J Clin Aesthet Dermatol. 2021;14(10):25–26.
 by Soumaya Gara, MD; Noureddine Litaiem, MD; Takwa Bacha, MD; Yosra Jmour, MD; and Faten Zeglaoui, MD
by Soumaya Gara, MD; Noureddine Litaiem, MD; Takwa Bacha, MD; Yosra Jmour, MD; and Faten Zeglaoui, MD
All authors are with the Department of Dermatology, Charles Nicolle Hospital in Tunis, Tunisia.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Mycosis fungoides (MF) is the most common type of cutaneous T cell lymphoma. Phototherapy is a first-line treatment option of early stages MF. The present study aimed at assessing the efficacy of phototherapy in Tunisian patients with MF treated with phototherapy and evaluate the efficacy of maintenance phase.
Key words: Mycosis fungoides, PUVA therapy, NB-UVB therapy, maintenance phase
Phototherapy has been a mainstay of treatment of mycosis fungoides (MF) and is recommended as a first-line therapy for early-stage (Ia–IIa) MF. However, there is no internationally standardized phototherapy regimen schedule for MF, and questions regarding the efficacy of maintenance phototherapy persist.1
To assess the efficacy of maintenance phototherapy, we performed a retrospective cohort study including patients diagnosed with early-stage MF over a 12-year period.
Methods
Patients underwent a whole-body phototherapy course (three sessions/week) of psoralen (0.6 mg/kg) and ultraviolet (UV) A (PUVA) therapy (320–400 nm) or narrow-band UV B (NB-UVB) therapy (311–313 nm). The regimen was sustained until complete clinical clearance (induction phase), then continued for four weeks (consolidation phase). Maintenance therapy was applied at the end of the consolidation phase once weekly for two weeks, followed by once every two weeks for eight weeks and then once every four weeks. A complete response was defined as clearing of 100% of lesions, a partial response was defined as clearing of 50% to 99% of lesions, and a therapeutic failure was defined as clearing of less than 50% of lesions. Patients who received treatments prior to phototherapy were excluded from the study.
Results
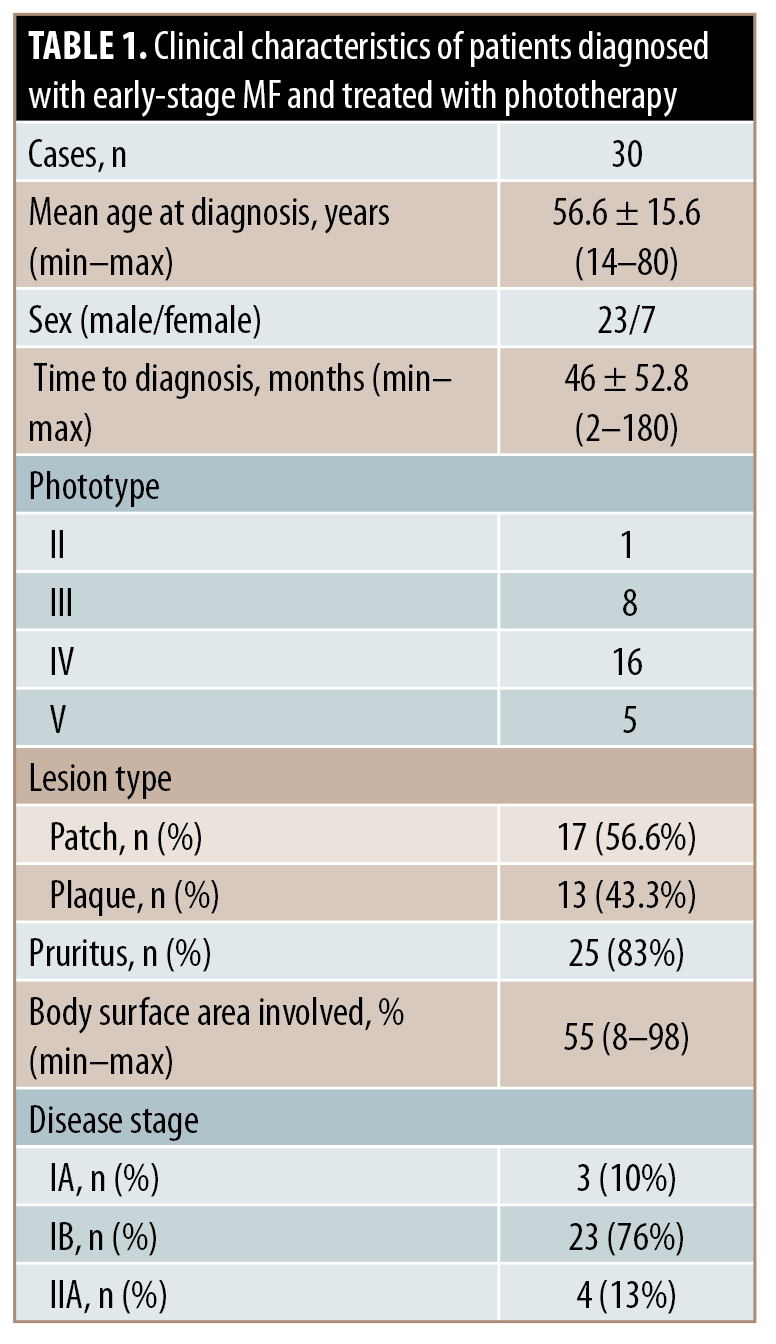
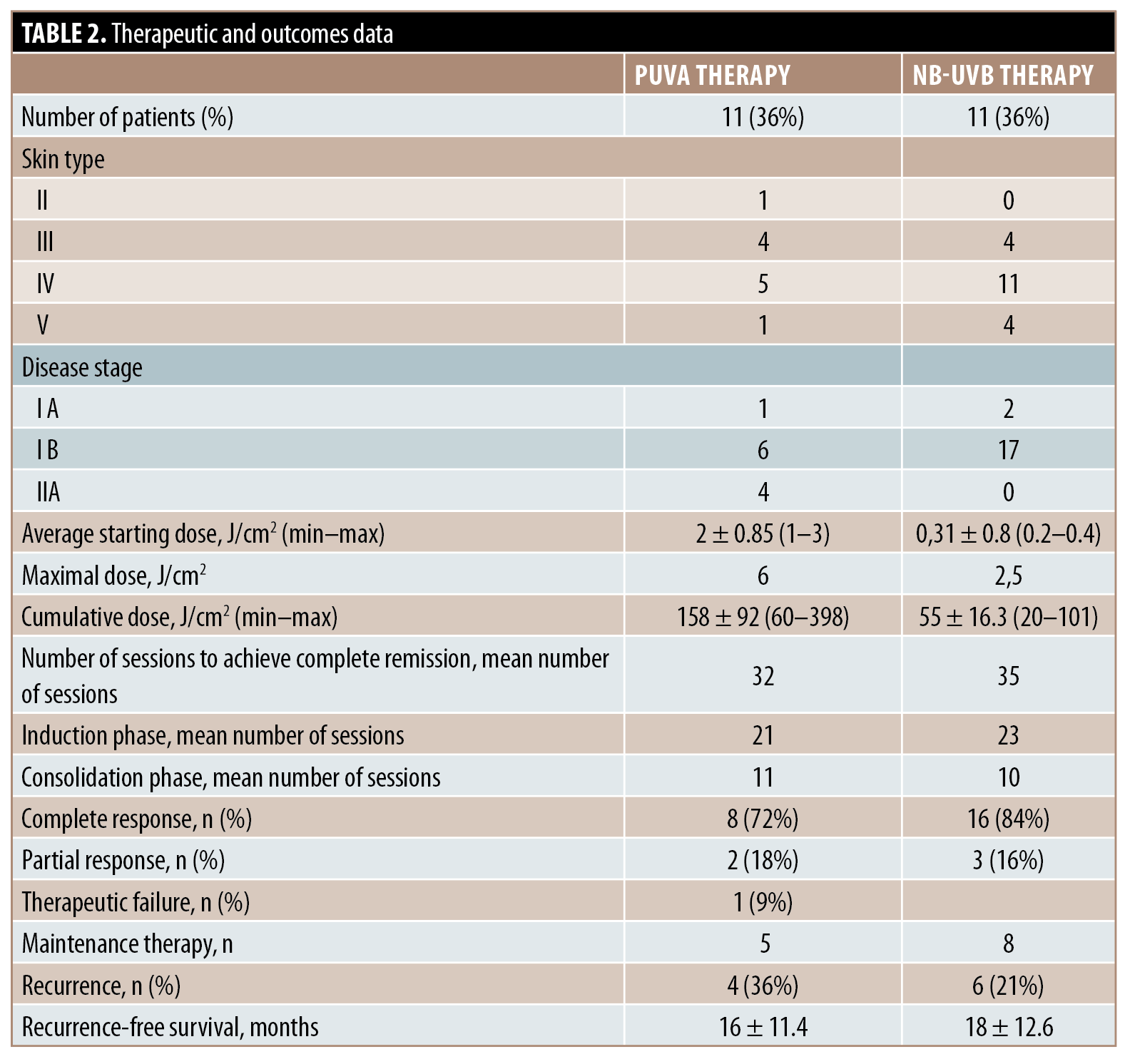
Thirty patients were enrolled. The demographic data and clinical features are shown in Table 1. PUVA therapy and NB-UVB therapy were applied in 11 and 19 cases, respectively. Therapeutic and outcomes data are summarized in Table 2. There was no significant association between rates of recurrence and maintenance phototherapy (P=0.64). There was no significant difference between recurrence-free survival in patients who received maintenance therapy and those who underwent follow-up in both the PUVA (P=0.63) and NB-UVB (P=0.3) subgroups.
Discussion
In this study, response rates were comparable with previous reports, despite including an important proportion of patients with dark skin types.2 Recurrence rates and recurrence-free survival were not significantly different between the groups according to the receipt of maintenance therapy.
The benefits of maintenance PUVA therapy are still contested. To date, only rare single-center studies have evaluated recurrence rates and recurrence-free survival in patients who received maintenance therapy,3,4 and none of these studies reported a significant difference in either recurrence rate or recurrence-free survival. Maintenance NB-UVB therapy is also still debated. Encouraging results were reported in a limited number of studies.5 These results should, however, be interpreted with caution due to distinct maintenance regimens.
There are several concerns regarding maintenance phototherapy. Evidence of its efficacy is limited. Besides, it was hypothesized that UV phototherapy may promote the selection of treatment-resistant clones. Most importantly, UV phototherapy (especially PUVA) is linked with skin aging and increased incidence of non-melanoma skin cancers in a dose-dependent manner. Patients with early-stage MF have a life expectancy similar to that of an age- and sex-matched population. Therefore, maintenance therapy could expose patients to treatment-related adverse events without offering significant clinical advantages.
Limitations. Limitations of the present study include the relatively small number of enrolled patients as well as its retrospective and monocentric nature, which may lead to missing data and selection bias. Our results may also be confounded by indication as maintenance therapy could have been encouraged for patients with more extensive or severe disease.
Conclusion
Phototherapy is a safe and effective treatment option for patients with early-stage MF, regardless of patient phototype. The treatment regimen should include induction and consolidation phases; the aim of this latter phase is to treat subclinical lymphoma. Evidence supporting the use of maintenance phase for the treatment of early-stage MF is still lacking.
References
- Grandi V, Fava P, Rupoli S, et al. Standardization of regimens in narrowband UVB and PUVA in early stage mycosis fungoides: position paper from the Italian Task Force for Cutaneous Lymphomas. J Eur Acad Dermatol Venereol. 2018;32(5):683–691.
- Almohideb M, Walsh S, Walsh S, et al. Bath psoralen-ultraviolet A and narrowband ultraviolet B phototherapy as initial therapy for early-stage mycosis fungoides: a retrospective cohort of 267 cases at the University of Toronto. Clin Lymphoma Myeloma Leuk. 2017;17(9):604–612.
- Sánchez MA, González T, Gaitán MF, et al. Is PUVA maintenance therapy necessary in patients with early-stage mycosis fungoides? Evaluation of a treatment guideline over a 28-month follow-up. Int J Dermatol. 2011;50(9):1086–1093.
- Hernández Z, Peñate Y, Hernández-Machín B, et al. Treatment of stage Ia and Ib mycosis fungoides with psoralen UVA monotherapy: an observational study in tertiary hospitals in the Canary Islands. Int J Dermatol. 2014;53(11):1417–1422.
- Vieyra-Garcia P, Fink-Puches R, Porkert S, et al. Evaluation of low-dose, low-frequency oral psoralen-UV-A treatment with or without maintenance on early-stage mycosis fungoides: a randomized clinical trial. JAMA Dermatol. 2019;155(5):538–547.

