J Clin Aesthet Dermatol. 2019;12(1):35–37
 by Blake Sampson, MD, and Bethany K. H. Lewis, MD, MPH
by Blake Sampson, MD, and Bethany K. H. Lewis, MD, MPH
Dr. Sampson is with the Oregon Medical Group in Eugene/Springfield, Oregon (with the Department of Dermatology at University of Utah in Salt Lake City, Utah (at the time of this work). Dr. Lewis is with the Department of Dermatology at University of Utah in Salt Lake City, Utah.
Funding: No funding was provided for the preparation of this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: We present a case of medication-associated paronychia involving multiple toenails in a patient undergoing hepatitis C (HCV) therapy with ledipasvir/sofosbuvir. The patient was treated conservatively with topical mupirocin, clobetasol ointment, and acetic acid soaks, resulting in symptom improvement and control. This topical regimen was maintainined throughout the remaining weeks of the patient’s antiviral course, with complete symptom resolution occurring only after completion of his ledipasvir/sofosbuvir treatment.
Keywords: Paronychia, lepidasvir, sofosbuvir, Harvoni®, hepatitis C, HCV
The hepatitis C virus (HCV) is an enveloped ribonucleic acid (RNA) virus that can cause both acute and chronic hepatitis infections, which can lead to severe complications, including cirrhosis and hepatocellular carcinoma, in approximately 15 to 30 percent of patients.1 The World Health Organization estimates between 130 and 150 million people globally have a chronic HCV infection.2 The HCV genome encodes a single polyprotein that is cleaved by cellular and viral proteases into at least 10 individual proteins, including nonstructural proteins NS5A and NS5B, which are essential proteins for viral RNA replication.3–10
The recent development of direct-acting antiviral (DAA) medications targeted against NS5A (ledipasvir) and NS5B (sofosbuvir) have revolutionized the treatment of chronic HCV.11,12 In particular, the fixed-dose combination of ledipasvir 90mg/sofosbuvir 400mg (Harvoni®; Gilead, Foster City, California) has proven to be highly efficacious against certain genotypes of HCV, including genotype 1.13–16
A recent meta-analysis found that the incidence of cutaneous adverse events (CAEs) in 843 patients treated with ledipasvir/sofosbuvir was 3.9 percent.17 Reported CAEs associated with DAAs were generalized rash, vesicles, bullae, ulcerations, drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, erythema multiforme, and toxic epidermal necrolysis. Here, we present a case of paronychia associated with ledipasvir/sofosbuvir, a previously unreported adverse effect.
Case Presentation
A 34-year-old man currently undergoing treatment with ledipasvir/sofosbuvir for chronic genotype 1a HCV infection was referred for evaluation and consideration of surgical management of paronychia involving multiple sites. The patient had a history of HCV cirrhosis, end-stage liver disease, and dialysis-dependent renal insufficiency status after liver and kidney transplantations.
Upon presentation to our clinic, the patient had completed 16 weeks of a planned 24-week course of daily ledipasvir/sofosbuvir. He reported a three-week history of progressively worsening, exquisitely tender “ingrown toenails,” which were severely inhibiting his mobility and adversely impacting his quality of life. He also endorsed having had an “ingrown nail” on his right third finger several weeks prior to presentation.
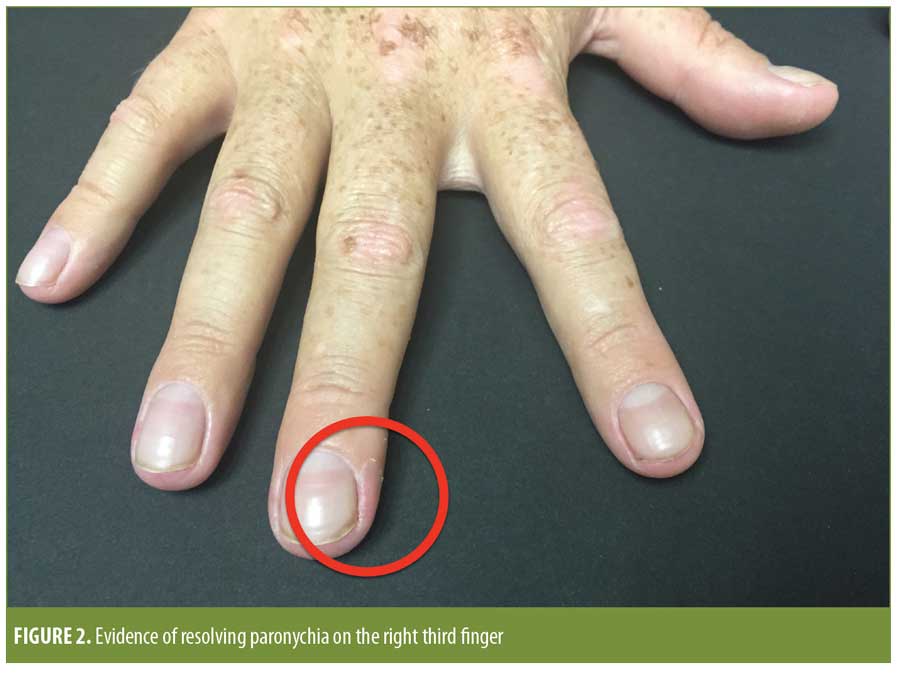
On examination, he was found to have erythematous, edematous periungual plaques with purulent exudate involving the medial and lateral nailfolds of the bilateral great toes (Figure 1). Superficial desquamation of the periungual area of the right third finger was consistent with a history of resolving paronychia (Figure 2).
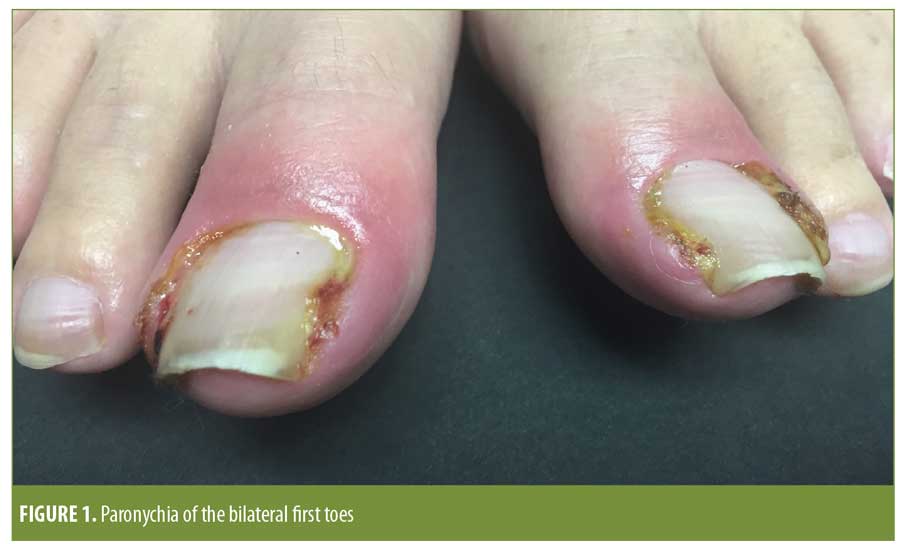
Based on the timing, multifocal and bilateral distribution on the toenails, and the lack of preceding trauma to the nailfolds, a diagnosis of medication-associated paronychia related to his ongoing ledipasvir/sofosbuvir treatment was suspected. The patient was treated conservatively with mupirocin 2% ointment once daily in the morning, clobetasol 0.05% ointment once daily in the evening, and acetic acid soaks twice daily. Wound cultures at the time of presentation grew modest (2+) Staphylococcus aureus; although this was felt to be secondary, the patient was continued on a 10-day course of amoxicillin/clavulanate because it had been previously started for a different diagnosis (otitis media).
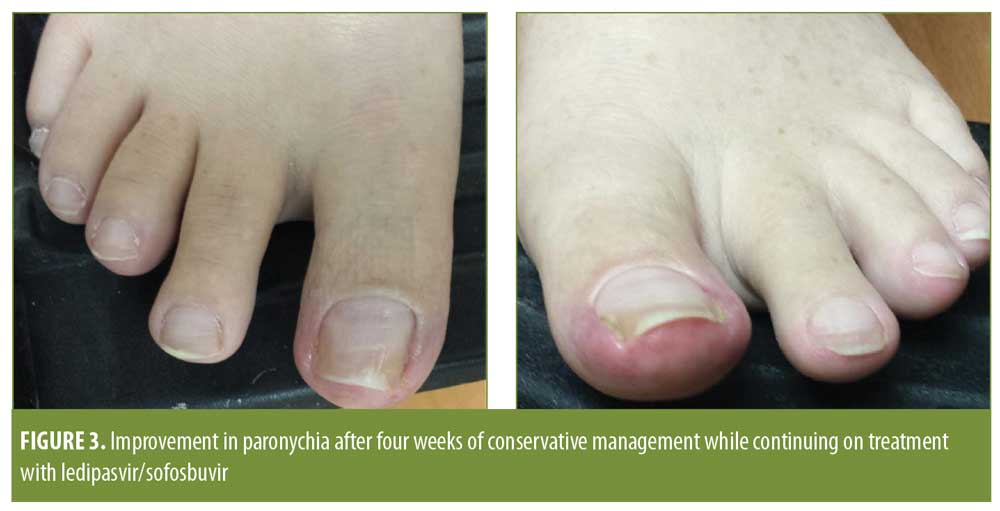
Four-week follow-up revealed improvement, but not full clearance, of the paronychia with conservative management (Figure 3). The patient’s symptoms were well-controlled with continuation of his topical regimen for the remaining four weeks of his antiviral course, with complete symptom resolution occurring only after completion of his ledipasvir/sofosbuvir treatment.
Discussion
Paronychia is an inflammatory process involving the nail folds that can be acute or chronic.18 Acute paronychia typically develops in association with direct or indirect trauma to the cuticle or nail fold, which predisposes it to bacterial inoculation and subsequent infection, most commonly with Staphylococcus aureus. Characteristically, acute paronychia develops rapidly, involves only one nail, and is associated with significant edema, erythema, and tenderness.19,20 Chronic paronychia is commonly associated with a history of continuous immersion in water or contact with irritating chemicals. It typically presents over weeks to months as a retraction of the proximal nail fold and absence of the adjacent cuticle on the thumb, second, or third fingers of the dominant hand, with a history of recurrent, self-limited exacerbations of erythema, tenderness, and swelling.20 There is often nail dystrophy, such as thickening and discoloration of the nail plate, resulting from chronic inflammation of the nail matrix. Colonization of chronic paronycha lesions with Candida albicans is common.18
In addition to moisture, irritants, and allergens, paronychia has been associated with several systemic medications, including isotretinoin, antiretrovirals, epidermal growth factor receptor inhibitors, methotrexate, capecitabine, and sirolimus.18,20–26 The pathogenesis of these associations is unclear; however, in the case of systemic retinoids, it has been proposed that induction of nail fragility leads to the penetration of nail fragments into the periungual tissue and subsequent formation of pseudopyogenic granulomas, which are commonly seen with ingrowing toenails.27 Antiretroviral protease inhibitors might have a “retinoid-like” effect due to homologous portions of the amino acid sequences of cellular retinoid acid binding protein 1 (CRABP1) and of the catalytic site of HIV-1 protease.28 In one study, which reported on six patients and reviewed data from an additional 34 patients from the literature, 37 of the 40 patients treated with the antiretroviral medications lamivudine and indinavir, either alone or in combination, had involvement of the toenails, which is otherwise an uncommon location for acute or bacterial paronychia.22
Paronychia is a known side effect associated with antiretrovirals but which is previously unreported in this new class of DAAs used for HCV. Our patient’s simultaneous bilateral involvement of the great toenails, multifocal involvement of the toenails and fingernails, presentation during antiviral course, lack of complete resolution until medication completion, and lack of typical risk factors led us to conclude his paronychia was medication-related. Ledipasvir/sofosbuvir is an increasingly prescribed potentially life-saving medication for millions of HCV patients, and we highlight our case to increase awareness of this side effect that has the potential to severely impact patient quality of life.
Many treatments have been attempted for medication-associated paronychia, with the most effective being corticosteroid ointment in combination with phenol chemical matricectomy.29 Although this patient received a concomitant 10-day course of oral antibiotics for his otitis media, his paronychia symptoms did not abate during the course and we do not advocate the use of systemic antibiotics for medication-induced paronychia. Even if cultures often grow bacteria, these are likely secondary or incidental growth, and drug-induced and irritative paronychias do not require systemic antibiotics. While cessation of the offending agent offers a simple resolution, in many patients, discontinuation of critical medications might not be a realistic option. As demonstrated in this case, prompt recognition of medication-associated paronychia and the initiation of conservative therapy with topical corticosteroids and topical antimicrobial measures might avoid invasive surgical management and control symptoms to enable continuation of the offending medication.
References
- Lo SY, Li HC. Hepatitis C virus: virology, diagnosis and treatment. World J Hepatol. 2015;7(10):
1377–1389. - World Health Organization. Hepatitis C fact sheet. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed 29 Sep 2017.
- Fridell RA, Qiu D, Valera L, et al. Distinct functions of NS5A in hepatitis C virus RNA replication uncovered by studies with the NS5A inhibitor BMS-790052. J Virol. 2011;85(14):7312–7320.
- Poenisch M, Bartenschlager R. New insights into structure and replication of the hepatitis C virus and clinical implications. Semin Liver Dis. 2010;30(4):333–347.
- Suzuki T, Ishii K, Aizaki H, Wakita T. Hepatitis C viral life cycle. Adv Drug Deliv Rev. 2007;59(12):
1200–1212. - Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290(5498):1972–1974.
- Lohmann V, Körner F, Koch J, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285(5424):110–113.
- Behrens SE, Tomei L, De Francesco R. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 1996;15(1):12–22.
- Huang Y, Staschke K, De Francesco R, Tan SL. Phosphorylation of hepatitis C virus NS5A nonstructural protein: a new paradigm for phosphorylation-dependent viral RNA replication?. Virology. 2007;364(1):1–9.
- Macdonald A, Harris M. Hepatitis C virus NS5A: tales of a promiscuous protein. J Gen Virol. 2004;85
(Pt 9):2485–2502. - Bourliere M, Oules V, Ansaldi C, et al. Sofosbuvir as backbone of interferon free treatments. Dig Liver Dis. 2014;46(Suppl 5):S212–S220.
- Link JO, Taylor JG, Xu L, et al. Discovery of ledipasvir (GS-5885): a potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J Med Chem. 2014;57(5):2033–2046.
- Gilead Sciences Inc. Harvoni (ledipasvir and sofosbuvir) tablets, for oral use. U.S. prescribing information. Gilead Sciences Inc., Foster City, California; 2014.
- Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493.
- Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898.
- Younossi ZM, Stepanova M, Marcellin P, et al. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: results from the ION-1, -2, and -3 clinical trials. Hepatology. 2015;61(6):1798–1808.
- Patel P, Malik K, Krishnamurthy K. Cutaneous adverse events in chronic hepatitis C patients treated with new direct-acting antivirals: a systematic review and meta-analysis. J Cutan Med Surg. 2016;20(1):58–66.
- Tosti A, Piraccini BM, Schaffer JV, et al. Nail disorders. In: Bolognia J, Jorizzo J, Schaffer J (eds). Dermatology. 3rd ed. Vol 1. New York, NY: Saunders Elsevier; 2012:1129–1147.
- Jebson PJ. Infections of the fingertip. Paronychias and felons. Hand Clin. 1998;14(4):547–555.
- Rigopoulos D, Larios G, Gregoriou S, Alevizos A. Acute and chronic paronychia. Am Fam Physician. 2008;77(3):339–346.
- Blumental G. Paronychia and pyogenic granuloma-like lesions with isotretinoin. J Am Acad Dermatol. 1984;10(4):677–678.
- Tosti A, Piraccini BM, D’Antuono A, et al. Paronychia associated with antiretroviral therapy. Br J Dermatol. 1999;140(6):1165–1168.
- Lacouture ME, Lai SE. The PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) syndrome. Br J Dermatol. 2006;155(4):852–854.
- Wantzin GL, Thomsen K. Acute paronychia after high-dose methotrexate therapy. Arch Dermatol. 1983;119(7):623–624.
- Sastre J, Grávalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist. 2012;17(3):339–345.
- Sibaud V, Dalenc F, Mourey L, Chevreau C. Paronychia and pyogenic granuloma induced by new anticancer mTOR inhibitors. Acta Derm Venereol. 2011;91(5):584–585.
- Baran R. Retinoids and the nails. J Dermatol Treat. 1990;1:151–154.
- Bouscarat F, Bouchard C, Bouhour D. Paronychia and pyogenicgranuloma of the great toes in patients treated with indinavir. N Engl J Med. 1998;338(24):1776–1777.
- Goto H, Yoshikawa S, Mori K, et al. Effective treatments for paronychia caused by oncology pharmacotherapy. J Dermatol. 2016;43(6):670–673.

