such topical technologies have been developed, a critical review of their safety and efficacy has not been recently conducted.
This literature review will provide a focused overview of open dressings used after laser treatments, with a special emphasis on ablative laser resurfacing. These topical treatments might benefit patients by enhancing cosmetic results, speeding wound healing, and reducing adverse effects, such as erythema and dyspigmentation.11 It is important to also note that an ideal postresurfacing topical agent does not lead to complications. Our review includes the following topicals: thermal spring water (TSW) (Avene Thermal Spring Water; Pierre Fabre Dermo-Cosmetique, Paris, France), conjugated linolenic acid (CLA) (Pomega MD; Pomega Inc., San Anselmo, California), vitamin C/vitamin E/ferulic acid (VCEF) serum (CE Ferulic; SkinCeuticals, Dallas, Texas), tripeptide/hexapeptide technology-containing products (Regenerating Skin Nectar, Restorative Neck Complex, and Ultra-nourishing Moisturizer with TriHex Technology; ALASTIN Skincare, Inc., Carlsbad, California), growth factor serum and gel (TNS Essential Serum and Recovery Complex; Skin Medica, Irvine, California), recombinant human epidermal growth factor ointment and gel (rhEGF; Easyef; Daewoong Pharmaceutical Co., Ltd., Hyderabad, India), red deer umbilical cord lining mesenchymal stem cell extract cream and serum (RCE; Calecim Multiaction Cream and Serum; CellResearch Corporation, Singapore), silicone-based gel (Stratacel; Stratapharma AG, Basel, Switzerland), and microparticulate (1-3/1-6 beta-glucan) gel (Glucoprime gel/TR-987; TR Therapeutics, Reston, Virginia).
Methods
We performed a PubMed search of the generic name of each of the aforementioned topicals and included literature relevant to laser procedures. All relevant literature was included up to September 16, 2019. References of included articles were also explored further. Additionally, relevant basic scientific literature was included. Lastly, for the growth factor serum, which had limited laser-related data, other aesthetic human studies were included.
Results
Our search of the PubMed database yielded 62 results, out of which 17 clinical studies were included in this publication. Table 1 provides a summary of the mechanism of action and highlights for each reviewed topical agent.
TSW. TSW, which is characterized by a low salt content, has been shown to have anti-inflammatory properties. Specifically, TSW inhibits basophil and mast cell degranulation.12,13 Some studies have suggested that it might reduce adverse effects associated with laser treatments when applied postoperatively.
Garcia et al14 compared TSW to a comparative water spray after photodynamic therapy (PDT) for 25 patients with acne or photodamage. Both sprays were used four times daily for a week. Patient and investigator pain assessments showed a significant decrease of pain in the TSW group on Day 2 relative to on Day 0 (p=0.015 and p=0.021), which was not seen in the comparative water group. Additionally, an investigator assessment of pruritus demonstrated a significant reduction in the TSW group compared to in the control group on Days 4 and 7 (p=0.0385).14 These findings indicate that TSW helps to reduce adverse effects in patients treated with PDT for acne or photodamage.
Barolet et al15 performed a split-face comparative study of 20 patients with bilateral dermal melasma treated with fractional resurfacing followed by the application of moisturizer to both sides of the face with frequent spray application of TSW unilaterally. Patients noted a significant reduction in pain, cutaneous dryness, and erythema on the TSW side relative to the control side (p<0.05). Additionally, an investigator assessment found marked reduction of erythema on the treatment side (p<0.01).
Sulimovic et al16 performed a randomized, open-label, multicentric, two-parallel-group study of 74 patients who were treated with CO2 or Er:YAG laser therapy on the face for various indications, such as photoaging and acne scars. The patients were split into two groups. Both groups were instructed to apply sterile petrolatum ointment postprocedure. However, one of the two groups was also instructed to apply TSW prior to the application of the petrolatum ointment. Patients in the TSW group experienced statistically significant reductions in erythema (p<0.04), pruritus (p<0.045), and stinging and tightening (p<0.05).
The aforementioned studies suggest that TSW might play a role in reducing adverse effects following laser-resurfacing and PDT.
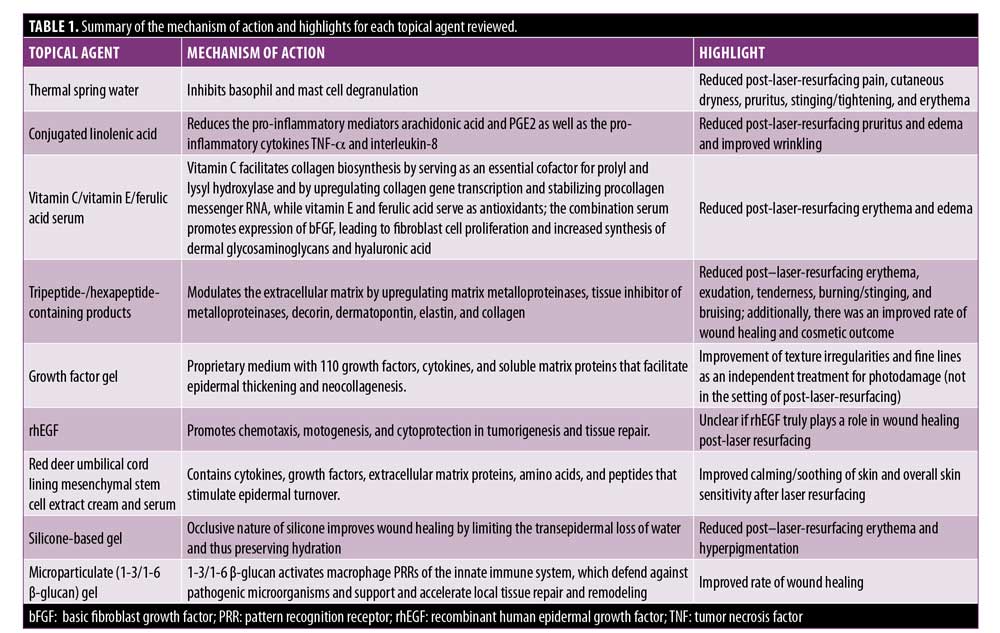
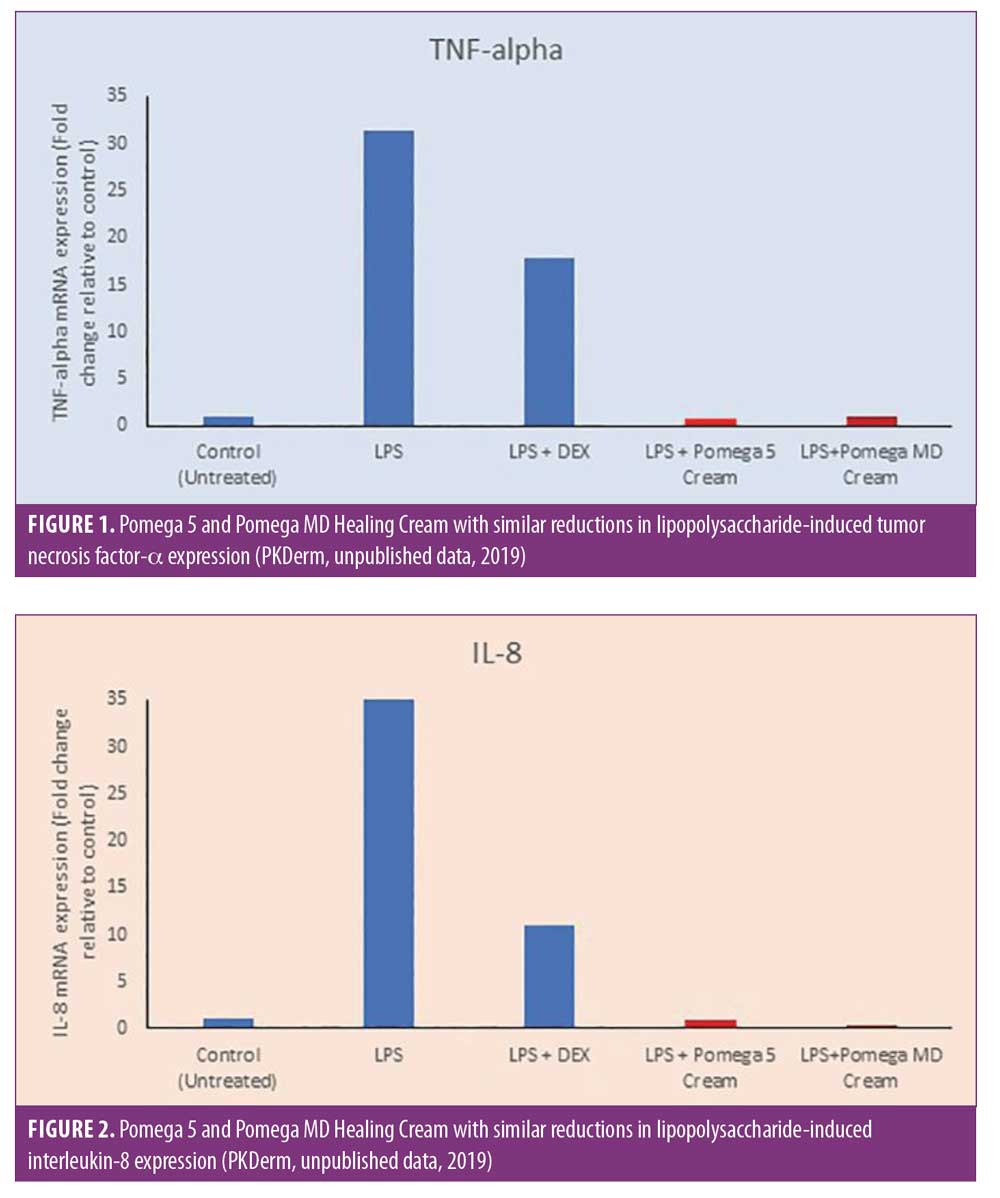
CLA. CLA, a component of pomegranate (Punica granatum L.) seed oil, has demonstrated antioxidant and anti-inflammatory properties in breast cancer lines.17 In a murine model, it has also been shown to reduce pro-inflammatory mediators produced by keratinocytes, such as arachidonic acid and PGE2.18 Unpublished data from an in-vitro three-dimensional reconstructed human epidermis model developed by PKDerm (Grasse, France) demonstrated a reduction in gene expression of lipopolysaccharide-induced pro-inflammatory cytokines (tumor necrosis factor-alpha and interleukin-8) compared to dexamethasone, which served as a positive control of anti-inflammation (Figures 1 and 2) (PKDerm, unpublished data, 2019). Recently, CLA was investigated as a post-laser-resurfacing topical in a randomized, controlled trial.19
Wu et al19 enrolled 34 patients who had fractionated CO2 laser resurfacing to the face with 24 randomized to using CLA, while 10 were randomized to using 1% dimethicone ointment (Vaniply; Pharmaceutical Specialties, Inc., Rochester, Minnesota). Based on the subjects’ pre-existing wrinkles and elastosis, a combination of Active FX and Deep FX modes were used. After laser resurfacing, either the test product or standard-of-care control was applied to the treated areas. The test regimen included a two-phase system, with CLA from promegranate seed oil serving as the main active ingredient in each phase. For the first two weeks after treatment, the test subjects applied a thick occlusive ointment, followed by two weeks of applying a concentrated repair serum and a healing cream, while the control group applied the 1% dimethicone ointment for the entire four-week span. The topical CLA healing regimen was well tolerated, with no increased adverse effects relative to the 1% dimethicone ointment. Subjects using the topical CLA healing regimen experienced significantly reduced itching on postprocedure Days 1 and 3 (p=0.03 and p=0.04) relative to baseline. The CLA group also had significantly reduced edema at postprocedure Day 3 (p=0.04) relative to baseline. However, no statistical difference between the CLA healing regimen and 1% dimethicone ointment treatment groups were noted through Day 30 in terms of physician assessments of edema, crusting, exudation, and erythema. The topical CLA regimen led to significantly improved wrinkling as quickly as two weeks postprocedure compared to the 1% dimethicone treatment group. These findings suggest that CLA might be beneficial after resurfacing procedures in reducing pruritus and further improving cosmetic outcome.
VCEF serum. Vitamin C (i.e., ascorbic acid) is an essential cofactor for the enzymes prolyl and lysyl hydroxylase in the biosynthesis of collagen. Prolyl and lysyl hydroxylase aid in the wound-healing process.20 One of the mechanisms through which vitamin C aids in collagen synthesis is through its direct effect on activating collagen-gene regulation by increasing transcription rate and stabilizing procollagen messenger RNA.21,22 Additionally, vitamin C also works indirectly through vitamin E on the cell membrane, as vitamin E is a major antioxidant of the cell membrane and is regenerated by ascorbic acid.23
Topical use of this compound is currently restricted by its instability in traditional formulations and delivery.24 Vitamin C in a purified form (L-ascorbic acid) at significant concentrations (10%–20%) and at an acidic pH (<3.5) has been shown to penetrate the epidermis into the skin.25 It has also been shown to reduce erythema after CO2 laser resurfacing.26
VCEF are antioxidants that help protect skin by neutralizing free radicals. When fractionally ablative lasers are used on the skin, the cutaneous antioxidant needs are conceivably increased, and thus, it might be beneficial to enrich the environment with significant amounts of vitamin C to the deeper layers of skin to promote the healing process. A prospective, single arm, split-face, double-blinded, controlled pilot study of 15 patients applying VCEF serum to one side and vehicle on the other side of the face after treatment with full-face fractional ablative CO2 laser (UltraPulse Active FX; Lumenis Santa Clara, California) resulted in decreased edema and erythema earlier in the postoperative period.27 Although not statistically significant, trends of decreasing erythema and edema at 24 to 48 hours were seen on the VCEF serum side. Interestingly, levels of basic fibroblast growth factor (bFGF) expression, as measured by real-time polymerase chain reaction from punch biopsies, were significantly higher in the VCEF side. bFGF has a wide spectrum of activity, including promoting fibroblast cell proliferation and increasing synthesis of dermal glycosaminoglycans and hyaluronic acid.27 Additionally, it promotes wound healing by stimulating fibroblast proliferation and inhibiting the expression of matrix metalloproteinase-1, also referred to as collagenase-1.27 Importantly, this is the first study to show that the application of VCEF serum after ablative laser resurfacing correlates with increased wound healing through specific molecular mechanisms of action during the initial days after laser-induced injury.27 Of note, there were no reports of irritation or dermatitis during the study.
In summary, the role of vitamin C in wound healing is well known but is limited by molecular instability and poor topical penetration. VCEF serum might help to decrease erythema and edema after resurfacing procedures. It also has been shown to promote wound healing through measurable differences in levels of bFGF expression. Although there were no reports of irritation or dermatitis during the study, in our clinical experience, vitamin C solution might not be an ideal wound-healing agent in the setting of ablative laser procedures, as it can cause pain or sensitivity due to its inherent acidity. However, it might be tolerated well when used in conjunction with nonablative laser procedures.
Tripeptide/hexapeptide technology. Tripeptide/hexapeptide technology, which has been incorporated in a gel, neck cream, and moisturizer by ALASTIN Skincare, Inc., is designed to optimize the results of laser-resurfacing procedures by modulating the extracellular matrix.28 Gene-expression studies of this product show an upregulation of matrix metalloproteinases, tissue inhibitor of metalloproteinases, decorin, dermatopontin, elastin, and collagen. These changes were reflected in histological studies of the extracellular matrix.28
Gold et al29 performed an open-label nonrandomized pilot study of 10 healthy female subjects with Fitzpatrick Skin Types II and III and aged 44 to 59 years (mean age: 51.3 years) to evaluate the efficacy of two tripeptide- and hexapeptide-containing products in improving healing and aesthetic outcomes of radiofrequency (RF) microneedling of photoaged neck skin.RF microneedling was performed using a commercially available device (Intensif; Endymed 3Deep Skin Science, Freehold, New Jersey). Patients used two products: 1) tripeptide-/hexapeptide-containing gel applied twice daily starting two weeks before RF microneedling and continued for one week posttreatment; and 2) tripeptide-/hexapeptide-containing cream applied twice daily starting on posttreatment Day 7 through the end of the study. The mean baseline Investigator Photodamage Assessment score was 2.3 and indicative of moderately severe photodamaged skin, but decreased steadily and was statistically significant at posttreatment Days 30 (1.6) and 90 (1.6). The skin tone, smoothness, texture, dryness/flakiness, blotchiness, and overall appearance parameters of the Investigator Global Assessment were also significantly improved by posttreatment Day 30. Subject skin quality questionnaire parameters also improved by posttreatment Day 30, with sustained improvement at Day 90. The RF microneedling procedure was well tolerated by patients, with mostly mild-to-moderate symptoms that resolved by posttreatment Day 7. However, this study was limited by its small sample size and lack of a true control group.
A prior study by Wilson et al30 evaluated the use of topical products with tripeptide and hexapeptide with fractionated CO2 laser resurfacing. The topical product studied was a tripeptide/hexapeptide system (Procedure Enhancement Invasive System) that included a cleanser (Gentle Cleanser), healing gel (Regenerating Skin Nectar), occlusive ointment (Soothe+Protect Recovery Balm), moisturizing cream (Ultranourishing Moisturizer with TriHex Technology), and sunscreen (Broad Spectrum SPF 30+). All products were from ALASTIN Skincare®, Carlsbad, California. This system of topical products was compared to the investigators’ standard of care with 1% dimethicone-based ointment (Vaniply; Pharmaceutical Specialties, Inc.), petrolatum-based cream (Vanicream; Pharmaceutical Specialties, Inc.), sunscreen (Broad Spectrum SPF 30+; ALASTIN Skincare®), and a gentle cleanser (CeraVe® Foaming Facial Cleanser, Valeant Pharmaceuticals North America, Bridgewater, New Jersey). Ten patients were randomized to use the tripeptide/hexapeptide system and five patients were randomized to use the standard of care. Three weeks prior to the laser procedure, subjects in the treatment group were instructed to perform twice-daily face washing with a gentle cleanser followed by application of the gel, ointment, and sunscreen. Patients in the standard-of-care group were instructed to perform twice-daily face washing followed by application of petrolatum-based moisturizer and sunscreen. Investigator-rated healing was better in the tripeptide/hexapeptide treatment group and achieved statistically significant superiority at postprocedure Day 7 (p=0.01). Furthermore, patients in the tripeptide/hexapeptide treatment group had significantly less erythema, exudation, tenderness, and burning/stinging at postprocedure Day 3 (p=0.02, p=0.01, p=0.02, and p=0.03, respectively). Given the risk of bruising with nerve block injections prior to resurfacing, the tripeptide/hexapeptide group ended up experiencing less bruising at Day 13 (p=0.03). Subject satisfaction was greater in the tri/hexapeptide regimen group at Day 28 and significantly greater at Day 84 (p=0.03).
A study using the same tripeptide/hexapeptide technology as the Wilson study was conducted by Robinson and Frulla.31 However, in this study, 10 patients had split-face or decollete comparisons performed, with one side treated with the same regimen as the Wilson study and the other treated with a substituted petrolatum-based ointment (Aquaphor Healing Ointment; Beiersdorf AG, Hamburg, Germany). Blinded investigator assessments determined that the side treated with tripeptide/hexapeptide technology had significantly superior healing after four days posttreatment as well as improvements in lentigines and texture (p=0.035). A blinded investigator assessment also confirmed a significant improvement in lentigines and texture on the treatment side at Day 4 (p=0.043 and p=0.009). Subjects also reported significantly greater satisfaction with the tripeptide/hexapeptide regimen at 14 days postprocedure and were significantly more likely to want to continue the regimen and recommend the treatment regimen to others. Subjects were able to continue with their regular lifestyle after 1 to 2 days, which is likely to make patients more receptive to receiving further treatments and more likely to comply with the treatment regimen, possibly leading to better outcomes. Therefore, tripeptide-/hexapeptide-containing products seemed to not only reduce adverse effects of laser resurfacing treatments but also improve wound healing and cosmetic results.
Growth Factor Serum and Gel (TNS Essential Serum and Recovery Complex)
TNS is a proprietary conditioned medium obtained from neonatal foreskin fibroblast culture that contains 110 growth factors, cytokines, and soluble matrix proteins.32,33 TNS comes in both gel (TNS Recovery Complex) and serum (TNS Essential Serum) forms. There are a few studies investigating the use of growth factor gel for the treatment of photodamage. One study included 40 patients who applied growth factor gel twice daily for 60 days and showed an improvement of texture irregularities and fine lines, especially in the periorbital area (p=0.003).33 Biopsy samples demonstrated thickening of the epidermis and neocollagenesis of the grenz zone. Mehta et al,34 in a double-blind, randomized, vehicle-controlled study involving 60 patients, noted greater improvement of photodamage in the growth factor gel group at three months’ postapplication. Growth factor gel could potentially play a role post-laser resurfacing, and such studies in the future may be helpful.
rhEGF. Epidermal growth factors promote chemotaxis, motogenesis, and cytoprotection in tumorigenesis and tissue repair.35 rhEGF is a human-derived growth factor that has been studied for its role in wound healing. In a systematic review and meta-analysis, Yang et al36 found that diabetic foot ulcers treated with topicals containing rhEGF had a significantly greater complete healing rate compared to placebo.
Techapichetvanich et al37 performed a randomized split-face study in Thailand that included 19 patients treated with fractional ablative CO2 laser application to the bilateral cheeks.37 One side of the face was then treated with petrolatum ointment and the other side was treated with rhEGF ointment (Easyef; Daewoong Pharmaceutical Co., Ltd., Hyderabad, India). Although the incidence of postinflammatory hyperpigmentation (PIH) was slightly lower on the rhEGF-treated side (52.6% vs. 57.9%), this finding lacked statistical significance. There was also no statistically significant difference in the duration of scab shedding, duration of postlaser erythema, erythema index, and transepidermal water loss. Meanwhile, another study performed in Thailand compared rhEGF gel (REGEND 150; Bharat Biotech International Ltd.) to aloe vera gel in treating striae alba after laser resurfacing with a fractional ablative CO2 laser.38 Although there were no objective statistically significant differences between the two sides, participants reported greater and statistically significant improvements on the rhEGF side. Despite improvements in diabetic foot ulcers using rhEGF-based topicals, it remains unclear whether rhEGF truly plays a role in wound healing following laser-resurfacing.
RCE. RCE contains cytokines, growth factors, extracellular matrix proteins, amino acids, and peptides that stimulate epidermal turnover.39 RCE has been formulated in a cell-free proprietary protein-mix cream and serum (Calecim Multiaction Cream and Serum; CellResearch Corporation, Singapore). Alhaddad et al40 compared the efficacy of RCE cream in a transepidermal vehicle versus the vehicle alone for facial rejuvenation. Forty patients with moderate-to-severe facial wrinkling secondary to photodamage participated in this 12-week prospective, randomized, double-blind, split-face clinical trial. There were no significant differences in the percent improvement between the active cream and vehicle in terms of wrinkling, laxity, dyschromia, erythema, or texture.40 However, at Week 12, patients noted significantly better skin tone/turgor (p=0.022) as well as overall satisfaction (p=0.036), with the active cream relative to the vehicle.
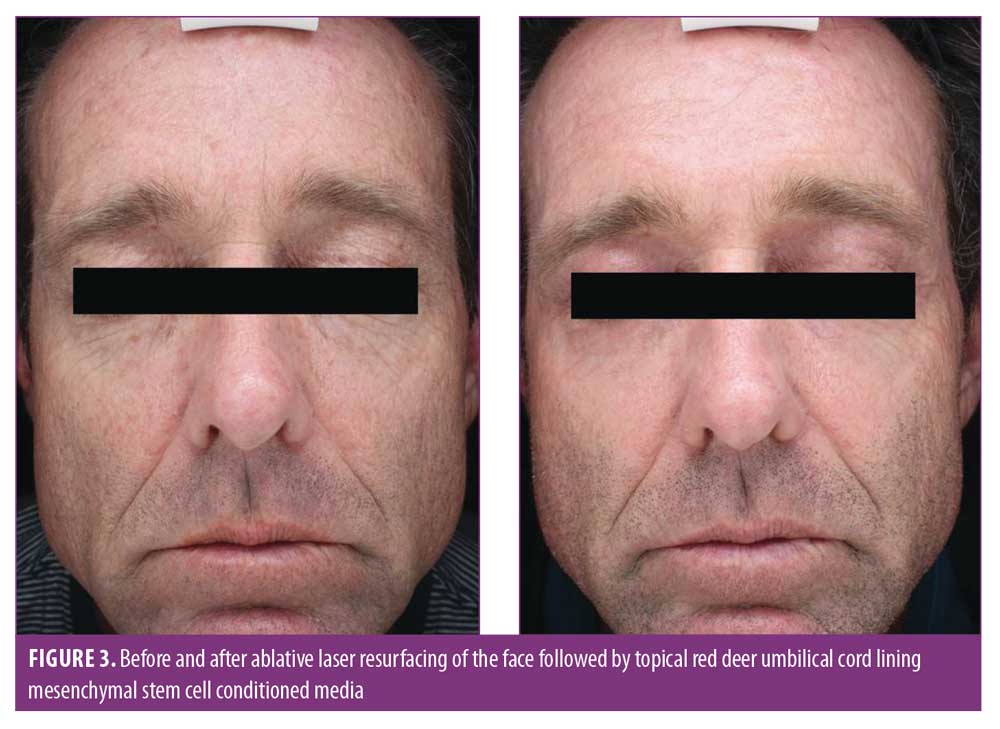
Our team, in a prospective, double-blind, placebo-controlled, randomized clinical trial investigated the use of RCE compared to the vehicle alone applied before and after ablative CO2 laser treatments for moderate-to-severe photodamage.41 The control group comprised five subjects, who applied a preprocedure cream (vehicle), postprocedure serum and cream (vehicle), gentle cleanser (Cetaphil Cleanser; Galderma, Lausanne, Switzerland), and broad-spectrum sunscreen (EltaMD UV Daily Broad-Spectrum SPF 40; EltaMD Skin Care, New York, New York). The treatment group comprised 15 patients who applied a preprocedure cream (Calecim Multiaction Cream), postprocedure cream and serum (Calecim Multiaction Cream and Serum), the same cleanser, and the same sunscreen. Although there was no statistically significant difference between both groups in terms of improvement of photodamage, erythema, edema, or xerosis, patients in the treatment group at various time points throughout the postoperative study were significantly more satisfied overall (Figure 3). Patients in the treatment group also experienced statistically significant calming and soothing as well as less skin sensitivity compared to the control group. Based on these findings, RCE cream and serum might be beneficial following ablative laser resurfacing in reducing adverse effects such as skin sensitivity and discomfort. However, further studies with larger sample sizes in the future might help to validate our findings. In addition, the use of a split-face study for this and other growth factors may not be ideal, since the growth factor media effects might cross over to the placebo side.
Silicone-based gel. Topical silicone has, for the past several years, played a role in scar management by specifically reducing scar size, improving scar texture and color, and decreasing scar-related symptoms, such as erythema, pain, and pruritus.42–45 In one study, Khamthara et al46 found that silicone gel with cyclopentasiloxane incorporating vitamin C decreased skin roughness in patients who underwent Er:YAG laser resurfacing (p<0.05).46 Some authors have proposed that the occlusive nature of silicone improves wound healing by limiting the transepidermal loss of water and thus preserving hydration.42
A proprietary silicone-based gel (Stratacel; Stratapharma AG, Basel, Switzerland) has been used to improve the appearance of scars. Yeh et al47 conducted a randomized, open-label, split-face study comparing petrolatum-based ointment and silicone-based gel. A total of 10 patients underwent Er:YAG fractional laser resurfacing, which was followed by application of silicone gel to one side of the face and petrolatum-based ointment to the other. The silicone-based gel group experienced less erythema and hyperpigmentation compared to the control group.47
Marini,48 in a split-face, single-blinded, prospective observational study, compared silicone-based gel to spring water and ointment (Vaseline; Unilever, London, England) in patients who had full-face CO2 fractional ablative treatments. The silicone-based gel group experienced the greatest reduction in superficial melanin content, skin porphyrins, and superficial hemoglobin.48 These findings suggest that silicone-based gel might be useful following laser resurfacing to minimize adverse effects.
Microparticulate (1-3/1-6 beta-glucan) gel (Glucoprime gel/TR-987). TR-987 0.1% gel (TissueRepair, TR Therapeutics Inc.) is a novel nonabsorbable gel that consists of microparticulate high-molecular-weight 1-3/1-6 beta-glucan extracted from yeast, Saccharomyces cerevisiae. The molecule 1-3/1-6 beta-glucan activates macrophage pattern recognition receptors (PRRs) of the innate immune system, which function to defend against pathogenic microorganisms and also support and accelerate local tissue repair and remodeling.
A Phase II, double-blind, randomized, placebo-controlled study (NCT00792688) compared 0.1% and 1.0% active gel with placebo following cosmetic CO2 laser resurfacing of the lower eyelids in 26 subjects. The topical was applied daily for five days. Time to complete wound closure was reduced with 0.1% and 1.0% gels versus placebo (mean days to complete re-epithelization was 10.9 and 13.1 vs.16.3 days; p=0.0062 and p=0.0331, respectively), with comparable safety and tolerability.49
We have conducted an unpublished, prospective, double-blind, placebo-controlled, randomized clinical trial of 42 subjects randomized to five-day treatment with 0.1% active gel or vehicle control applied to the chest after CO2 resurfacing.50 Blinded evaluators reported significant differences in wrinkles and elastosis using the Fitzpatrick-Goldman Wrinkle and Elastosis Scale. At Day 28 postprocedure, 71 percent (15/21) of subjects achieved at least a three-point reduction (p=0.13) in elastosis using the active gel compared to those who received the placebo vehicle (n=7/21; 33% of subjects). The percent of subjects achieving at least a one-point reduction in wrinkling relative to baseline was 81 percent (n=17/21) in the active gel group and 48 percent (n=10/21) in the placebo vehicle group (p=0.052). However, wrinkle and elastosis reduction was not found to be statistically significant at the end of the study (Day 104).
Microparticulate 1-3/1-6 beta-glucan gel might therefore shorten wound-healing time, as well as increase the rate of physiologic dermal collagen remodeling after fractional ablative laser resurfacing, but more published data is necessary to confirm these findings.
Conclusion
Based on the Oxford Center of Evidence Based Medicine guidelines, most studies in our review of literature were found to have a level of evidence below Level 1, which prevents us from offering a strong recommendation of any particular topical (Table 2). Some of the topicals, including VCEF, RCE cream and serum, and microparticulate (1-3/1-6 beta-glucan) gel, were found to have Level 1B evidence in regards to improved post-laser-resurfacing recovery or cosmetic benefit. However, this level of evidence is limited by the paucity of clinical studies. VCEF had only a single supporting Level 1B study, where investigators found reduced erythema and edema compared to the control side in patients who underwent facial ablative laser treatments, but this finding was not statistically significant. Despite promising data from Alhaddad et al40 demonstrating that RCE cream and serum improved investigator-assessed skin turgor and tone, this study investigated the treatment of photodamage not in the context of laser resurfacing. Data from our clinical trial did, however, demonstrate that application of RCE-containing topicals before and after CO2 laser treatments reduced skin sensitivity and soothed the skin relative to the vehicle control.41 Meanwhile, microparticulate (1-3/1-6 beta-glucan) gel underwent two Level 1B supporting studies. In the study by Joseph et al,49 microparticulate (1-3/1-6 beta-glucan) gel 0.1% and 1% gels following laser resurfacing hastened re-epithelization of the lower eyelids. In the unpublished study performed by our clinic, application of microparticulate (1-3/1-6 beta-glucan) gel 0.1% following laser resurfacing of the chest statistically improved elastosis at Day 28 based on the Fitzpatrick-Goldman Wrinkle and Elastosis Scale. Although growth factor serum had Level 1B evidence, it was studied in regard to photodamage, not postablative laser care. Therefore, studies in the setting of laser resurfacing are required. In summary, RCE cream and serum can reduce adverse effects after full-face laser resurfacing, while microparticulate (1-3/1-6 beta-glucan) gel shortens the recovery period of periorbital resurfacing and improves the cosmesis of chest resurfacing.
Despite lower levels of evidence, it appears that CLA, tripeptide-/hexapeptide-containing products, TSW, and silicone-based gel improve postoperative adverse effects following laser resurfacing procedures with statistical significance. Despite its Level 2B evidence, CLA improved the overall cosmetic outcome of laser resurfacing with statistical significance. Although studies are generally limited and the highest level of evidence recorded was 2B, tripeptide-/hexapeptide-containing products are unique in that they appear to benefit postresurfacing patients in three aspects: by reducing adverse effects, facilitating wound healing, and improving cosmetics outcomes. Further studies of tripeptide-/hexapeptide-containing products would be beneficial in further exploring these possible benefits.
In regard to rhEGF, although subjects expressed improvement with application after laser resurfacing, there was no objective, statistically significant improvement with the topical.
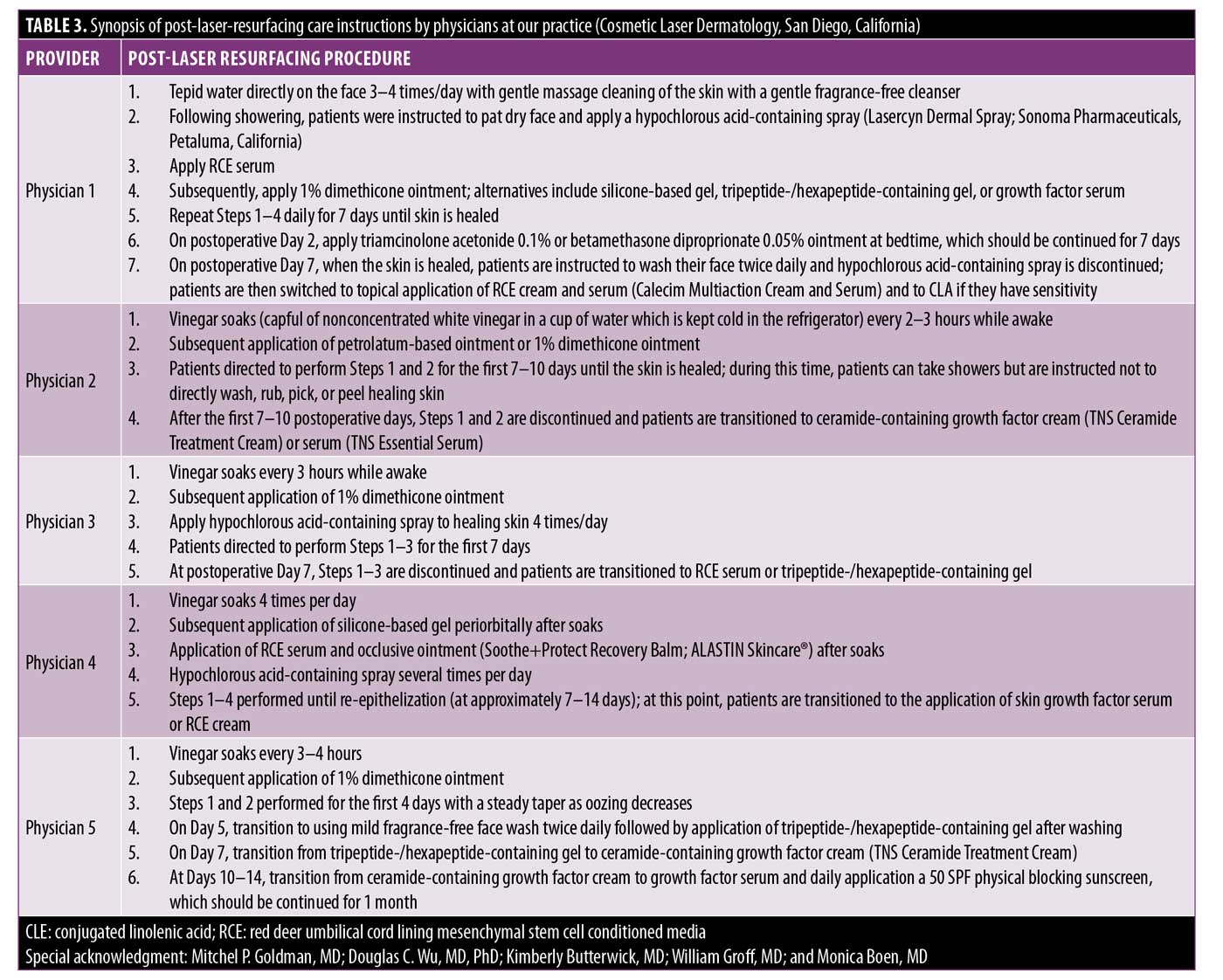
Given the lack of comparative studies and strong levels of evidence, it is difficult to propose a recommendation supporting a particular postresurfacing topical. At our practice, Cosmetic Laser Dermatology (San Diego, California), we have several highly experienced cosmetic dermatologists who all routinely perform laser resurfacing. Each dermatologist has their own post-laser resurfacing protocol, employing different topical regimens based on individualized clinical experiences (Table 3). Additionally, our physicians tailor post-laser resurfacing treatment to each patient to address prolonged erythema, the onset of acne, any tendency toward hyperpigmentation, and excessive dryness. We hope that this overview might offer practitioners with insight regarding post-laser resurfacing topical regimens as we await further clinical studies.
References
- Ruiz-Esparza J, Barba Gomez JM, Gomez de la Torre OL. Wound care after laser skin resurfacing. A combination of open and closed methods using a new polyethylene mask. Dermatol Surg. 1998;24(1):79–81.
- Suarez M, Fulton, Jr. JE. A novel occlusive dressing for skin resurfacing. Dermatol Surg. 1998;24(5):567–570.
- VanderKam VM, Achauer BM, Finnie G. Use of a semipermeable dressing (Biobrane) following laser resurfacing of the face. Plast Surg Nurs. 1997;17(3):177–179.
- Newman JP, Koch RJ, Goode RL. Closed dressings after laser skin resurfacing. Arch Otolaryngol Head Neck Surg. 1998;124(7):751–757.
- Concannon MJ, Malaney KB, Wiemer MS, Puckett CL. Omiderm: an inexpensive dressing after CO2 laser resurfacing. Plast Reconstr Surg. 1998;101(7):1981–1983.
- de Oliveira Gonzalez, Costa TF, de Araújo Andrade Z, Peixoto Medrado ARA. Wound healing—a literature review. An Bras Dermatol. 2016;91(5):614–620.
- Mendonca RJ, Coutinho-Netto J. Cellular aspects of wound healing. An Bras Dermatol. 2009;84(3):257–262.
- Goldman MP, Roberts 3rd TL, Skover G, et al. Optimizing wound healing in the face after laser abrasion. J Am Acad Dermatol. 2002;46(3):399–407.
- Jalkanen M. Haapanen T, Lyytikäinen AM, Larjava H. Wound fluids mediate granulation tissue growth phases. Cell Biol Int Rep. 1983;7(9):745–753.
- Davidson JM. Wound repair. J Hand Ther. 1998;11(2):80–94.
- de Vries FMC, Meulendijks AM, Driessen RJB, et al. The efficacy and safety of non-pharmacological therapies for the treatment of acne vulgaris: a systematic review and best-evidence synthesis. J Eur Acad Dermatol Venereol. 2018;32(7):1195–1203.
- Sainte-Laudy J, Sambucy JL. Inhibition of basophil degranulation by Avène Spring Water. Int Immunother. 1987;4:307–312.
- Sainte-Laudy J, Gall Y, Soto P. Inhibition of human basophil and rat mast cell activation by Avène Spring Water. Agents Action. 1993;38:228–230.
- Garcia BD, Goldman MP, Gold MH. Comparison of pre- and/or postphotodynamic therapy and intense pulsed light treatment protocols for the reduction of postprocedure-associated symptoms and enhancement of therapeutic efficacy. J Drugs Dermatol. 2007;6(9): 924–928.
- Barolet D, Lussier I, Mery S, Merial-Kieny C. Beneficial effects of spraying low mineral content thermal spring water after fractional photothermolysis in patients with dermal melasma. J Cosmet Dermatol. 2009;8(2): 114–118.
- Sulimovic L, Licu D, Ledo E, et al. Efficacy and safety of a topically applied Avène spring water spray in the healing of facial skin after laser resurfacing. Dermatol Surg. 2002;28(5):415–418; discussion 418.
- Costantini S, Rusolo F, De Vito V, et al. Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules. 2014;19(6):8644–8660.
- Liu KL, Belury MA. Conjugated linoleic acid reduces arachidonic acid content and PGE2 synthesis in murine keratinocytes. Cancer Lett. 1998’127(1–2):15–22.
- Wu DC, Goldman MP. A topical anti-inflammatory healing regimen utilizing conjugated linolenic acid for use post-ablative laser resurfacing of the face: a randomized, controlled trial. J Clin Aesthet Dermatol. 2017;10(10):12–17.
- Nusgens BV, Humbert P, Rougier A, et al. Stimulation of collagen biosynthesis by topically applied vitamin C. Eur J Dermatol. 2002;12(4):XXXII–XXXIV.
- Murad S, Grove D, Lindberg KA, et al. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879–2882.
- Phillips CL, Tajima S, Pinnell SR. Ascorbic acid and transforming growth factor-beta 1 increase collagen biosynthesis via different mechanisms: coordinate regulation of pro alpha 1(I) and Pro alpha 1(III) collagens. Arch Biochem Biophys. 1992;295(2):397–403.
- Colven RM, Pinnell SR. Topical vitamin C in aging. Clin Dermatol. 1996;14(2):227–234.
- Starr NJ, Hamid KA, Wibawa J, et al. Enhanced vitamin C skin permeation from supramolecular hydrogels, illustrated using in situ ToF-SIMS 3D chemical profiling. Int J Pharm. 2019;563:21–29.
- Pinnell SR, Yang H, Omar M, et al. Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 2001;27(2):137–142.
- Alster TS, West TB. Effect of topical vitamin C on postoperative carbon dioxide laser resurfacing erythema. Dermatol Surg. 1998;24(3):331–334.
- Waibel JS, Mi Q-S, Ozog D, et al. Laser-assisted delivery of vitamin C, vitamin E, and ferulic acid formula serum decreases fractional laser postoperative recovery by increased beta fibroblast growth factor expression. Lasers Surg Med. 2016;48(3):238–244.
- Widgerow AD, Fabi SG, Palestine RF, et al. Extracellular Matrix Modulation: Optimizing Skin Care and Rejuvenation Procedures. J Drugs Dermatol. 2016;15(4 Suppl):s63–s71.
- Gold MG, Sensing W, Biron JA. A topical regimen improves skin healing and aesthetic outcomes when combined with a radiofrequency microneedling procedure. J Cosmet Dermatol. 2019 Jul 2. Epub ahead of print.
- Vanaman Wilson MJ, Bolton J, and Fabi SG. A randomized, single-blinded trial of a tripeptide/hexapeptide healing regimen following laser resurfacing of the face. J Cosmet Dermatol. 2017;16(2):217–222.
- Robinson DM, Frulla AP. Randomized, split-face/decollete comparative trial of procedure enhancement system for fractional non-ablative laser resurfacing treatment. J Drugs Dermatol. 2017;16(7):707–710.
- Knighton DR, Ciresi K, Fiegel VD, et al. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet. 1990;170(1):56–60.
- Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J Cosmet Laser Ther. 2003;5(1): 25–34.
- Mehta RC, Smith SR, Grove GL, et al. Reduction in facial photodamage by a topical growth factor product. J Drugs Dermatol. 2008;7(9):864–871.
- Berlanga-Acosta J, Gavilondo-Cowley J, López-Saura P, et al. Epidermal growth factor in clinical practice – a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6(5): 331–346.
- Yang S, Geng Z, Ma K, et al. Efficacy of topical recombinant human epidermal growth factor for treatment of diabetic foot ulcer: a systematic review and meta-analysis. Int J Low Extrem Wounds. 2016;15(2):120–125.
- Techapichetvanich T, Wanitphakdeedecha R, Iamphonrat T, et al. The effects of recombinant human epidermal growth factor containing ointment on wound healing and post inflammatory hyperpigmentation prevention after fractional ablative skin resurfacing: a split-face randomized controlled study. J Cosmet Dermatol. 2018;17(5):756–761.
- Disphanurat W, Kaewkes A, Suthiwartnarueput W. Comparison between topical recombinant human epidermal growth factor and Aloe vera gel in combination with ablative fractional carbon dioxide laser as treatment for striae alba: a randomized double-blind trial. Lasers Surg Med. 2020;52(2):166–175.
- Lim IJ, Thang Phan T. Epithelial and mesenchymal stem cells from the umbilical cord lining membrane. Cell Transplant. 2014;23(4–5):497–503.
- Alhaddad M, Boen M, Wu DC, Goldman MP. Red deer umbilical cord lining mesenchymal stem cell extract cream for rejuvenation of the face. J Drugs Dermatol. 2019;18(4):363–366.
- Hoss E, Kollipara R, Alhaddad M, Boen M, Goldman MP. Red Deer Umbilical Cord-Derived Stem Cell Conditioned Media Combined With Ablative Resurfacing of the Face. J Drugs Dermatol. 2020 Nov 1;19(11):1044–1048.
- Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg. 2008;32(1):82–92.
- Chernoff WG, Cramer H, Su-Huang S. The efficacy of topical silicone gel elastomers in the treatment of hypertrophic scars, keloid scars, and post-laser exfoliation erythema. Aesthetic Plast Surg. 2007;31(5):495–500.
- Chan KY, Lau CL, Chan SMA, et al. A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg. 2005;116(4):1013–1020; discussion 1021–1022.
- Signorini M, Clementoni MT. Clinical evaluation of a new self-drying silicone gel in the treatment of scars: a preliminary report. Aesthetic Plast Surg. 2007;31(2):183–187.
- Khamthara J, Kumtornrut C, Pongpairoj K, Asawanonda P. Silicone gel enhances the efficacy of Er:YAG laser treatment for atrophic acne scars: A randomized, split-face, evaluator-blinded, placebo-controlled, comparative trial. J Cosmet Laser Ther. 2018;20(2):96–101.
- Yeh LC, Gonzalez N, and Goldberg DJ. Comparison of a novel wound dressing vs current clinical practice after laser resurfacing. J Cosmet Dermatol. 2019;18(4):1020–1024.
- Marini L. Advanced film-forming gel formula vs spring thermal water and white petrolatum as primary dressings after full-face ablative fractional CO2 laser resurfacing: a comparative split-face pilot study. J Eur Acad Dermatol Venereol. 2018;32(1):113–116.
- ClinicalTrials.gov. Efficacy Study of GLYC-101 to Evaluate Outcomes After Laser Ablation. Available at: http://clinicaltrials.gov/show/NCT00792688.
- Kollipara R, Boen M, Alhaddad M, et al. TR-987 0.1% Active Gel (Glucoprime) vs. Placebo Gel Following Fractionated CO2 Laser Resurfacing of the Chest (Abstract). 24th World Congress of Dermatology Milan 2019, Milan, Italy.

