 J Clin Aesthet Dermatol. 2021;14(10):19–24.
J Clin Aesthet Dermatol. 2021;14(10):19–24.
by Barry I. Galitzer, MD
Dr. Galitzer is with the Skin Center in Fort Lauderdale, Florida.
FUNDING: This study was supported by Biofrontera, Inc.
DISCLOSURES: The author reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Actinic keratoses (AKs) are sun-induced cutaneous lesions that may progress to squamous cell carcinoma (SCC). Photodynamic therapy (PDT) is an ideal treatment option for AKs because it allows for treatment of field cancerization, selective destruction of diseased tissue, good cosmetic outcomes, and limited downtime.
Objectives. This study sought to determine the efficacy and safety of pretreating AKs of the dorsal hands and forearms with adapalene gel, an inexpensive and over-the-counter retinoid, prior to debridement of the target area and PDT with aminolevulinic hydrochloride acid (ALA, 10%) gel and narrowband red light.
Methods. Fifteen patients with AK lesions of the right or left dorsal hands or forearms were pretreated with adapalene gel (0.1%) twice daily for one week prior to ALA-PDT. The other hand or forearm was treated with ALA-PDT (standard therapy), but not pretreated. For PDT, all treated areas were debrided with sandpaper, degreased with acetone, incubated for one hour with 10% ALA gel under occlusion, and illuminated with narrowband red light (~635 nm). All patients experienced one PDT treatment session.
Results. Eight weeks after treatment, 12 subjects in the adapalene-pretreated group achieved 50% to 100% clearance compared to 10 subjects in the standard therapy group. The median lesion count reduction in the adapalene-pretreated group was -79% compared to -57% in the standard therapy group, and this difference was significant (P=0.0164). The treatment was well-tolerated and the level of patient satisfaction was high.
Conclusion. Pretreatment with adapalene gel twice daily for one week may enhance efficacy in a single ALA-PDT treatment of AK lesions of the dorsal hand or forearm.
Key words: Cutaneous lesions, field cancerization, photosensitizing agent, protoporphyrin, lesion count
Actinic keratoses (AKs) are sun-induced cutaneous lesions that may progress to squamous cell carcinoma (SCC).1 Although the risk that a single AK lesion will progress to SCC is variable,2,3 patients often have multiple AK lesions, and the overall risk of progression increased. Therefore, the primary goal of AK treatment is to completely clear all visible and subclinical lesions.4
Lesion-directed therapies, such as cryotherapy, curettage, and lasers, although effective against visible AKs, do not address field cancerization and may lead to high recurrence rates.5 Photodynamic therapy (PDT) is an ideal treatment option for AKs because it allows for treatment of field cancerization, selective destruction of diseased tissue, good cosmetic outcomes, and limited downtime.6
In PDT, a photosensitizing agent is applied topically and activated by visible light. Aminolevulinic acid (ALA) is a precursor in the heme pathway and is converted to the photosensitizer protoporphyrin IX (PpIX) in the skin. When exposed to red or blue light, PpIX generates reactive oxygen species, which are cytotoxic and produce an inflammatory response.7
The objective of the present study was to determine the efficacy and safety of pretreating AKs of the dorsal hands or forearm with adapalene gel (0.1%) prior to broad-area PDT with ALA (10%) gel and narrowband red light. Adapalene was chosen as the retinoid to be used in this study because it is inexpensive and available over the counter.
Methods
Subjects. Nine men and six women (n=15 participants) aged (mean ± standard deviation) 65.4 ± 9.3 years with AK lesions on the dorsal hand or forearm were enrolled in this study. Eligible individuals had at least five AK lesions (grade 1 or 2) in an area that included the extensor surface of the hand/forearm between the elbow and the base of the fingers. Skin types were II (n=11) and III (n=4). Women were not pregnant, were surgically sterile, or were using a medically acceptable form of birth control.
Individuals were excluded if they had porphyrin abnormalities, sensitivity to trial constituents, or a skin condition that could interfere with clinical evaluations; used tanning salons or photosensitizing drugs; had undergone recent procedures or taken topical medications directed at the treatment area; were participating in other trials; or had human immunodeficiency viral infection or another condition that required immunosuppressive therapy. All subjects provided signed informed consent for treatment, and this study was approved by an institutional review board.
Treatment. This was a single-center, randomized, bilateral comparison (right vs. left) study. At the first visit (Visit 1), qualified subjects were randomized so that one dorsal hand or forearm was pretreated with adapalene gel (0.1%, Differin Gel; Galderma Laboratories, LP, Fort Worth, Texas) twice daily for one week and the other dorsal hand or forearm was not pretreated. Seven days later (Visit 2), both the pretreated area and corresponding untreated area were debrided with sandpaper and scrubbed twice with acetone before the application of 10% ALA gel (Ameluz®; Biofrontera Inc., Woburn, Massachusetts) over the entire surface. Ameluz® gel, in combination with PDT using a narrowband red light (BF-RhodoLED® lamp; Biofrontera Inc.), is indicated for lesion-directed and field-directed treatment of AKs of mild-to-moderate severity on the face and scalp.
The ALA-treated areas were occluded with plastic wrap for one hour. Care was taken to avoid sunlight or high-intensity light radiation. After one hour, the occlusive dressing was removed and the treated area was cleaned with a mild cleanser, washed thoroughly with water, and patted dry.
Treatment areas were then exposed to red light continuously for 10 minutes. Discomfort during light exposure was graded by the subject on a scale of 0 to 10 immediately after treatment. The emitted wavelength of the light source (~635 nm) and light dose (37 J/cm2) were fixed by the manufacturer. Discomfort (stinging/burning) during light exposure was minimized by constant air flow with an integrated fan. Immediately after light treatment, the treated areas were cleaned with a mild cleanser and water and patted dry. Sunblock (Vanicream Sport SPF 35; Pharmaceutical Specialties Inc., Rochester, Minnesota) was applied, and each subject was advised to protect the treated skin from sunlight or prolonged or intense light by wearing protective clothing and applying sunblock regularly for the next 48 hours.
One or two days after PDT, subjects were contacted by telephone (Visit 3) and asked to report adverse events or other issues related to the study. Eight weeks later, subjects received a final clinical evaluation (Visit 4) in the physician’s office and were asked to comment on their satisfaction with results, acceptability and convenience of treatment, and adverse events.
Evaluation of the results. Since data were not always normally distributed, as shown by the Shapiro–Wilk test, nonparametric methods were used to test for significant differences between adapalene and standard therapy (ALA-PDT) results. The cutoff level for significance was P less than or equal to 0.05. When nonindependent comparisons were made, Bonferroni correction was applied, in which the cutoff value was divided by the number of nonindependent comparisons to arrive at a new cutoff value. For example, if two nonindependent comparisons were made, the cutoff value was 0.05/2=0.025.
Lesions were graded during Visit 1 according to the scale in Table 1.8
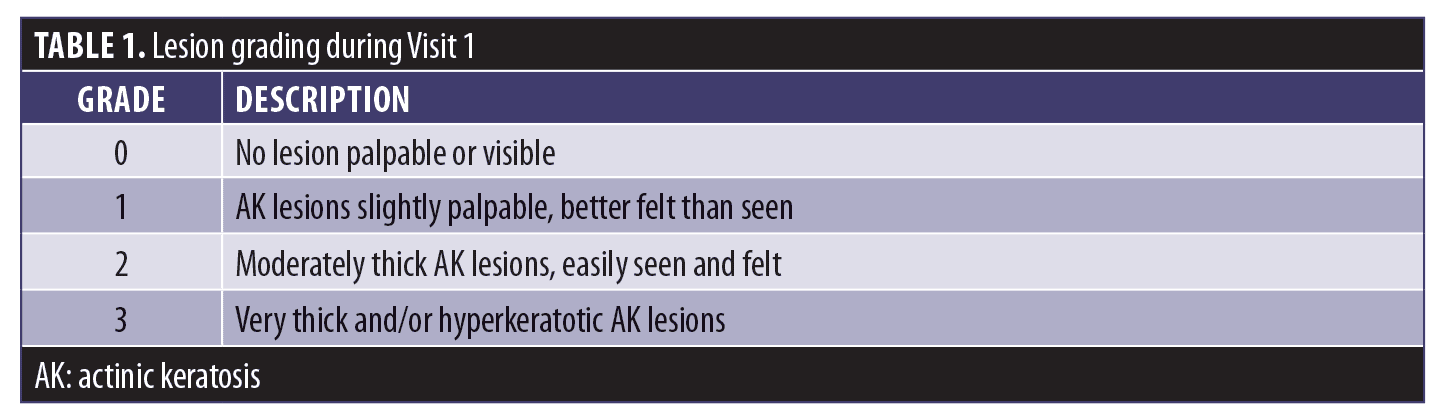
The efficacy of treatment was evaluated by three methods; the first aimed to compare lesion counts of the treatment area before ALA-PDT (Visit 1=baseline) with counts at Visit 2 (immediately before ALA-PDT) and at Visit 4 (end of study, eight weeks after PDT) for both the adapalene and standard therapy treatment arms.
The second method was to compare the proportions of responders falling into six categories of percent change of lesion count from baseline (-100%, -75% to -99%, -50% to -74%, -25% to -49%, 0% to -24%, and <0%) using Pearson’s chi-squared test. In these cases, negative percentages indicated improvement. The percent change in lesion count (LC) for each subject was calculated for each subject using the following formula:
Change (%) = (LC [Visit 2 or Visit 4] – LC [Visit 1]) × 100)/LC (Visit 1)
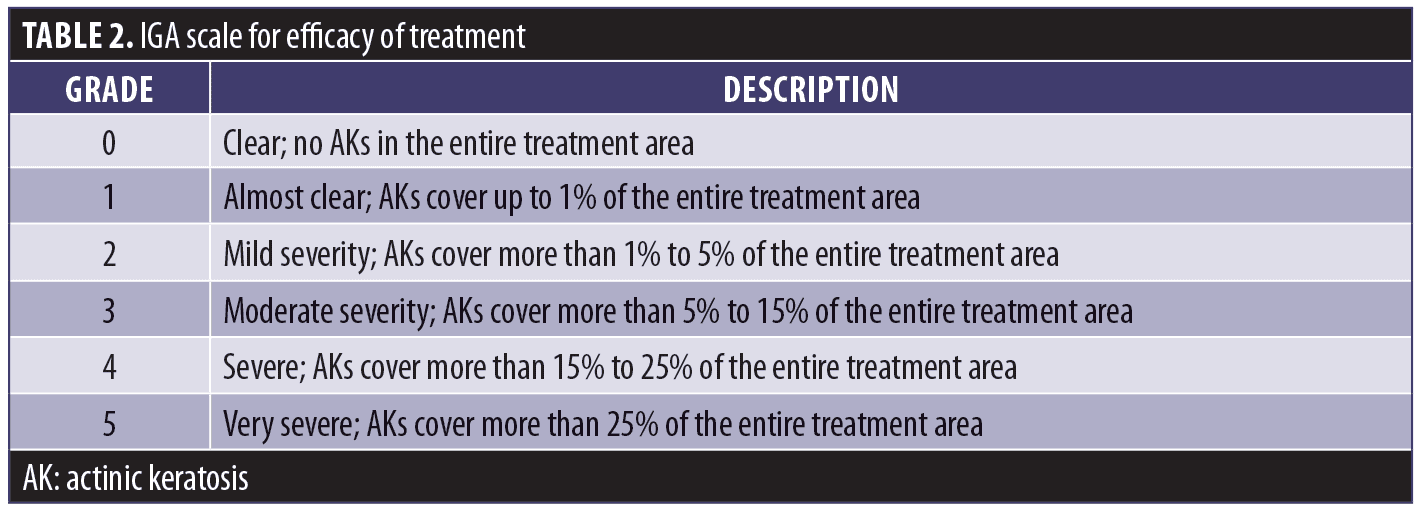
The third was to compare the Investigator Global Assessment (IGA) scores of the adapalene arm and the standard therapy arm at Visit 4. Differences were tested for significance using the Wilcoxon signed-rank test.
The IGA scale for efficacy is shown in Table 2.
Safety. Tolerability and adverse events (erythema, edema, stinging/burning, itching, scaling, crusting, vesicles) were assessed as mild, moderate, or severe.
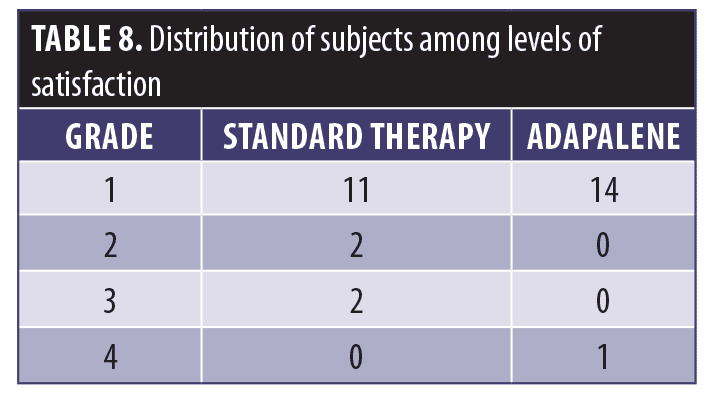
Satisfaction. Subject satisfaction was determined at the end of the study according to a four-point scale, where one point indicated excellent satisfaction, two points indicated moderate satisfaction, three points indicated slight satisfaction, and four points indicated dissatisfaction/no satisfaction at all. Differences in the proportions of responders falling into the four categories were tested for significance with Pearson’s chi-squared test.
The convenience and acceptability of the treatment were determined by asking subjects to evaluate in-office time, adverse effects (pain, crusts), and duration of adverse effects associated with treatment.
Results
All 15 subjects completed the study. At Visit 1, 176 lesions were included on the adapalene side and 185 lesions were included on the standard therapy side. Three subjects were late (3–16 weeks) for their final visit. Lesions in the treatment area were of grade 1 or 2 and slightly palpable.
Efficacy. The total AK lesion clearance rate eight weeks after PDT was 64.8% on the adapalene-pretreated side compared to 54.6% of AKs on the standard therapy side. AK lesion counts at Visit 1 were compared with counts at both Visit 2 and Visit 4 by Friedman’s test, a nonparametric counterpart of analysis of variance. Variation was expressed as an interquartile range (IQR) (the 75th percentile minus the 25th percentile), which corresponds to the parametric standard deviation. The results are shown in Table 3.
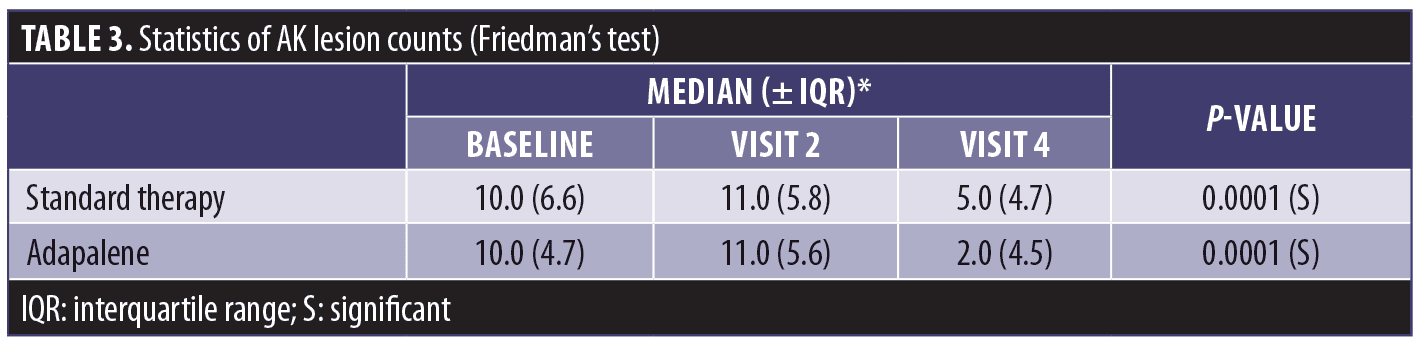
Friedman’s test showed that, for both the standard therapy and adapalene-pretreated groups, the AK lesion counts among the three time points for each group did not share the same median value (P=0.0001). Separate comparisons with baseline by the Wilcoxon signed-rank test revealed that, in the adapalene group, the median at Visit 2 did not differ significantly from the Visit 1 baseline (P=0.1602) while the Visit 4 value was significantly lower (P=0.0002). In the standard therapy treatment arm, the corresponding values were P=0.1783 and P=0.0002, respectively.
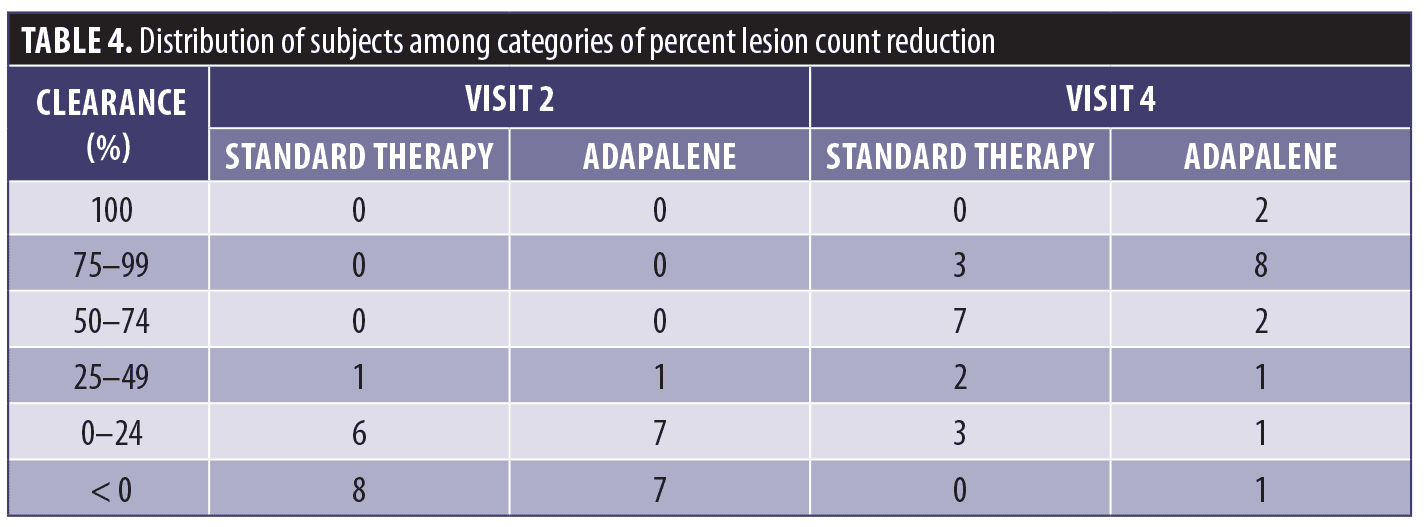
Proportions of responders falling into six categories of percent change in lesion counts from baseline were compared using Pearson’s chi-squared test (Table 4). Although differences were not significant, for Visit 4, 50% to 100% clearance was recorded for 12 subjects in the adapalene group compared to 10 subjects in the standard therapy group.
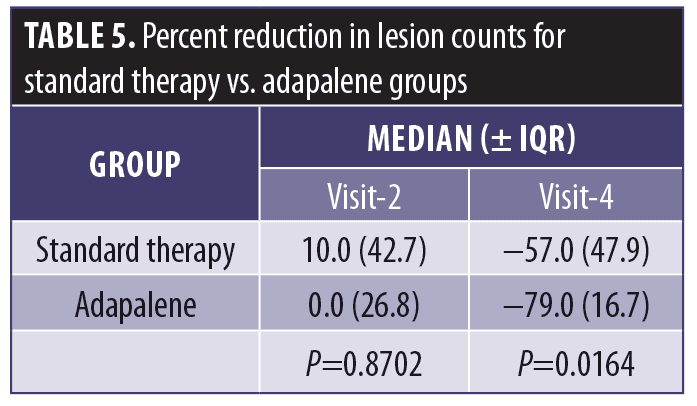
Lesion count reductions with adapalene and standard therapy were compared with each other directly (Table 5). At Visit 4, the median percent reduction in the adapalene group was greater than that in the standard therapy group (-79% vs. -57%, respectively), representing a difference that was statistically significant (P=0.0164).
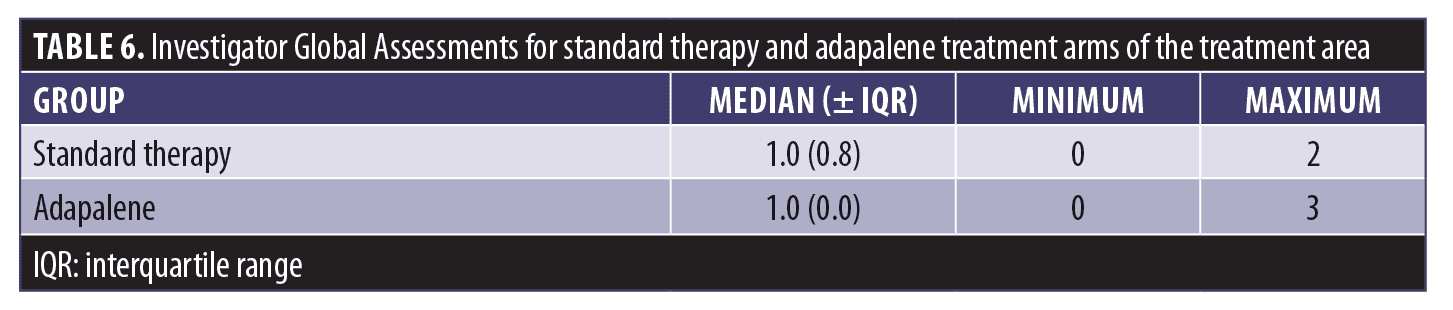
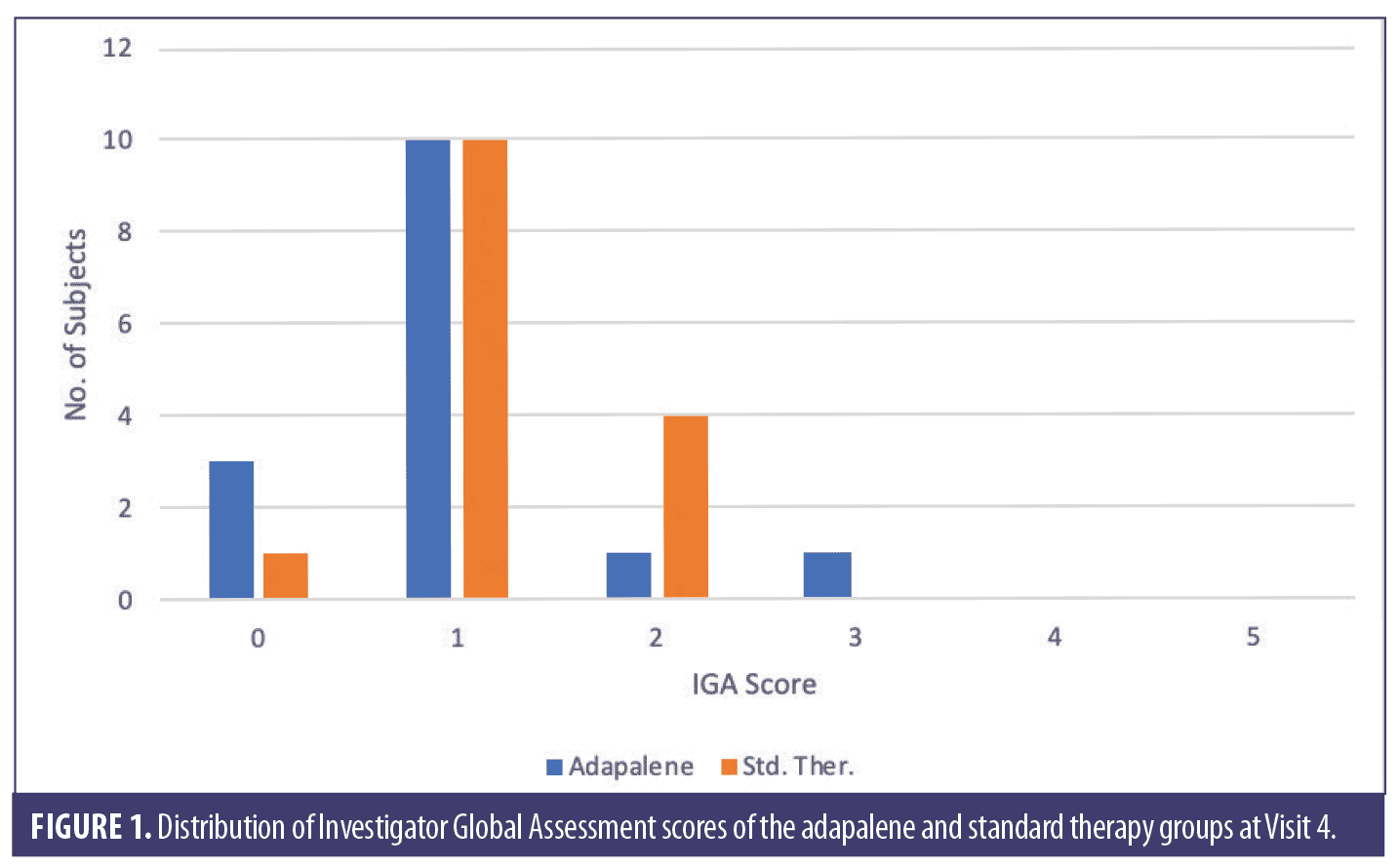
Eight weeks after PDT, IGA values for the standard therapy and adapalene groups were assessed and results were compared using the Wilcoxon signed-rank test. The median values did not differ significantly (Table 6). The proportions of subjects among the six IGA grades (grades 0–5) of the adapalene group did not differ significantly from the proportions in the standard therapy group as shown by the Pearson’s chi-squared test (Figure 1). Three adapalene-pretreated subjects scored zero points (clear) compared to just one subject who did so in the standard therapy group.
Safety. Subjects scored tolerability parameters (erythema, edema, induration, stinging/burning, itching, scaling, crusting, vesicles) five minutes after light treatment. Most subjects (n=13) reported “mild” for all parameters on both standard therapy and adapalene sides. One subject reported moderate erythema, stinging/burning, and itching, while another reported moderate itching on both the standard therapy and adapalene sides.
At Visit 3 (done by telephone 24–48 hours after PDT), subjects reported discomfort (n=4); itching (n=1); slight burning, erythema, and tingling (n=4); or none of the aforementioned (n=6).
At Visit 4 (eight weeks after PDT), erythema, edema, induration, stinging/burning, itching, scaling, crusting, and vesicles were absent in all subjects.
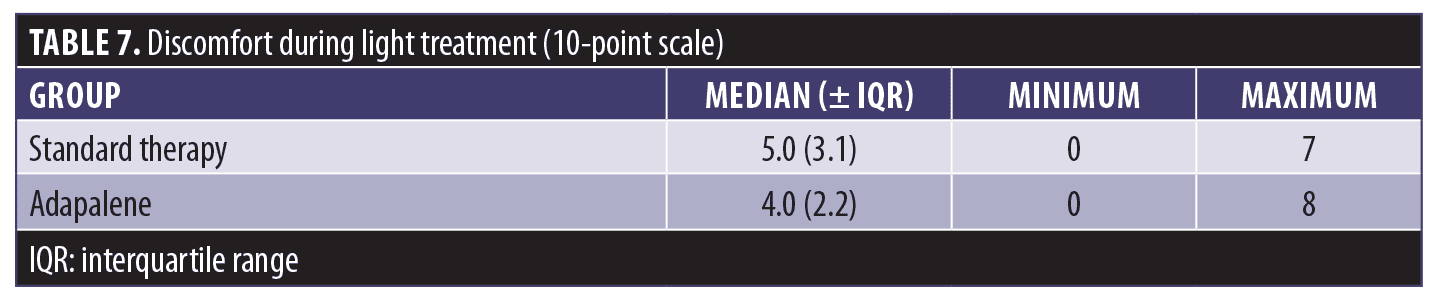
Discomfort during red light illumination (Table 7) was slightly greater with the standard therapy, but the difference did not achieve significance. Scores varied from 0 to 8 points for the adapalene treatment and from 0 to 7 points for standard therapy.
Satisfaction. As shown in Table 8, although differences in the proportions of responders in the four categories were not significant, 14 subjects rated the adapalene-pretreated side as “excellent” compared to 11 subjects on the standard therapy side.
Regarding convenience and acceptability of the treatment, all subjects agreed that the in-office time, side effects, and duration of side effects were acceptable.
Clinical examples are shown in Figures 2 through 4.
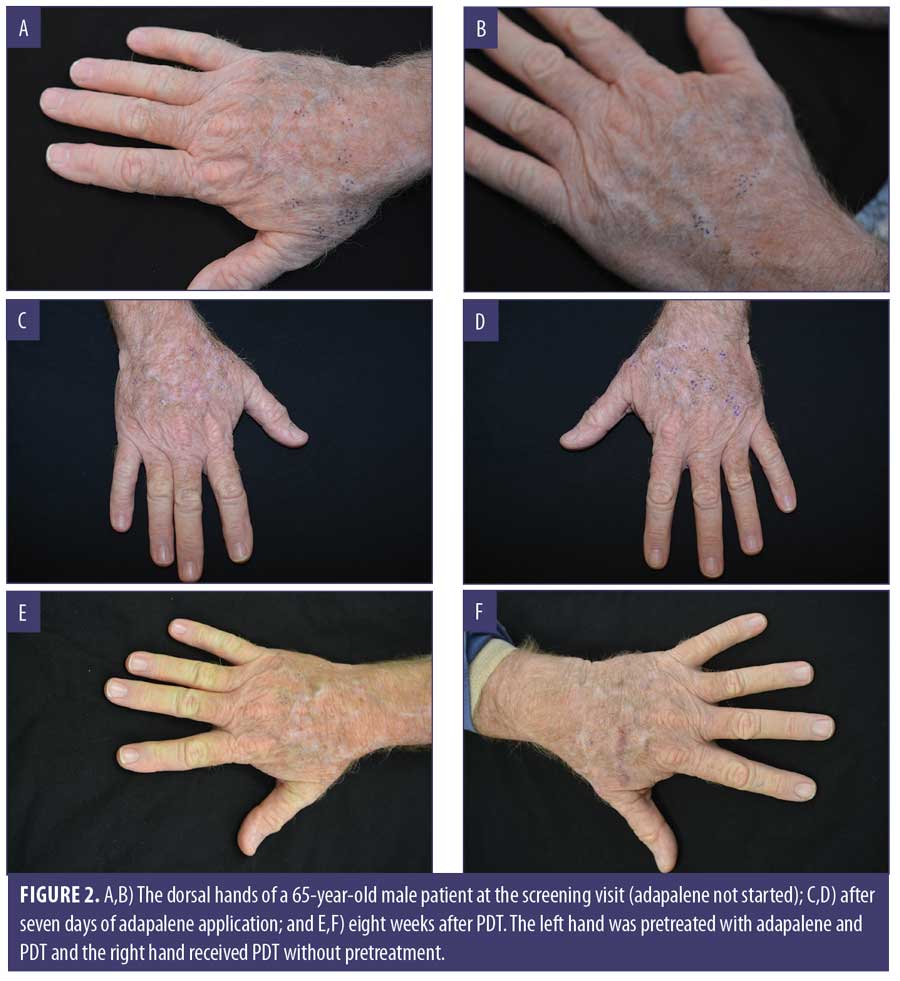
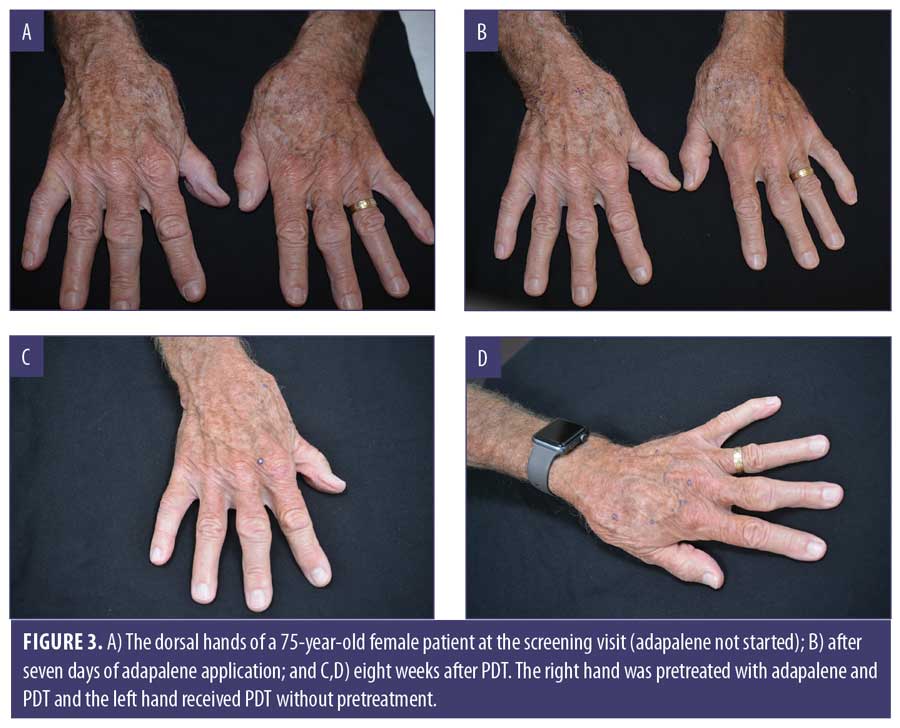
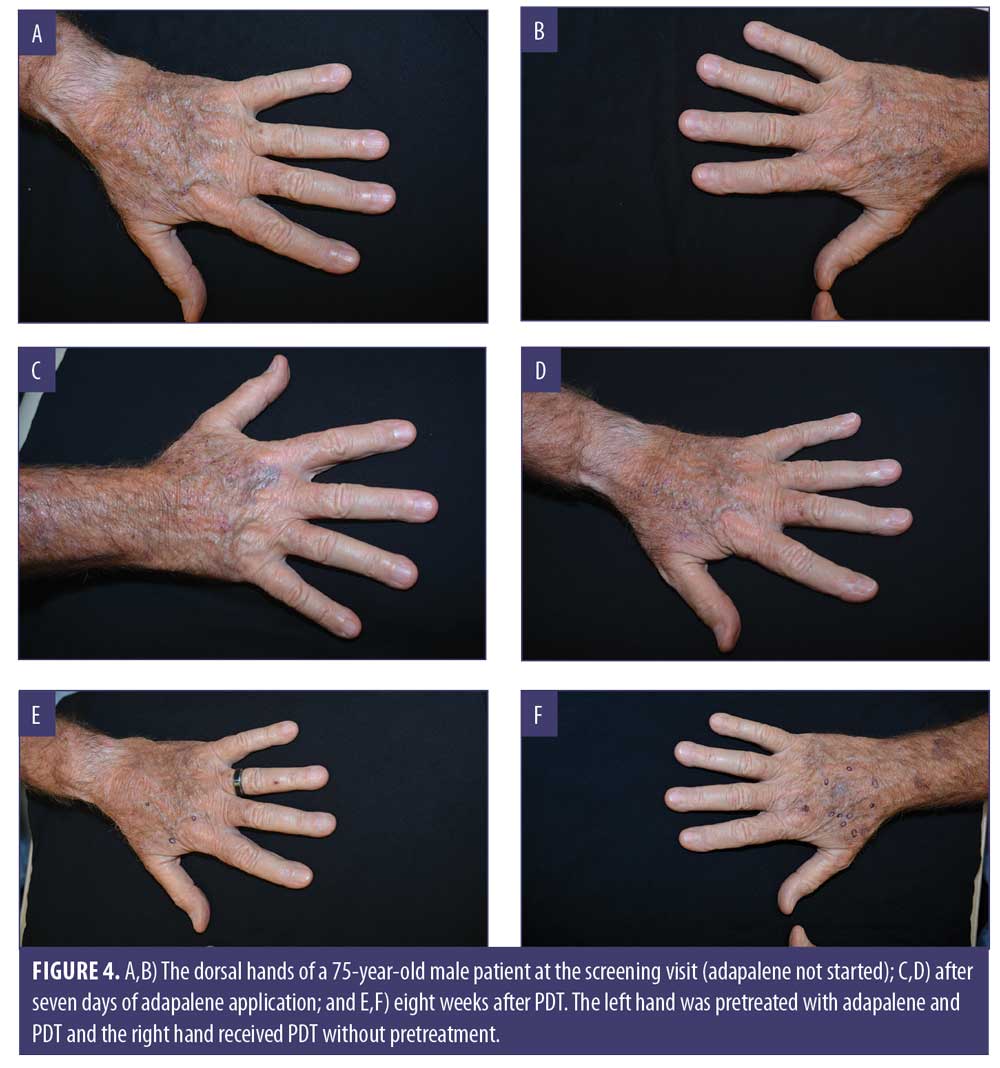
Discussion
In this study, AK lesions of the dorsal hand and forearm were treated with PDT using 10% ALA gel in combination with red light and adapalene pretreatment. At eight weeks (Visit 4), the total AK lesion count for the adapalene-pretreated group decreased by 64.8% compared to 54.6% for the standard therapy group. Both reductions were significant (P=0.0002) compared to baseline. A lesion clearance rate of at least 50% was achieved by 12 subjects in the adapalene group compared to 10 subjects in the standard therapy group. A lesion clearance rate of at least 75% clearance was achieved by 10 subjects in the adapalene group compared to three subjects in the standard therapy group. Three adapalene-pretreated subjects achieved an IGA score of 0 points (clear) at Visit 4 compared to a single subject in the standard therapy group.
These data suggest that pretreatment with adapalene may, in most cases, enhance efficacy over standard therapy. This trend is supported by the data in Table 5, in which the median percent reduction in the adapalene-pretreated group exceeded that in the standard therapy group (-79% vs. -57%, respectively), and the difference was statistically significant (P=0.0164).
It is possible that a keratolytic action on the skin due to adapalene pretreatment may have improved penetration of ALA through the stratum corneum, thus enhancing the efficacy. The decision to pretreat with adapalene was based on its documented efficacy for the treatment of AK9,10; the well-established efficacy and safety of the ALA 10% gel-red light combination for PDT11–14; and the encouraging results of an earlier study in which AK lesions on the hand and forearm were pretreated with another retinoid, tazarotene, before ALA-PDT.15
Variations from the 2011 study15 include the use of adapalene, a retinoid available without prescription; debridement of the area to be treated before ALA application; use of ALA 10% gel rather than ALA 20% gel; and illumination with red instead of blue light.
In the 2011 study,15 AKs of the dorsal hand or forearm were pretreated with tazaratene gel (0.1%) twice daily for one week and the other hand or forearm was not pretreated. After seven days, hands or forearms were treated with 20% ALA, incubated for one hour, and irradiated with blue light. Efficacy and safety were evaluated within 48 hours and eight weeks, same as in the present study.
In the present study, adapalene was chosen for pretreatment over tazarotene for two reasons: it is less expensive than tazarotene and is available over the counter. The decision to use adapalene versus tazarotene did not consider differences in potencies, mechanism of action, or impact on retinoid receptors. Tazarotene requires a prescription and it may be difficult to obtain coverage from insurance companies for its use. For these reasons, most providers would choose the adapalene protocol with ALA 10% gel and red light over the tazarotene protocol with ALA 20% and blue light. Since both adapalene and tazarotene are retinoids, we hypothesized that the less expensive and more readily available adapalene followed by debridement of the target area would improve the ALA 10% gel standard protocol.
Differences in lesion count reduction percentages between the pretreated and non-pretreated groups at eight weeks were also compared in both studies. In the 2011 study,15 the difference between the two groups was of borderline significance (P=0.0547), whereas the corresponding difference in the present study was significant (P=0.0164), suggesting that the combination of adapalene pretreatment, debridement, ALA 10% gel, and red light in the present study offers superior effectiveness.
According to the labels, the original PDT protocol for AKs on the face and scalp recommends a three-hour incubation with 10% ALA gel and 14- to 18-hour incubation with 20% ALA gel. However, shorter contact times, such as one hour in the present study, have been reported16,17 using 20% ALA. Touma et al,16 in an 18-patient study, reported a significant reduction in nonhypertrophic AK lesions of the face after one, two, and three hours of ALA incubation. Pretreatment with 40% urea cream for seven days had no significant effect on the results. In contrast, Gilbert,17 in a study of 15 patients with multiple and diffuse facial AK lesions, pretreated with 5-fluorouracil nightly for five days before ALA-PDT. In this study, ALA was incubated for 30 to 45 minutes under occlusion and low-intensity visible light. Then, ALA was removed and the treated area was illuminated with a single pass of 560 to 1,200-nm broadband light. At one month and one year later, 90% of AK lesions were resolved in all but one patient.
Further support for short incubation times is available from Warren et al,18 who, using noninvasive fluorescence monitoring, demonstrated PpIX accumulation in 63 AK lesions and adjacent skin (of the face and scalp) during the first two hours after topical application of 20% ALA. During this time, PpIX fluorescence rose one SD above baseline in half of all lesions at 20 minutes and in all lesions at two hours. All AK lesions were resolved at two hours, posttreatment erythema correlated with PpIX accumulation, and field cancerization was evident by the elevated PpIX levels observed in areas of photodamaged skin that appeared normal.
Subsequent studies have focused on the use of various agents, such as imiquimod,19 diclofenac gel,1 and 5-fluorouracil,20 in sequential use with PDT to further enhance PDT results, all with varying levels of success. The use of physical pretreatment methods (fractional laser [ablative and nonablative], microdermabrasion, microneedling, and curettage) has been reviewed.21 Disadvantages are operator dependency for curettage, microneedling, and microdermabrasion and the expense and treatment-induced discomfort associated with fractional lasers.
In contrast to the aforementioned studies, AK lesions in the present study were treated with 10% ALA, and the lesions were located on the extremities which, due to a thicker stratum corneum, are more difficult to treat than facial AKs.
Limitations. Limitations of the present study are the small number of patients, possible noncompliance in self-treatment with adapalene, and potential uncertainty in lesion counts before and after treatment.
Conclusions
Pretreatment with adapalene gel (0.1%) for one week may enhance efficacy in a single ALA-PDT treatment of AK lesions of the dorsal hand and forearm. Debridement before ALA application, one-hour incubation with occlusion, and the use of red light constitute an improved standard therapy with 10% ALA. Future studies should include more patients, longer pretreatment and incubation times, heating the treated area, and longer follow-up periods to assess the need for retreatment.
Acknowledgments
The author thanks Kristie Murias, Kellie Buglione, Jennifer Colon, and Summil Valdes for their technical assistance.
References
- Van der Geer S, Krekels GA. Treatment of actinic keratoses on the dorsum of the hands: ALA-PDT versus diclofenac 3% gel followed by ALA-PDT. A placebo-controlled, double-blind, pilot study. J Dermatolog Treat. 2009;20(5):259–265.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 Pt 2):23–24.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115(11):2523–2530.
- Wolf JE Jr, Rigel DS. Understanding efficacy end-points in studies of field-directed therapy for actinic keratosis. Int J Dermatol. 2013;52(9):1063–1070.
- Stockfleth E. The importance of treating the field in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31 Suppl 2:8–11.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26(9):1063–1066.
- Ohgari Y, Nakayasu Y, Kitajima S, et al. Mechanisms involved in delta-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochem Pharmacol. 2005;71(1–2):42–49.
- Olsen EA, Abernethy ML, Kulp-Shorten C, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991;24(5 Pt 1):738–743.
- Kang S, Goldfarb MT, Weiss JS, et al. Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: a randomized trial. J Am Acad Dermatol. 2003;49(1):83–90.
- Bagatin E, Gonçalves HS, Sato M, et al. Comparable efficacy of adapalene 0.3% gel and tretinoin 0.05% cream as treatment for cutaneous photoaging. Eur J Dermatol. 2018;28(3):343–350.
- Szeimies RM, Radny P, Sebastian M, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br J Dermatol. 2010;163(2):386–394.
- Dirschka T, Radny P, Dominicus R, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol. 2012;166(1):137–146.
- Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz(®)) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED(®) lamp. Br J Dermatol. 2016;175(4):696–705.
- Dirschka T, Ulrich M, Reinhold U, et al. Photodynamic therapy with BF-200 ALA for the treatment of mild to severe actinic keratosis in extremities and trunk/back. Results of a randomized phase III trial. Data on file. Biofrontera Inc. 2017.
- Galitzer BI. Effect of retinoid pretreatment on outcomes of patients treated by photodynamic therapy for actinic keratosis of the hand and forearm. J Drugs Dermatol. 2011;10(10):1124–1132.
- Touma D, Yaar M, Whitehead S, et al. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol. 2004;140(1):33–40.
- Gilbert DJ. Treatment of actinic keratoses with sequential combination of 5-fluorouracil and photodynamic therapy. J Drugs Dermatol. 2005;4(2):161–163.
- Warren CB, Lohser S, Wene LC, et al. Noninvasive fluorescence monitoring of protoporphyrin IX production and clinical outcomes in actinic keratoses following short-contact application of 5-aminolevulinate. J Biomed Opt. 2010;15(5):051607.
- Shaffelburg M. Treatment of actinic keratoses with sequential use of photodynamic therapy; and imiquimod 5% cream. J Drugs Dermatol. 2009;8(1):35–39.
- Maytin EV, Anand S, Riha M, et al. 5-Fluorouracil enhances protoporphyrin IX accumulation and lesion clearance during photodynamic therapy of actinic keratoses: a mechanism-based clinical trial. Clin Cancer Res. 2018;24(13):3026–3035.
- Bay C, Lerche CM, Ferrick B, et al. Comparison of physical pretreatment regimens to enhance protoporphyrin IX uptake in photodynamic therapy: a randomized clinical trial. JAMA Dermatol. 2017;153(4):270–278.

