J Clin Aesthet Dermatol. 2021;14(10):27–31.
 by Yan Wu, MD, PhD; Chengxin Li, MD; Julia Garcia, PhD; and Sarah Baradaran, PharmD
by Yan Wu, MD, PhD; Chengxin Li, MD; Julia Garcia, PhD; and Sarah Baradaran, PharmD
Dr. Wu is with Peking University First Hospital in Beijing, China. Dr. Li is with the Chinese PLA General Hospital in Beijing, China. Drs. Garcia and Baradaran are with Allergan Aesthetics, an AbbVie Company, in Irvine, California.
FUNDING: This study, as well as writing and editorial support of this manuscript provided by Peloton Advantage, LLC, an OPEN Health company, was funded by Allergan Aesthetics, an AbbVie Company.
DISCLOSURES: Dr. Garcia is an employee of AbbVie and may own stock in the company. Dr. Baradaran was an employee of Allergan (prior to its acquisition by AbbVie) when the work was completed. The other authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Crow’s feet lines (CFLs) can impact the emotional state, self-perception, and consciousness regarding appearance of patients.
Objective. This study sought to assess patient-reported outcomes after onabotulinumtoxinA treatment for CFLs among Chinese subjects.
Methods. A five-month, double-blind, randomized, parallel-group, placebo-controlled Phase III clinical study was conducted including Chinese adults with moderate-to-severe CFLs at maximum smile. Subjects were randomized 3:1 to 24 U of onabotulinumtoxinA or placebo and completed the 11-item Facial Line Outcomes (FLO-11) questionnaire and Facial Line Satisfaction Questionnaire (FLSQ) at baseline; on Days 8, 15, and 30; and monthly thereafter until Day 150. Item-level and/or domain analyses for the FLO-11 and FLSQ were conducted.
Results. Of 417 treated subjects, 316 received onabotulinumtoxinA and 101 received placebo. For all 10 validated stand-alone FLO-11 items, there was a significantly greater proportion of responders in the onabotulinumtoxinA group versus placebo (P<0.001) at Day 30 that was maintained through Day 150. Significant improvements at Day 30 were reported for all FLSQ items and the FLSQ Follow-up Impact Domain (P less than or equal to 0.01).
Conclusion. FLO-11 and FLSQ data indicated high satisfaction and significant improvements in appearance-related and emotional impacts through Day 150 in patients treated with onabotulinumtoxinA for moderate-to-severe CFLs in Chinese subjects. Trial
Registration: ClinicalTrials.gov identifier no. NCT02195687
Key Words: Patient satisfaction, treatment outcome, surveys and questionnaires, Asia, eyes, skin aging
Lateral canthal lines, i.e., crow’s feet lines (CFLs), are wrinkles that form around the eyes during smiling, laughing, or squinting.1–3 CFLs may impact daily mental and emotional status and self-perception.3 In a recent study, Asian-American women reported that CFLs were among their most bothersome signs of aging and one of their top priorities for treatment.4
OnabotulinumtoxinA (Botox® Cosmetic; Allergan Aesthetics, an AbbVie Company, Irvine, California, USA) is approved for the treatment of CFLs in more than 55 countries.5 Traditionally, clinical trials of facial aesthetic procedures, including botulinum toxin injections for treating CFLs, have focused on safety and efficacy endpoints. However, measures of subject satisfaction and emotional, psychological, and appearance-related impacts may also provide useful information and signal the effectiveness of botulinum toxin injections and other facial aesthetic treatments.6 In real-world practice, subject satisfaction drives successful results and repeat treatments.7 Therefore, patient-reported outcomes (PROs) have become a foundation for the clinical study of facial aesthetic treatments.6,8
Multiple PROs, including the Facial Line Satisfaction Questionnaire (FLSQ) and the 11-item Facial Line Outcomes (FLO-11) questionnaire,9 have been developed and validated for use in clinical trials.10 Both the FLSQ and the FLO-11 assess the psychological impact of and satisfaction with upper facial lines (crow’s feet, forehead, and glabellar lines) separately and in combination.9,10 The FLO-11 and FLSQ results of subjects with upper facial lines treated with onabotulinumtoxinA have been reported previously.5,11
A pivotal trial conducted in China demonstrated the safety and efficacy of onabotulinumtoxinA in Chinese subjects with moderate-to-severe CFLs while also demonstrating improvements in PROs (including FLO-11 and FLSQ) related to overall satisfaction with onabotulinumtoxinA for the treatment of CFLs.5 The present analysis reports additional FLO-11 and FLSQ item-level and impact domain data from this study related to the effect of onabotulinumtoxinA on appearance, emotional state, and satisfaction with treatment among Chinese subjects.
Methods
Full study methods were published previously.5 Briefly, a five-month, double-blind, randomized, parallel-group, placebo-controlled Phase III clinical study randomized Chinese subjects aged 18 years and older with moderate-to-severe CFLs at maximum smile to 24 U of onabotulinumtoxinA (six intramuscular 0.1-mL injections, three per side) or placebo. The proportion of subjects who achieved a CFL severity of none or mild at maximum smile on the Facial Wrinkle Scale with Asian Photonumeric Guide (FWS-A) at Day 30 was the primary endpoint.5
PROs. PRO endpoints of the study included data from both the FLO-11 and the FLSQ. The 11 items of the FLO-11 questionnaire evaluate the impact of CFLs on the appearance and emotional status of subjects and are scored on an 11-point scale (0 [not at all] to 10 [very much]),9 based on subjects’ perceptions when they complete the questionnaire. The FLSQ consists of the nine-item Baseline version and 11-item Follow-up version.10 The Baseline version measures treatment expectations related to CFLs, and the Follow-up version measures satisfaction with CFLs treatment results. FLSQ Impact Domain items explore appearance-related psychological impacts of treatment.12
The FLO-11 and FLSQ questionnaires were administered at baseline; on Days 8, 15, and 30; and monthly thereafter until Day 150. Item-level and/or domain analyses for the FLO-11 and FLSQ were conducted. Responders to validated stand-alone FLO-11 Items 1 through 10 (Item 11 is not validated for stand-alone use) were defined as follows: for all items except Item 8, individuals with baseline FLO-11 scores of at least two points who had at least a two-point improvement from baseline for each item, and for Item 8 (looking tired), individuals with a baseline score of at least three points who experienced at least a three-point improvement. These definitions were based on a previously conducted minimal important difference threshold analysis (data on file, Allergan Aesthetics).
Specific FLSQ-validated stand-alone items (Baseline Items 1 and 4–6, Follow-up Items 2–5 and 11, Baseline Impact Domain Items 7–9, and Follow-up Impact Domain Items 6–8) were also assessed. Impact FLSQ items were rated on a five-point Likert-type scale, with answers ranging from very dissatisfied to very satisfied. Responders to FLSQ Follow-up version stand-alone validated items were as follows: for Items 2 to 5, individuals who rated themselves as mostly or very satisfied; for item 11, individuals rating treatment as meeting or exceeding their expectations. Impact Domain score responders were defined as subjects who experienced at least a 20-point improvement from baseline at Day 30. This definition is consistent with the threshold for meaningful change from a minimal important difference analysis of the FLSQ Impact Domain (data on file, Allergan Aesthetics)10 and with existing pivotal trials of onabotulinumtoxinA in facial lines.13,14
Statistical analyses. Analyses were performed on the modified intent-to-treat (mITT) population, defined as all randomized subjects who received at least one injection of the study drug. Between-group comparisons were analyzed using the Wilcoxon rank-sum test. Distributions of FLO-11 and FLSQ results were summarized by treatment group, with Baseline item results summarized by treatment group for the mITT population. Baseline and change from baseline analyses for each FLO-11 item were based on an analysis of variance. To obtain FLSQ Impact Domain scores, the results of Impact Domain items were averaged and standardized to a score ranging from 0 (best) to 100 (worst) points.
Results
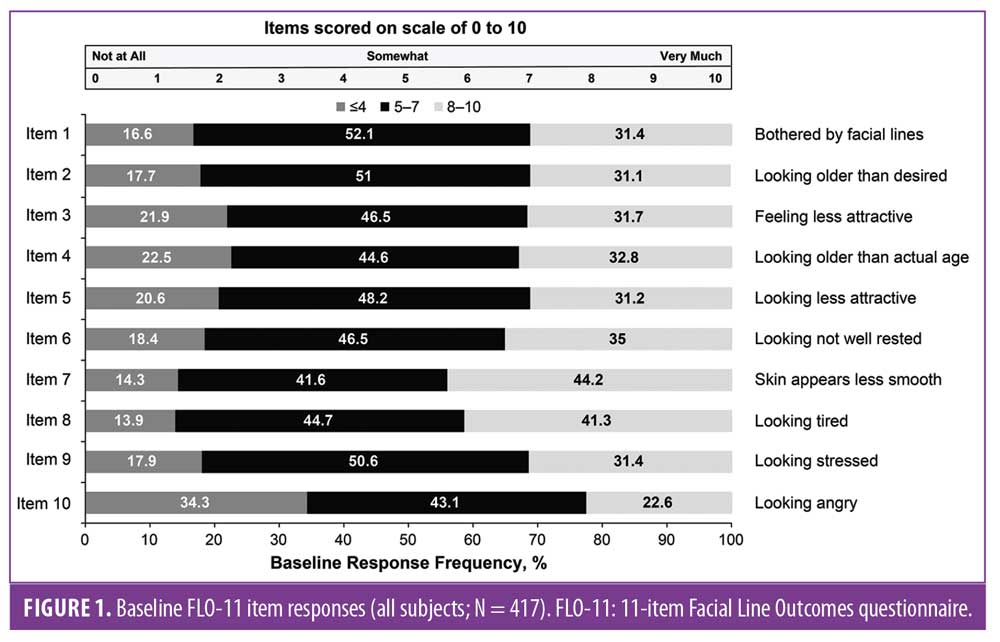
Demographics and baseline characteristics. Demographics and baseline characteristics were reported previously;5 briefly, the mITT population included 417 subjects (316 in the onabotulinumtoxinA group and 101 in the placebo group), and the mean age was 46.4 years (range, 23–74 years). The majority of subjects were female (86.3%), and similar proportions had moderate (48.4%) or severe (51.6%) baseline FWS-A CFL severity ratings at maximum smile. Baseline scores for each validated FLO-11 item were similar and indicated that moderate-to-severe CFLs impacted the appearance and emotional status of most subjects (Figure 1).
PROs. Our report concentrates on the results of validated stand-alone items from the FLO-11 questionnaire (Items 1–10) and of items and domains from the FLSQ Baseline and Follow-up versions that were validated for stand-alone use and are appropriate for use in clinical trial settings. Results of FLSQ Item 5 (satisfaction with CFL treatment effect) and FLO-11 total score (appearance and emotional impact of CFLs across all 11 items combined) were previously published.5
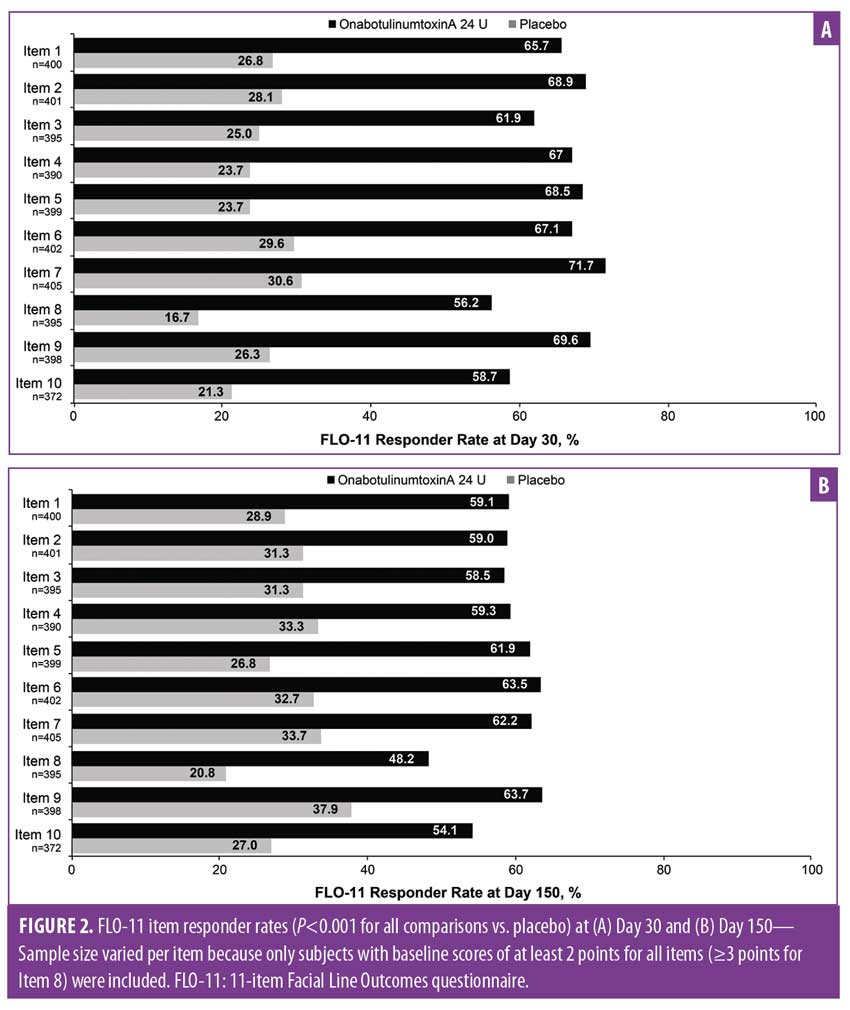
For all 10 validated stand-alone FLO-11 items, the proportion of responders at Day 30 (Figure 2A) was maintained through Day 150 (Figure 2B). The proportion of responders was significantly greater in the 24-U onabotulinumtoxinA group than in the placebo group (P<0.001), including the FLO-11 items on looking older than desired (Item 2), looking older than actual age (Item 4), and looking tired (Item 8).
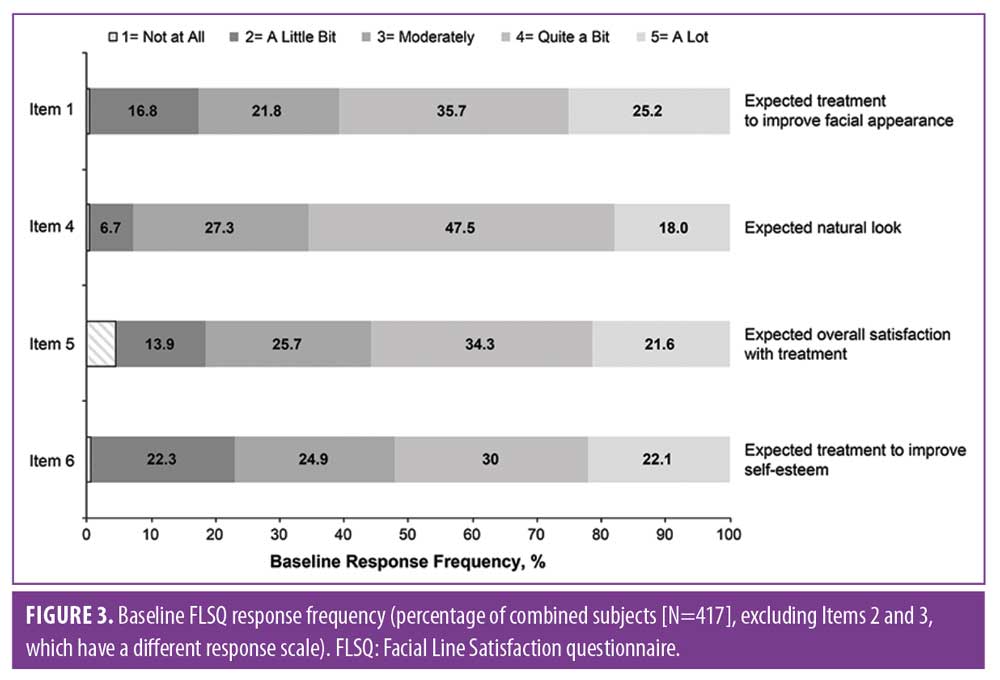
Baseline FLSQ responses were also similar across groups and demonstrated that moderate-to-severe CFLs impacted the appearance and emotional status of the majority of subjects (Figure 3). Significant improvements at Day 30 (P?0.01 for all comparisons between onabotulinumtoxinA and placebo) were reported for all FLSQ items (Figure 4). Corresponding data for the FLSQ Impact Domain also demonstrated the impact of appearance and emotional status at baseline (Figure 5A); significant improvements at Day 30 (P?0.01 for all comparisons between onabotulinumtoxinA and placebo) are also shown for the FLSQ Follow-up Impact Domain (Figure 5B).
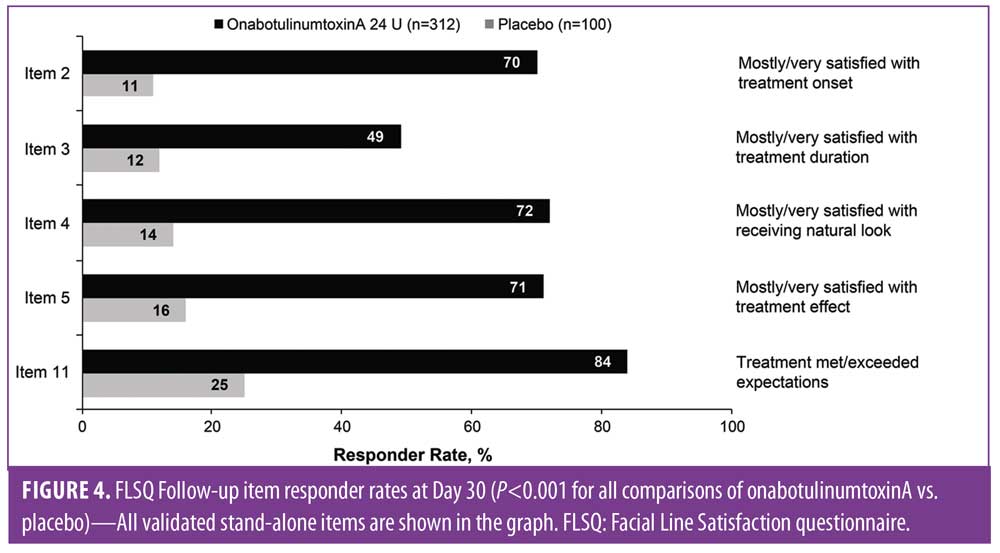
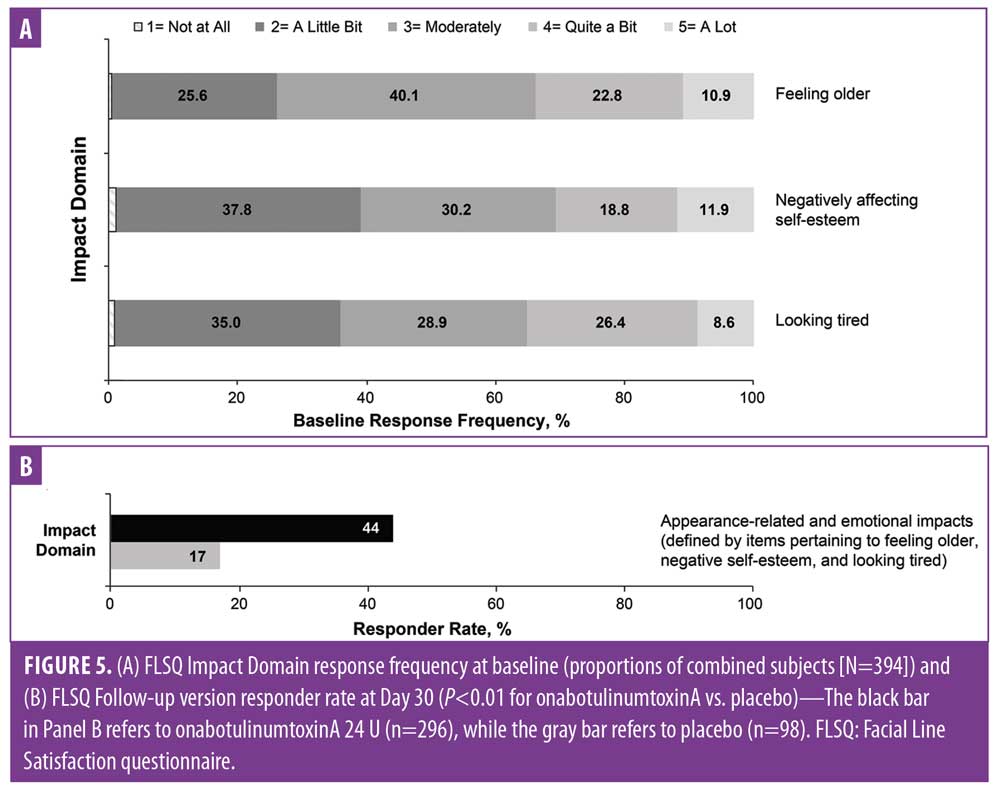
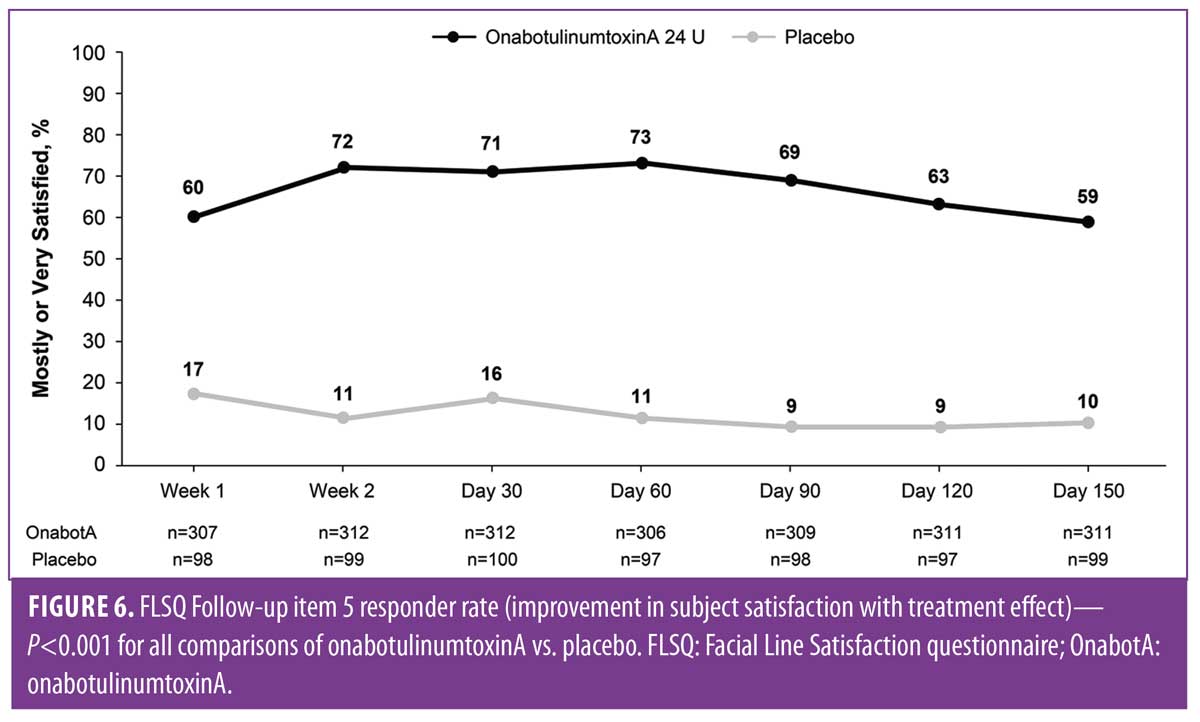
Significant improvements were maintained through Day 150 for 24 U of onabotulinumtoxinA versus placebo (P<0.001) on the FLSQ Follow-up item on satisfaction with onabotulinumtoxinA treatment effect (Item 5; Figure 6), as well as for all other FLSQ items (P?0.01).
Discussion
These FLO-11 and FLSQ data demonstrate a high level of subject satisfaction and significant improvements in appearance-related and emotional impacts following treatment with onabotulinumtoxinA compared to placebo in Chinese subjects with moderate-to-severe CFLs at maximum smile. Significant improvements in nearly all FLSQ satisfaction measures were maintained through Day 150. Improvements in appearance-related and emotional impacts were also significant, providing further evidence of satisfaction with onabotulinumtoxinA for the treatment of CFLs among Chinese subjects. Previously published results of this study showed significantly greater overall subject satisfaction with treatment effect (FLSQ Item 5) with onabotulinumtoxinA versus placebo at all time points from Day 30 through Day 150 after treatment, as well as a significantly greater mean change from baseline in total FLO-11 scores by Day 30.5 OnabotulinumtoxinA was effective in reducing moderate-to-severe CFLs in Chinese subjects, with all efficacy endpoints met and statistically significant (P<0.001) improvements in CFLs severity, as measured by the FWS-A, achieved versus placebo at Day 30 (63.9% vs. 5%) and maintained through Day 150.5 These findings suggest that subject-perceived treatment benefits are consistent with efficacy data and should be considered when treating subjects with CFLs. These data are meaningful because real-world patients with positive self-reported outcomes may be more likely to repeat or continue treatment.15
Other studies have similarly reported efficacy of onabotulinumtoxinA for addressing CFLs in Asian subjects, along with simultaneous PRO improvements. For example, a 13-month, double-blind, Phase III study of Japanese subjects (N=101) demonstrated a high proportion (81%) of FLSQ overall satisfaction responders in those who received onabotulinumtoxinA (n=53) versus placebo (n=48) for CFLs and glabellar lines for up to five treatments, while also demonstrating that most subjects (85–98%) were also responders on the investigator-rated FWS-A.16 Comparable PRO results and efficacy findings were reported in Japanese subjects who were treated with onabotulinumtoxinA for moderate-to-severe CFLs.17 Significant efficacy results coupled with favorable PRO findings have also been noted in North American and European study populations who received injectable treatments for CFLs.15,18,19 In two separate, previously conducted studies, proportions of subjects who showed improvement on FLO-11 Items 2 (looking older), 5 (looking less attractive), and 8 (looking tired) were significantly greater with onabotulinumtoxinA versus placebo at Day 30 and were sustained up to Day 90 or Day 150.18,20,21 However, impacts of facial lines may vary among ethnic groups, with reports from international studies indicating that Asian subjects generally perceive themselves as having less severe age-related facial characteristics than White subjects.22,23 Previously published data also suggested that the severity of baseline CFLs among Asian subjects may be lower than that in the general population, possibly increasing the likelihood that Asian subjects may more readily achieve CFL severity ratings of none or mild after treatment and achieve longer duration of treatment effect than non-Asian subjects.5,16 Taken together, these factors may have contributed to the findings of the present study. It is also important for clinicians to be aware that the pattern of CFLs and facial skin or anatomy differ between Asian and non-Asian subjects,16,24,25 which may necessitate a customized injection pattern for Asian subjects.24,25
Conclusion
Results from FLO-11 and FLSQ data indicate a high level of satisfaction and significant improvements in appearance-related and emotional impacts with 24 U of onabotulinumtoxinA compared to placebo for the treatment of moderate-to-severe CFLs in Chinese subjects, which were maintained through Day 150, as measured from the subjects’ perspective using the validated FLO-11 questionnaire and FLSQ. These PRO data are important for comprehensive assessment of treatment efficacy in addition to the clinician’s perspective and provide information that may help clinicians with patient selection for CFL treatment. Additionally, the PRO findings presented here provide evidence that support a patient-centric approach to CFL treatment planning.
Acknowledgment
The authors wish to acknowledge Regina Kelly, MA, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, New Jersey, for writing and editorial support in preparing this manuscript.
References
- Cheon HI, Jung N, Won CH, et al. Efficacy and safety of prabotulinumtoxin A and onabotulinumtoxin A for crow’s feet: a phase 3, multicenter, randomized, double-blind, split-face study. Dermatol Surg. 2019;45(12):1610–1619.
- Lee JH, Hong G. Definitions of groove and hollowness of the infraorbital region and clinical treatment using soft-tissue filler. Arch Plast Surg. 2018;45(3):214–221.
- Dayan S, Yoelin SG, De Boulle K, Garcia JK. The psychological impacts of upper facial lines: a qualitative, patient-centered study. Aesthet Surg J Open Forum. 2019;1(2):1–10.
- Chiu A, Hui A, Mariwalla K, et al. Understanding the Asian American facial aesthetic patient [poster]. Presented at: Annual Meeting of the Skin of Color Seminar Series; May 5–6, 2018; New York, NY.
- Wu Y, Wang G, Li C, et al. Safety and efficacy of onabotulinumtoxinA for treatment of crow’s feet lines in Chinese subjects. Plast Reconstr Surg Glob Open. 2019;7:e2079.
- Ogilvie P, Rivkin AZ, Dayan S, et al. OnabotulinumtoxinA for treatment of forehead and glabellar lines: subject-reported satisfaction and impact from a phase 3 double-blind study. Dermatol Surg. 2019;45(5): 689–699.
- Stotland MA, Kowalski JW, Ray BB. Patient-reported benefit and satisfaction with botulinum toxin type A treatment of moderate to severe glabellar rhytides: results from a prospective open-label study. Plast Reconstr Surg. 2007;120(5):1386–1393.
- Rivkin AZ, Ogilvie P, Dayan S, et al. OnabotulinumtoxinA for simultaneous treatment of upper facial lines: subject-reported satisfaction and impact from a phase 3 study. Dermatol Surg. 2020;46(1):50–60.
- Yaworsky A, Daniels S, Tully S, et al. The impact of upper facial lines and psychological impact of crow’s feet lines: content validation of the Facial Line Outcomes (FLO-11) Questionnaire. J Cosmet Dermatol. 2014;13(4):297–306.
- Pompilus F, Burgess S, Hudgens S, et al. Development and validation of a novel patient-reported treatment satisfaction measure for hyperfunctional facial lines: Facial Line Satisfaction Questionnaire. J Cosmet Dermatol. 2015;14(4):274–285.
- Trindade de Almeida A, Carruthers J, Cox SE, et al. Patient satisfaction and safety with aesthetic onabotulinumtoxinA after at least 5 years: a retrospective cross-sectional analysis of 4,402 glabellar treatments. Dermatol Surg. 2015;41(Suppl 1):S19–S28.
- Palm MD, Few J, Patel T, et al. Efficacy, patient-reported outcomes, and safety for millennial subjects treated with onabotulinumtoxinA for moderate to severe horizontal forehead lines. Dermatol Surg. 2020;46(5):653–661.
- Fagien S, Cohen JL, Coleman W, et al. Forehead line treatment with onabotulinumtoxinA in subjects with forehead and glabellar facial rhytids: a phase 3 study. Dermatol Surg. 2017;43(Suppl 3):S274–S284.
- De Boulle KL, Werschler WP, Gold MH, et al. Phase 3 study of onabotulinumtoxinA distributed between frontalis, glabellar complex, and lateral canthal areas for treatment of upper facial lines. Dermatol Surg. 2018;44(11):1437–1448.
- Keaney TC, Cavallini M, Leys C, et al. Efficacy, patient-reported outcomes, and safety in male subjects treated with onabotulinumtoxinA for improvement of moderate to severe horizontal forehead lines. Dermatol Surg. 2020;46(2):229–239.
- Kawashima M, Harii K, Horiuchi Y, et al. Safety, efficacy, and patient satisfaction with onabotulinumtoxinA for the treatment of upper facial lines in Japanese subjects. Dermatol Surg. 2020;46(4):483–490.
- Harii K, Kawashima M, Furuyama N, et al. OnabotulinumtoxinA (Botox) in the treatment of crow’s feet lines in Japanese subjects. Aesthetic Plast Surg. 2017;41(5):1189–1197.
- Dayan S, Coleman WP, III, Dover JS, et al. Effects of onabotulinumtoxinA treatment for crow’s feet lines on patient-reported outcomes. Dermatol Surg. 2015;41(Suppl 1):S67–S74.
- Fabi SG, Champagne JP, Nettar KD, et al. Efficacy and safety of and patient satisfaction with injectable hyaluronic acid with 0.3% lidocaine hydrochloride for the treatment of superficial perioral lines or superficial lateral canthal lines. Dermatol Surg. 2013;39(11):1613–1620.
- Carruthers A, Bruce S, de Coninck A, et al. Efficacy and safety of onabotulinumtoxinA for the treatment of crow’s feet lines: a multicenter, randomized, controlled trial. Dermatol Surg. 2014;40(11):1181–1190.
- Moers-Carpi M, Carruthers J, Fagien S, et al. Efficacy and safety of onabotulinumtoxinA for treating crow’s feet lines alone or in combination with glabellar lines: a multicenter, randomized, controlled trial. Dermatol Surg. 2015;41(1):102–112.
- Rossi A, Eviatar J, Green JB, et al. Signs of facial aging in men in a diverse, multinational study: timing and preventive behaviors. Dermatol Surg. 2017;43(Suppl 2):S210–S220.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45(12):1635–1648.
- Park DH, Han DG, Shim JS, et al. Analysis of the patterns of lateral canthal rhytids and reference for botulinum toxin treatment in orientals. Aesthetic Plast Surg. 2012;36(5):1211–1215.
- Kane MAC, Cox SE, Jones D, et al. Heterogeneity of crow’s feet lines patterns in clinical trial subjects. Dermatol Surg. 2015;41(4):447–456.

