 J Clin Aesthet Dermatol. 2021;14(8):34–40.
J Clin Aesthet Dermatol. 2021;14(8):34–40.
by Sheila C. Barbarino, MD, FACS; Jani A. J. van Loghem, MD; Cheryl M. Burgess, MD, FAAD; and Niamh Corduff, MBBS, FRACS
Dr. Barbarino is with Barbarino Surgical Arts in Austin, Texas. Dr. Loghem is with the UMA Institute in Amsterdam, the Netherlands. Dr. Burgess is with the Center for Dermatology and Dermatologic Surgery in Washington, DC. Dr. Corduff is with the Cosmetic Refinement Clinic in Geelong, Australia.
FUNDING: No funding was provided for this study.
DISCLOSURES: Dr. Barbarino is a consultant for Merz, Lumenis, Alastin Skincare, Skinceuticals, Syneron/Candela, and Sinclair Pharmaceuticals. Dr. Burgess is a consultant for Merz and Allergan. Dr. van Loghem is a consultant for Merz, Allergan, and Ipsen. Dr. Corduff is a clinical advisor, investigator, and speaker for Merz and medical advisory board member for Establishment Labs.
ABSTRACT: Background. As aesthetic preferences have evolved and patients wish their muscles to be relaxed, but not frozen, a higher dilution of incobotulinumtoxinA (INCO) has allowed for increased spread using fewer units, yet no studies to date have investigated the efficacy, longevity, and safety of hyperdiluted INCO.
Objective. We evaluated the effect of incobotulinumtoxinA (INCO) in glabellar, forehead, and lateral periorbital lines using a high dilution.
Methods. Subjects with moderate-to-severe upper facial lines at rest according to the Merz Aesthetics Scales™ (Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) received 15U of INCO to the glabellar (n=4 injection sites), 10U to the rest of the forehead (n=10 injection sites), and 5U to the lateral periorbital lines (n=3 injection sites/eye). Primary outcomes were physician- and subject-rated improvement at one month using the Global Aesthetic Improvement Scale (GAIS) and changes in line severity using the Merz Aesthetics Scales™.
Results. The study included 15 women aged 35 to 65 years. At one month, physician GAIS scores indicated 91.2% of subjects were very much improved and 8.8% were much improved; 91.5%, 78.0%, and 57.6% of participants remained at least improved at four, five, and six months, respectively. Subject GAIS scores at one month were in agreement with physician scores. At one month, an improvement of at least one point in Merz Aesthetics Scales™ scores in glabellar, forehead, and lateral periorbital lines was reported in 88.9%, 98.3%, and 94.8% of participants, respectively. Subject satisfaction was high throughout the study. No treatment-related adverse events were observed.
Conclusions. Hyperdilute INCO was effective at improving overall appearance and reducing line severity in individuals with moderate-to-severe upper facial lines. Patient satisfaction was maintained up to six months and treatment was well tolerated.
Keywords: IncobotulinumtoxinA, hyperdiluted, glabellar lines, horizontal forehead lines, lateral periorbital lines
Botulinum neurotoxin injection has become the most popular nonsurgical aesthetic procedure worldwide,1,2 a position it has held in American plastic surgery practices since 1999.2 This minimally invasive procedure is effective for the temporary improvement of dynamic wrinkles and can also be used prophylactically in younger patients to prevent lines and wrinkles resulting from muscular activity.
The use of injectable botulinum neurotoxins for aesthetic purposes is evolving rapidly. While approved indications are still restricted to the treatment of glabellar lines, lateral periorbital lines, and horizontal forehead lines caused by muscle activity, such products are also widely used off-label for many other facial indications, ranging from eyebrow shaping to softening platysmal bands. There has also been a refinement of outcomes, with physicians now striving for neuromodulation softening rather than complete paralysis.3
Incobotulinumtoxin A (INCO) (Xeomin®; Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) is a botulinum toxin type A preparation that contains only the 150-kDa pure active neurotoxin moiety.4 Licensed indications for use vary and are specific to individual countries. In Europe, INCO has been available since 2005. It is licensed for the combined treatment of moderate-to-severe glabellar frown lines, lateral periorbital lines (crow’s feet), and upper facial lines in adults younger than 65 years. In the United States, the product was approved by the Food and Drug Administration (FDA) in 2011 for the treatment of moderate-to-severe glabellar frown lines with a retreatment interval of no less than three months.
For aesthetic use, INCO is supplied in a 50- or 100-U vial and should be reconstituted with sterile, preservative-free 0.9% saline. Dilution volumes for INCO reconstitution typically range from 0.25 to 5mL.5 To date, only a few studies have examined the efficacy and safety of botulinum neurotoxin dilutions.6–9 Two studies assessing INCO with dilution volumes ranging from 0.55 to 4mL 0.9% sodium chloride per 50-U vial found no differences in efficacy or safety between the higher and lower dilutions.8,9 As a result, the final dilution volume for INCO is largely a matter of personal preference. The original studies submitted for FDA approval incorporated 20U with a primary endpoint of responders at maximum frown.10,11 Since then, aesthetic preferences have changed and the modern patient prefers their muscles relaxed, but not frozen, maintaining a degree of facial expression. To provide this result, the corresponding author began treating her patients with a higher dilution, which increased spread using fewer units. This achieved the softer look desired by patients and resulted in high patient satisfaction. Lower dilutions with higher units are thought by some to ensure more accurate placement, fewer side effects from spread into adjacent muscles, and longer duration of effects.12,13 Others believe that higher dilutions are easier to work with, less wasteful, and that spread associated with higher dilutions can be used to the injector’s advantage—for example, in areas such as the forehead and lateral orbit, where a more homogeneous effect is required.13 Carruthers et al6 studied dilution volumes of onabotulinumtoxinA (ONA) in 80 women with moderate-to-severe glabellar lines and reported no significant difference between dilution groups (100, 33.3, 20, or 10 U/mL) in terms of either efficacy or adverse events.6
To date, no studies have investigated the efficacy, longevity, and safety of hyperdiluted INCO. This pilot study was conducted to follow a case series of patients injected with a high dilution volume of INCO and observe treatment efficacy, longevity, safety, and patient satisfaction up to six months.
Methods
This prospective, open-label study enrolled women presenting to a private aesthetic surgery practice seeking improvement in the appearance of their upper face. Eligible subjects were required to be between 22 to 65 years old, express a desire and willingness for correction of their upper face (i.e., glabellar lines, horizontal forehead, and lateral periorbital lines) with botulinum neurotoxin, and to have a baseline severity score of 2 or 3 for each area according to the relevant Merz Aesthetics Scales™ (MAS) (Merz Pharmaceuticals GmbH), where 0=no lines, 1=mild lines, 2=moderate lines, 3=severe lines, and 4=very severe lines.14 Patients who had received any neuromodulator or other aesthetic treatment to the area in the preceding six months (12 months in the case of dermal filler treatment in any of the areas treated in this study) were excluded. In addition, participants were required to remain on the same facial skincare regimen for 90 days prior to enrollment and for the duration of the study. Individuals who were not compatible with the prescribing criteria for the product and pregnant or breastfeeding women were also excluded.
All participants were required to show willingness and ability to comply with protocol requirements, including returning for follow-up visits and abstaining from undergoing any other procedures in the treated area throughout the study. All subjects provided signed, informed consent for the procedure and the subsequent use of identifiable photographs for scientific purposes. This study adhered to the tenets of the Declaration of Helsinki as amended in 2008 and was compliant with the Health Insurance Portability and Accountability Act, which ensures protection of individually identifiable health information, unless consented by the patient (institutional review board NO. PRO 00031715).
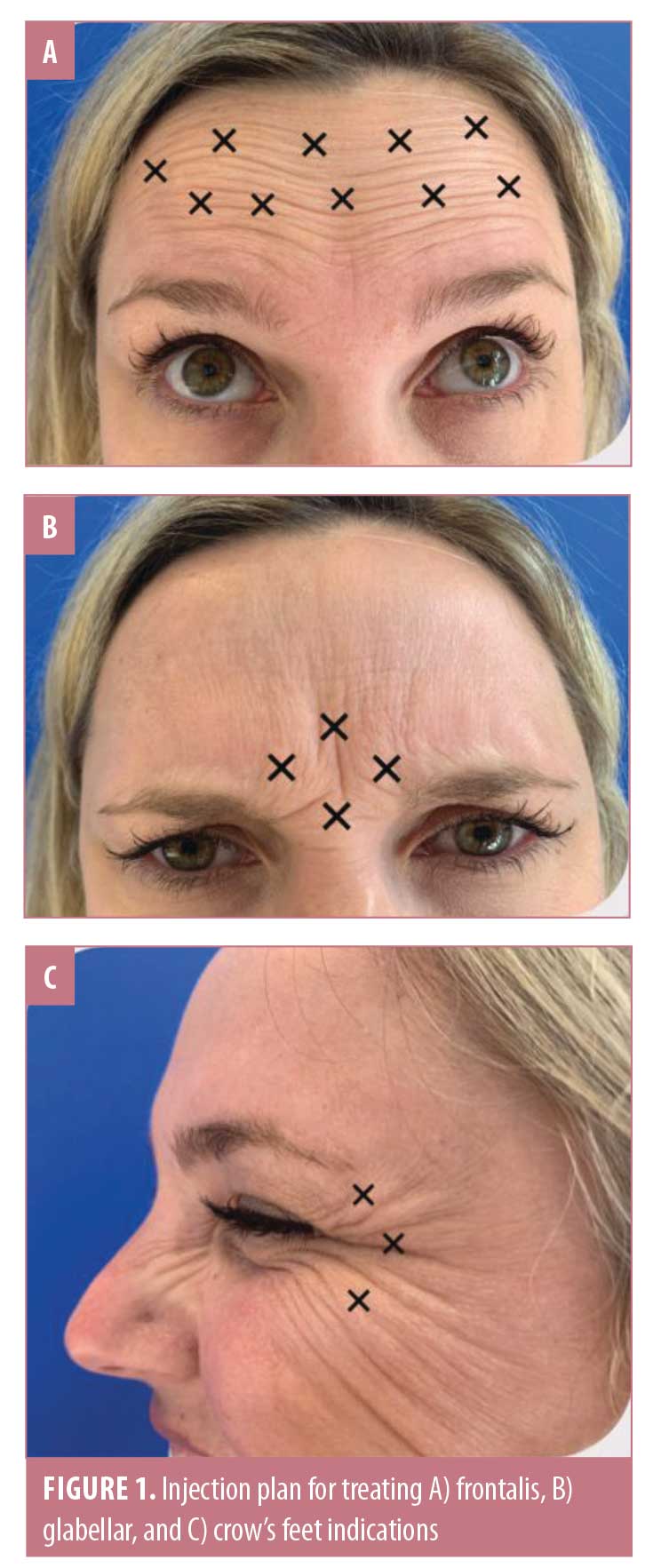
Each 100-U vial of INCO was hyperdiluted by reconstituting with 7.5mL of nonbacteriostatic, preservative-free 0.9% sodium chloride (NaCl). Each subject then received a total of 35U of INCO, including 15U to the glabellar area via four injections of 3.75U; 10 U over the rest of the forehead using 1U per injection (10 injection sites); and 5U to treat the lateral periorbital lines of each eye using 1.7U for each of three injection sites. Injections were performed with a 31-gauge needle and 3-cc syringe. The injection plans for each injection site are illustrated in Figure 1. Ice was applied to the injection sites pretreatment and for 10 minutes immediately postinjection to reduce possible swelling or bruising. Follow-up appointments were scheduled monthly up to six months postinjection. At each visit, photographs of the patient at rest, full frown, with raised eyebrows, and squinting were taken. All injections were performed by the same physician injector, all photographs were taken at each visit, and all photographic evaluations were completed by the same blinded evaluators at the end of the study. The treating physician and three others (two plastic surgeons and one dermatologist) evaluated each patient’s before and after photos and assessed improvement using a five-category Global Aesthetic Improvement Scale (GAIS) as follows: very much improved, much improved, improved, no change, and worse. Patient ratings of treatment effectiveness were also captured using the same GAIS scale.
The primary outcomes were independent, blinded evaluator-rated and subject-rated GAIS scores at Week 4 and evaluator-rated MAS scores at maximum frown at Week 4. Secondary outcomes included evaluator- and subject-rated GAIS scores and evaluator-rated glabella, forehead, and lateral periorbital line MAS scores at each posttreatment visit. In addition, subject satisfaction at one, three, and six months posttreatment was rated using the FACE-Q™ Satisfaction with Outcome and Perceived Age questionnaires.15,16
Results
The study enrolled 15 women ranging in age from 35 to 65 years, all of whom attended all study visits. At one month posttreatment, physician GAIS scores rated subject appearance as very much improved in 91.2 percent and much improved in 8.8 percent of patients (Figure 2). Figure 3 shows pretreatment and one-month posttreatment photos for each treatment area, illustrating a complete lack of dynamic wrinkles at maximum contraction.
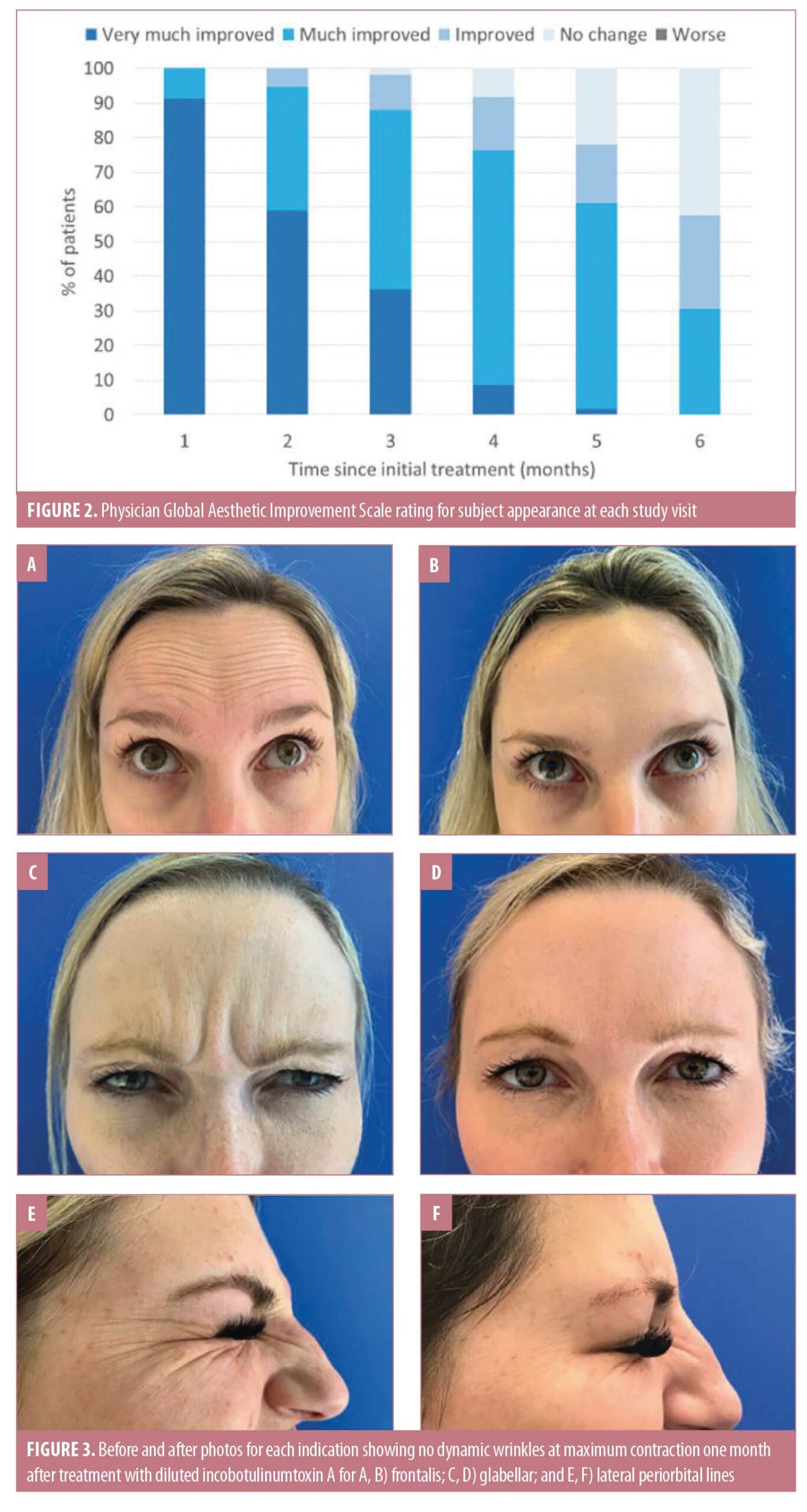
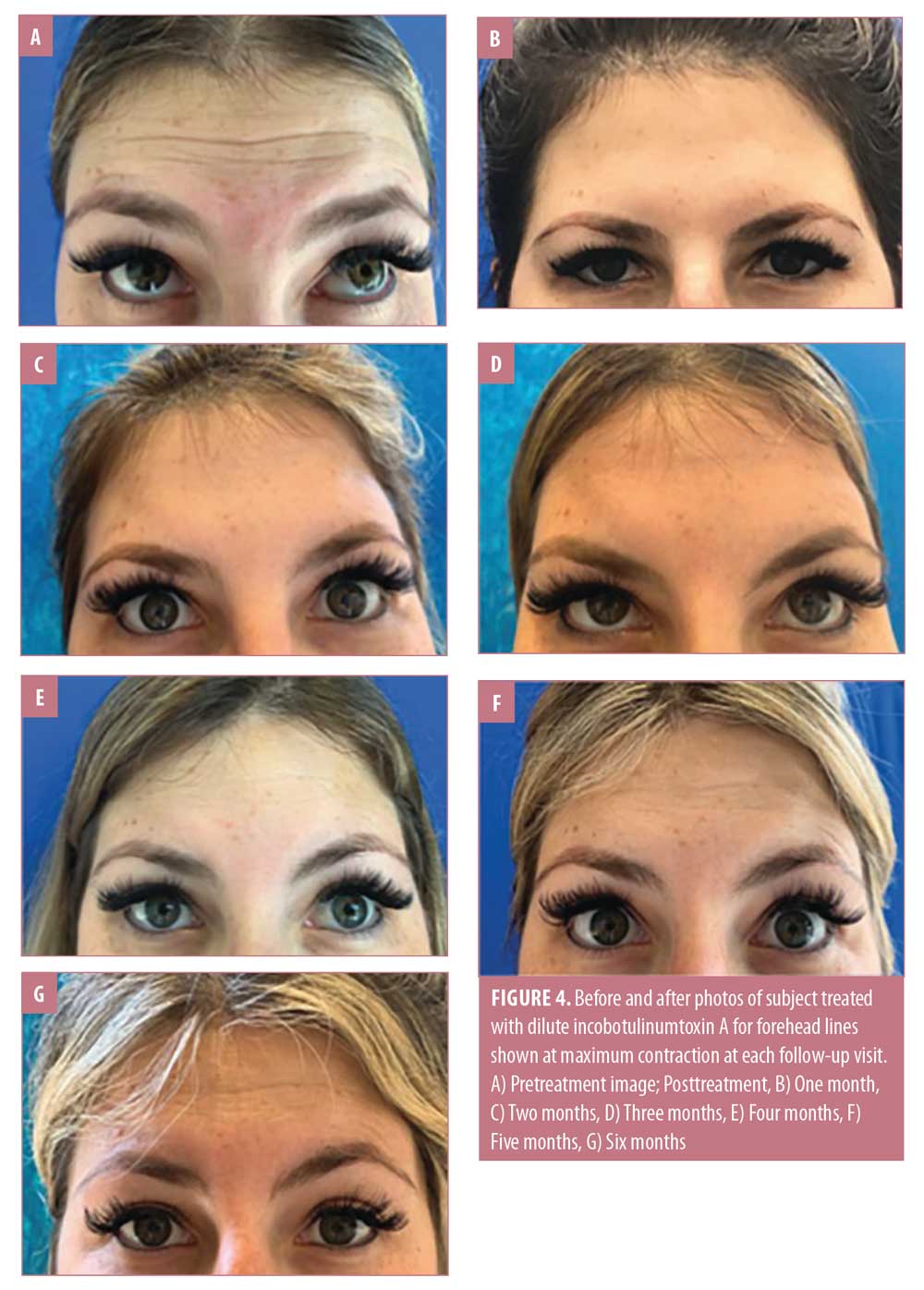
Physician GAIS scores indicated that the proportions of subjects whose appearance remained improved (improved, much improved, or very much improved) relative to at baseline was high at each subsequent visit, including 91.5 percent at four months, 78.0 percent at five months, and 57.6 percent at six months (Figure 2). Figure 4 presents photos of one patient who was treated for forehead lines and followed for six months from the initial injection, highlighting the longevity of the treatment.
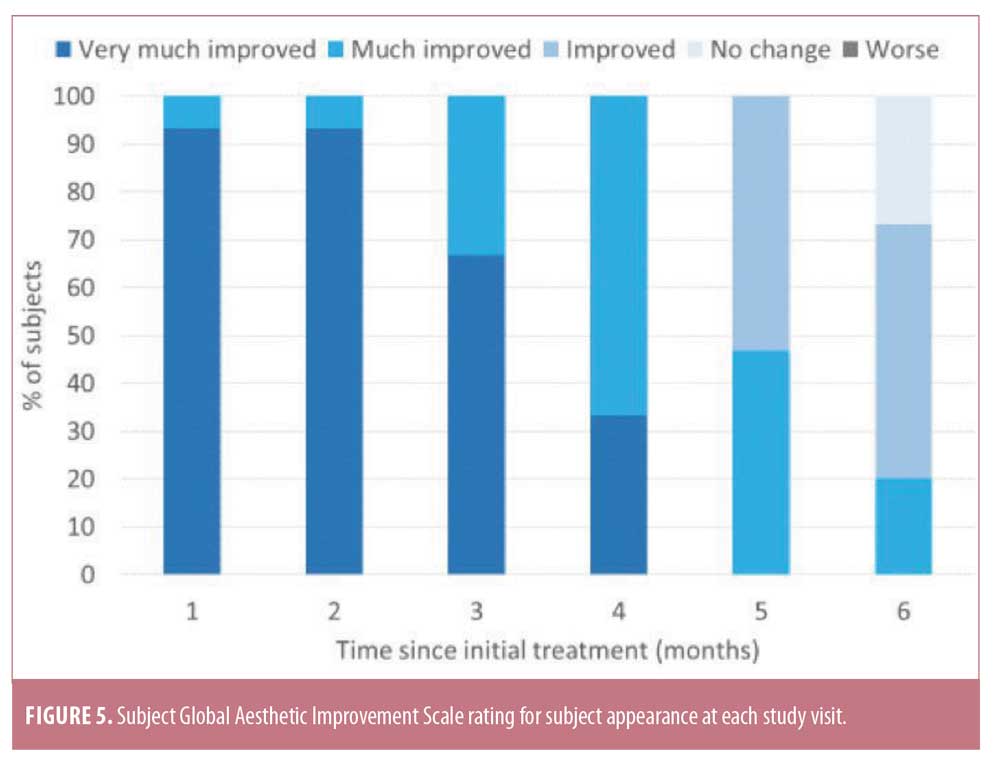
Subject GAIS results at one month were in agreement with physician GAIS scores, with 93.3 percent reporting their appearance as very much improved and 6.7 percent as much improved (Figure 5). An improved appearance continued to be reported by 100 percent of subjects at four months, with 33.3 percent reporting very much improved and 66.7 percent reporting much improved results, respectively. At five months, 100 percent of subjects continued to report an improved appearance, with 46.7 percent reporting much improved and 53.3 percent reporting improved results, while, at six months, 73.3 percent reported an improved appearance, with 20 percent reporting much improved and 53.3 percent reporting improved results. When interpreting these results, it should be kept in mind that this was a small pilot study and that these ratings are from the patient perspective.
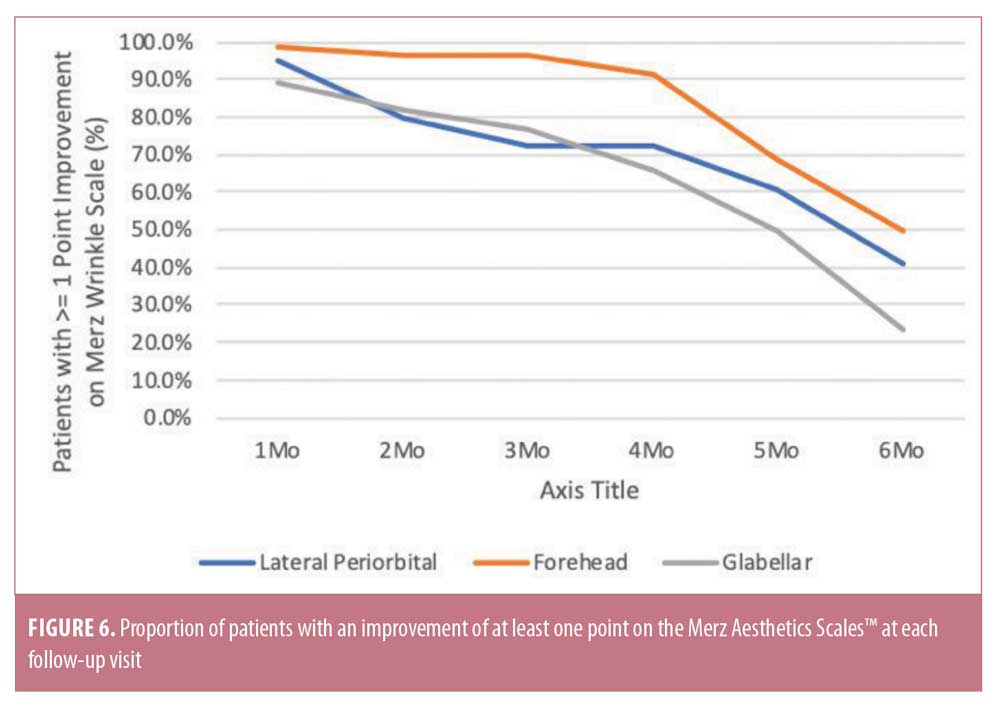
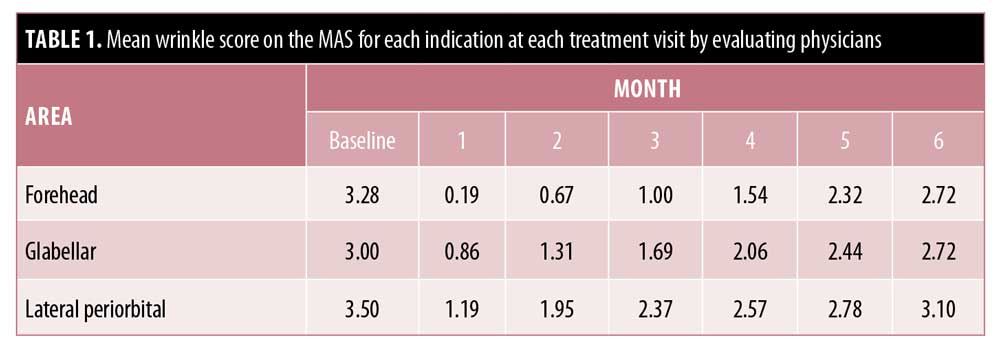
At baseline, the mean MAS scores for glabellar lines, horizontal forehead lines, and lateral periorbital lines when considering the physician ratings were 3.0, 3.28, and 3.5, respectively, indicating moderate-to-severe lines. All scores persistently remained below each patient’s initial baseline level at six months (Table 1). At one month, an improvement of at least one point in glabellar, forehead, and lateral periorbital lines was reported in 88.9 percent, 98.3 percent, and 94.8 percent of subjects, respectively. For the same indications, an improvement of at least one point was maintained at six months in 23.6 percent, 50.0 percent, and 41.1 percent of subjects. At all time points, the proportion of subjects with an improvement of at least one point was greatest for forehead lines (Figure 6).
All subjects reported improvement in their dynamic and static wrinkles in the three areas injected. Improvements were observed 3 to 7 days postinjection and were maintained through to six months. The FACE-Q™ Satisfaction with Outcome questionnaire, which rated subjects on a scale from one (most negative response) to four (most positive response), revealed that mean satisfaction was highest from 1 to 3 months (mean score: 3.7 points, range: 2.0–4.0). More than half of the participants (8/15) continued to have a score of 3.7 (range: 3.0–4.0) or more at six months, and all subjects had a score of 3.0 or more. The FACE-Q™ Perceived Age questionnaire revealed that, at one month after treatment, patients believed they looked on average 3.6 years younger than their true age; this increased to an average of 4.6 years younger than their true age at two months. At six months, patients believed they looked on average 2.7 years younger.
Treatment of all three areas was well tolerated and no adverse events suggestive of toxin spread distally to the sites of injection were reported. The injection of high-dilution INCO is necessarily associated with a larger injection volume and results in more postinjection wheals and bumps at the injection sites. However, these were short-lived and, with the application of ice posttreatment, resolved before the patients left the office.
Discussion
This case series demonstrated that the injection of INCO at a high dilution of 7.5mL of 0.9% NaCl per 100-U vial (1.3 U/0.1mL) was effective at improving patient appearance, with physician GAIS scores rating all patients as improved and 91.2 percent as very much improved at one month. Physician GAIS scores indicated that, in most patients, the appearance remained improved until at least five months and well over half (57.6%) of patients remained improved at six months. GAIS results reported by the patients were in agreement with the physicians’ evaluations, although patients tended to be less critical of their aesthetic improvement, with 73.3 percent continuing to report an improvement in their appearance at six months.
In this study, the injection of hyperdiluted INCO improved horizontal forehead, glabellar, and lateral periorbital lines both at rest and maximum contraction with a longevity in most subjects of at least four months. An interesting finding was that just under half the subjects continued to demonstrate an improvement of at least one point in their MAS score for horizontal forehead lines at six months. This area may therefore be particularly amenable to treatment with diluted INCO.
The duration of effect is an important measure, as it influences retreatment intervals as well as convenience and cost to the patient. A review by Flynn et al17 that examined the duration of effect in facial aesthetic applications for all toxins available at the time of writing (2010) reported that the duration of effect ranged from 3 to 5 months in female patients and from 4 to 6 months in male patients. In those studies that provided relapse rates, most patients had relapsed by six months. INCO was not included in the review by Flynn et al,17 but has since been widely studied for facial aesthetic indications in a range of placebo-controlled, randomized studies and head-to-head comparisons with other toxins.10,18–21 Comparator studies have shown that there is no difference in the duration of efficacy of treatment relative to ONA.18,21 One small, randomized, double-blind trial comparing the duration of the treatment effect of INCO, ONA, and abobotulinumtoxinA (ABO) for the treatment of glabellar frown lines appeared to show that INCO was associated with a longer duration of effect.22 In the current study, physician GAIS ratings showed that more than 90 percent of subjects remained improved relative to baseline at four months (the duration of most studies in the literature), and at least half remained improved at six months. All subjects reported improvement in their dynamic as well as static wrinkles in the three areas injected.
In the prescribing information for INCO, reconstitution volumes for a 100-U vial range from 0.5 to 5.0mL,5 but, to the authors’ knowledge, no studies have examined the optimal INCO dilution for aesthetic use. It is the authors’ belief that injectors generally continue to use the same dilution they were originally trained to use or the same dilution that their mentors used. A common concern is that higher dilutions will increase the tissue spread of botulinum neurotoxin, potentially influence the therapeutic effect, and cause an increase in unwanted side effects of the treatment.23 For therapeutic uses and particularly when treating larger muscles—for example, in patients with spasticity—many physicians use this to their advantage and recommend trying to increase the spreading characteristics of a toxin by using high-dilution volumes.24 However, data suggest such characteristics might differ between the botulinum toxin preparations, and particular studies using area of anhidrosis as an indication of tissue diffusion indicate that ABO appears to result in a wider spread than the other neurotoxins.25–29 There is no evidence, however, that higher reconstitution volumes result in larger action halos when treating the face with INCO or ONA. In a study designed to determine the most effective ONA dilution for the glabellar region, Carruthers et al6 found no evidence of larger action halos when using dilution volumes as high as 15mL per 100U.
The INCO prescribing information outside the United States recommends a total dose of 10 to 20U for the treatment of horizontal forehead lines. The current study used the lower end of this dosage range, injecting 10U over approximately 10 injection sites (depending on the size of the patient’s forehead). The same number of units is injected whether the toxin is reconstituted with 1mL, 2.5mL (the most commonly used dilution), or 7.5mL of NaCl—the only difference is the volume of NaCl. The precision of injection is as important as the number of units used. Injection of excess toxin is wasteful as, once the saturation point of the injected muscle is reached, further toxin will not provide additional benefit.30 A precise injection technique will provide the same result with fewer units as long as the minimal amount of units for the intended outcome are administered. Precision of injection also avoids unwanted effects, such as brow and eyelid ptosis.3 There is some evidence that INCO might have a narrower field of effect than ONA or ABO.3,29 The benefit is neuromodulation with greater precision, but an adjustment of strategy might be required for agents with a narrower field of effect by increasing dilution and/or adding additional injection points.3
There were no cases of eyelid or eyebrow ptosis with injection of high-dilution INCO in our study, which might be due to the injector never administering toxin below the lowest lateral forehead wrinkle. The higher volumes of toxin injected were associated with larger wheals at the site of injection than is commonly seen with lower volume dilutions, but these were short-lived and resolved within a maximum of 10 minutes after treatment. None of the wheals were apparent after topical application of ice and all had resolved well before the patients left the practice. There was no indication that the high dilution volumes used in this study were associated with more pain with injection.
Due to the subjective nature of cosmetic improvements, outcomes measured from a patient’s desire and satisfaction point of view are the most important variable in aesthetic medicine.31 Improvements were observed from three to seven days postinjection per patient report. What was interesting was that patient satisfaction was highest from around two months posttreatment, which reflects the desire of today’s patients to have a small amount of movement and not to appear completely devoid of expression. Patient satisfaction remained high to the end of follow-up at six months after initial treatment.
Limitations. This was a pilot study to assess patient satisfaction and efficacy with high-dilution INCO and has a number of limitations, mainly related to the small number of subjects studied. Following observations in the corresponding author’s practice, it was designed as a preliminary study to evaluate patient satisfaction with high-dilution INCO over six months. This study also used a validated scale to grade patient improvements relative to baseline administered by nontreating physicians not affiliated with the practice. Additional studies are necessary to determine whether our results can be replicated in other practices and determine which patients are best suited to treatment with high-dilution INCO (e.g., severity of presenting lines, different ethnic groups, and men). Longer-term studies will also be useful to determine the best repeat treatment interval for optimal effect. As stated earlier, the precision of the injection technique, including needle insertion placement and depth, is also important when performing neurotoxin procedures. The injection plans outlined in this paper were designed to ensure optimal placement and are similar to those recently reported with the One21 technique with INCO.32 The latter also recommends splitting the INCO dose over more injection points to provide more precision and longer lasting results and adjusting doses depending on the patient’s movement severity and size of the area being treated.32 Precision is essential when treating the face where the distance between targeted and nontargeted muscles can be incredibly small.
Conclusions
In this small case series, injection of high-dilution INCO was effective for the treatment of dynamic horizontal forehead lines, glabellar frown lines, and lateral periorbital lines and was associated with high patient satisfaction out to six months. Both patients and evaluating physicians indicated aesthetic results at six months were still improved relative to at baseline. There were no adverse events suggestive of a greater spread of toxin with the described high-dilution technique.
References
- International Society of Aesthetic Plastic Surgery (ISAPS). ISAPS international survey on aesthetic/cosmetic procedures performed in 2018. Available at: https://www.isaps.org/wp-content/uploads/2019/12/ISAPS-Global-Survey-Results-2018-new.pdf. Accessed June 2, 2020.
- The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics 2019. Available at: https://www.surgery.org/media/statistics. Accessed June 2, 2020.
- Kaminer MS, Cox SE, Fagien S, et al. Re-examining the optimal use of neuromodulators and the changing landscape: a Consensus panel update. J Drugs Dermatol. 2020;19(4):s5–s15.
- Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, et al. IncobotulinumtoxinA: a highly purified and precisely manufactured botulinum neurotoxin type A. J Drugs Dermatol. 2019;18(1):52–57.
- Xeomin prescribing information. XEOMIN—incobotulinumtoxina injection, powder, lyophilized, for solution. 2019 Merz Pharmaceuticals, LLC. Available at: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=ccdc3aae-6e2d-4cd0-a51c-8375bfee9458&type=display. Accessed June 2, 2020.
- Carruthers A, Carruthers J, Cohen J. Dilution volume of botulinum toxin type A for the treatment of glabellar rhytides: does it matter? Dermatol Surg. 2007;33(1 Spec No.):S97–S104.
- Abbasi NR, Durfee MA, Petrell K, et al. A small study of the relationship between abobotulinum toxin A concentration and forehead wrinkle reduction. Arch Dermatol. 2012;148(1):119–121.
- Muti GF, Basso M. Treatment of lateral periorbital lines with different dilutions of incobotulinumtoxinA. J Clin Aesthet Dermatol. 2017;10(9):27–29.
- Prager W, Zschocke I, Reich C, et al. Beeinflusst die Verdünnung das kosmetische Ergebnis von BoNT/A? Hautarzt. 2009;60: 815–820 [Article in German].
- Hanke CW, Narins RS, Brandt F, et al. A randomized, placebo-controlled, double-blind phase III trial investigating the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg. 2013;39(6):891–899.
- Carruthers A, Carruthers J, Coleman WP 3rd, et al. Multicenter, randomized, phase III study of a single dose of incobotulinumtoxinA, free from complexing proteins, in the treatment of glabellar frown lines. Dermatol Surg. 2013;39(4):551–558.
- Trindade De Almeida AR, Secco LC, Carruthers A. Handling botulinum toxins: an updated literature review. Dermatol Surg. 2011;37(H):1553–1565.
- de Sa Earp AP, Marmur ES. The five D’s of botulinum toxin: doses, dilution, diffusion, duration and dogma. J Cosmet Laser Ther. 2008;10(2):93–102.
- Flynn TC, Carruthers A, Carruthers J, et al. Validated assessment scales for the upper face. Dermatol Surg. 2012;38(2 Spec No.):309–319.
- Panchapakesan V, Klassen AF, Cano SJ, et al. Development and psychometric evaluation of the FACE-Q aging appraisal scale and patient-perceived age visual analog scale. Aesthet Surg J. 2013;33(8):1099–109.
- Klassen AF, Cano SJ, Schwitzer J, et al. FACE-Q scales for health-related quality of life, early life impact and satisfaction with outcomes and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135(2):375–386.
- Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. Am J Clin Dermatol. 2010;11(3):183–199.
- Kane MA, Gold MH, Coleman WP 3rd, et al. A randomized, double-blind trial to investigate the equivalence of incobotulinumtoxinA and onabotulinumtoxinA for glabellar frown lines. Dermatol Surg. 2015;41(11):1310–1319.
- Kerscher M, Rzany B, Prager W, et al. Efficacy and safety of incobotulinumtoxinA in the treatment of upper facial lines: results from a randomized, double-blind, placebo-controlled, phase III study. Dermatol Surg. 2015;41(10):1149–1157.
- Jones D, Carruthers J, Narins RS, et al. Efficacy of incobotulinumtoxinA for treatment of glabellar frown lines: a post hoc pooled analysis of 2 randomized, placebo-controlled, phase 3 trials. Dermatol Surg. 2014;40(7):776–785.
- Sattler G, Callander MJ, Grablowitz D, et al. Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg. 2010;36(Suppl 4):2146–2154.
- Rappl T, Parvixi D, Friedl H, et al. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double-blind study. Clin Cosmet Investig Dermatol. 2013;6:211–219.
- Hsu TS, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol. 2004;140(11):1351–1354.
- Lim EC, Seet RC. Botulinum toxin: description of injection techniques and examination of controversies surrounding toxin diffusion. Acta Neurol Scand. 2008;117(2):73–84.
- Trinidade de Almeida AR, Marques E, de Almeida J, et al. Pilot study comparing the diffusion of two formulations of botulinum toxin type A in patients with forehead hyperhidrosis. Dermatol Surg. 2007;33(1 Spec No):S37–S43.
- Cliff SH, Judodihardjo H, Eltringham E. Different formulations of botulinum toxin type A have different migration characteristics: a double-blind, randomized study. J Cosmet Dermatol. 2008;7(1):50–54.
- Hexsel D, Dal’Forno T, Hexsel C, et al. A randomized pilot study comparing the action halos of two commercial preparations of botulinum toxin type A. Dermatol Surg. 2008;34(1):52–59.
- Kerscher M, Roll S, Becker A, Wigger-Alberti W. Comparison of the spread of three botulinum toxin type A preparations. Arch Dermatol Res. 2012;304(2):155–161.
- da Costa A, Pereira ESP, Pereira MO, et al. Six-month comparative analysis monitoring the progression of the largest diameter of the sweating inhibition halo of different botulinum toxins Type-A. Aesthet Surg J. 2019;39(9):993–1004.
- Dressler D, Adib Saberi F. Towards a dose optimisation of botulinum toxin therapy for axillary hyperhidrosis: comparison of different Botox(®) doses. J Neural Transm (Vienna). 2013;120(11):1565–1567.
- Sears DE, Chung KC. A guide to interpreting a study of patient-reported outcomes. Plast Reconstr Surg. 2012;129(5):1200–1207.
- De Sanctis Pecora C. One21: A novel, customizable injection protocol for treatment of the forehead with incobotulinumtoxinA. Clin Cosmet Investig Dermatol. 2020;13: 127–136.

