 by Joseph M. Dyer, DO, and Richard A. Miller, DO
by Joseph M. Dyer, DO, and Richard A. Miller, DO
Drs. Dyer and Miller are with the Department of Dermatology at Largo Medical Center in Largo, Florida.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest to relevant to the content of this article.
Abstract: Thin skin and the appearance of bruises, seemingly unprovoked, are frequent complaints of elderly patients. Chronic cutaneous insufficiencies such as these are termed dermatoporosis. Although it is seldom the primary reason for consultation, dermatoporosis is associated with bleeding and healing complications and presents an opportunity for patient education and prevention. In this review, the authors explore the risk factors, pathogenetic mechanisms, clinical expression, and evidence-based therapies reported for chronic skin fragility due to aging.
Keywords: Dermatoporosis, skin fragility, geriatric dermatology, atrophic skin, chronic cutaneous insufficiency, senile purpura, skin tears, hyaluronic acid, CD44
J Clin Aesthet Dermatol. 2018;11(1):13–18
Introduction
In medical literature, there exists a variety of terms that give name to the failure of different organs after a slow decline over time. Terms such as congestive heart failure, chronic obstructive pulmonary disease, macular degeneration, and osteoporosis reflect declining function in the heart, lungs, eyes, and bones, respectively. However, the skin—the largest and most visible organ—lacked a nosologic analog until only recently. In 2007, Kaya and Saurat coined the term dermatoporosis to give name to the chronic cutaneous fragility of aging skin.1 It was their hope that dermatoporosis would convey the vulnerability in skin similar to the way osteoporosis does in bones, while also conveying the necessity for prevention. Key features of dermatoporosis are unsurprising to the geriatrician and include atrophic skin with solar purpura and white pseudoscars on the extremities of elderly patients. Skin lacerations and delayed healing are frequent features in dermatoporotic skin, leaving affected patients susceptible to bleeding complications and cutaneous infections.
The prevalence of dermatoporosis in the United States is likely to increase as those in the post-World War II “baby boom” cohort continue to age. The percentage of the United States population that is 65 years or older will shift from 13.1 percent in 2010 to an estimated 20.9 percent in 2050.9
In this article, we review updates in the literature on dermatoporosis since the seminal description by Kaya and Saurat, while also highlighting current concepts in epidemiology, pathogenesis, evaluation, prevention, and therapeutic measures.
Epidemiology
Demographics. Limited demographic data on dermatoporosis exist. One French study examined 202 subjects aged 60 to 80 years and determined the prevalence of dermatoporosis to be 32 percent.2 Another French study assessed 533 individuals aged 65 years or older by the use of a validated questionnaire and determined overall prevalence to be 37.5 percent.3
The frequency of solar purpura is 10 percent of those aged 70 to 90 years and is more common in women.1 Out of 198 consecutive patients aged 65 years or older admitted to a Polish hospital, 12.1 percent demonstrated purpuric lesions.4 Additionally, white pseudoscars are encountered in 20 to 40 percent of patients over the age of 70 years.1
Prevalence data from Australia estimated that skin tears occur in 5.5 percent of those individuals within the community setting and 41.5 percent of those within the long-term care setting.5,6 A Danish nursing home reported a skin tear prevalence of 4.6 percent,7 and a prospective cohort study following 368 Japanese in-patients for three months demonstrated a cumulative incidence of skin tears of 3.8 percent.8
Risk factors. Advancing age is the principle risk factor for developing dermatoporosis. Secondary risk factors include chronic actinic damage, genetic susceptibility, and long-term use of topical and systemic corticosteroids. One study determined chronic renal failure increased the risk of dermatoporosis five-fold, independent of age.2 Patients with chronic obstructive pulmonary disease were more likely to demonstrate senile purpura, with systemic corticosteroid use a possible confounder.4 These and other risk factors are summarized in Table 1.
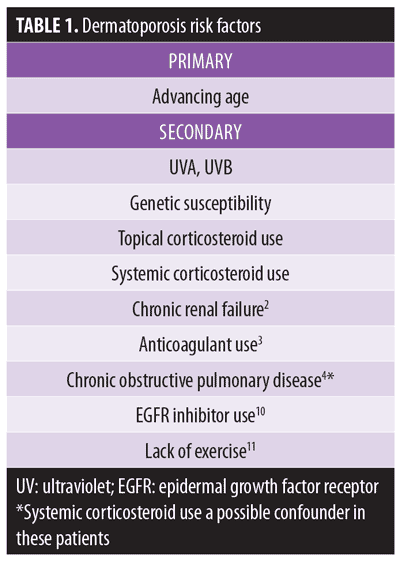
Besides dermatoporosis itself, impaired mental status, nutritional compromise, and a history of skin tears were identified as additional risk factors for dermatoporosis.12,13 In a Japanese cohort study,14 investigators measured the forearm dermal thickness of 149 patients 65 years of age or older using 20-MHz ultrasound scanner, and followed them for the development of skin tears. The researchers found dermal thickness to be a predictor of high risk of skin tears among elderly patients, with 0.80mm being the significant cutoff point.
Pathogenesis
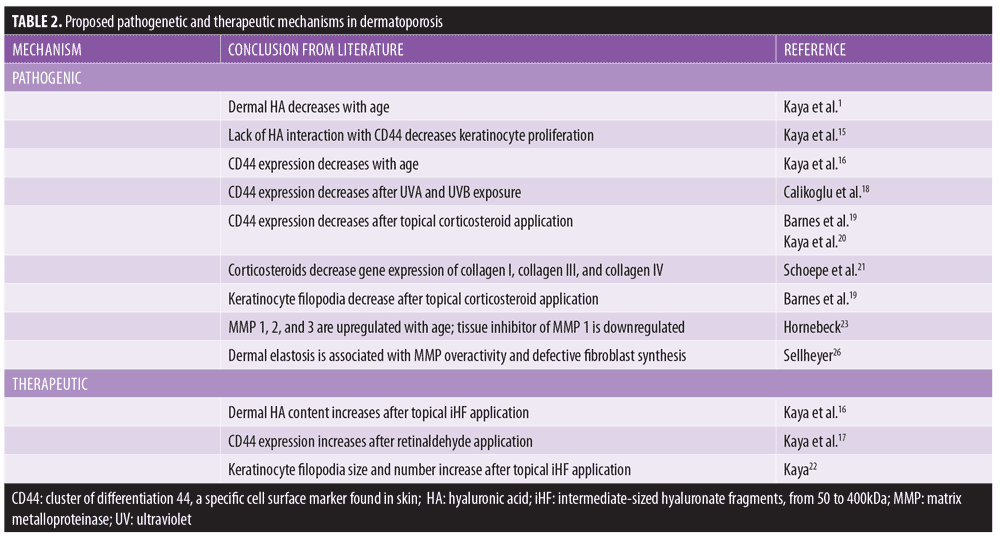
With age, skin becomes thinner and less capable of withstanding mechanical forces. The epidermis becomes attenuated, with effacement of rete ridges, as keratinocytes lose proliferative capacity. The dermis, chiefly comprised of extracellular matrix (ECM) with scattered fibroblasts and bolstered by an interwoven network of collagen and elastin fibers, loses volume.
The principle constituent of the ECM is hyaluronic acid (HA), a non-sulfated glycosaminoglycan produced mainly by fibroblasts. Hyaluronic acid is a highly hydrophilic substance that reduces friction between collagen fibers and allows resistance to shear forces. However, HA diminishes with advancing age, leaving skin vulnerable to tears from minimal trauma.1
HA also interacts with a cell surface receptor, CD44, which stimulates keratinocyte proliferation.15 Low levels of CD44 have been correlated with dermatoporotic skin when compared with young controls.16 Topical application of retinaldehyde increases expression of CD44 in skin,17 while ultraviolet (UV) light exposure decreases expression.18
Topical corticosteroids have been shown to reduce cutaneous CD44 expression,19 specifically an isotype called CD44v3 that is localized to keratinocyte membranes.20 Deficient CD44 is correlated with skin atrophy in this setting. Corticosteroids can also induce dermatoporotic changes through modulating gene expression of collagen I, collagen III, collagen IV, and matrix metalloproteinases (MMPs).21
A newly characterized organelle—the hyaluronosome—can be functionally deficient in patients with dermatoporosis.22 This organelle within keratinocyte membranes functions as a hyaluronate factory and contains CD44 and heparin-binding epidermal growth factor (HB-EGF).
Interestingly, these observations could be exploited for therapeutic gain. Topical application of intermediate-sized (50–400kDa) hyaluronate fragments (iHF) twice daily for one month led to epidermal hyperplasia and increased dermal HA content in a murine model.16 The use of small (1–50kDa) versus large (400–1,000kDa) hyaluronate fragments did not lead to similar results. After incubation with human keratinocytes in vitro, iHF induced HA production and increased number and size of keratinocyte filopodia.22
MMPs 1, 2, and 3 are upregulated in senescent skin, while a tissue inhibitor of MMP 1 is downregulated.23 This leads to the breakdown of collagen and elastin in the dermis with age. Ultraviolet (UV) irradiation has well-known deleterious effects on the skin. Ultraviolet B (UVB) damages skin cell deoxyribonucleic acid (DNA) directly by generating DNA photoproducts, most commonly cyclobutane dimers and 6,4-photoproducts.24 UVA, which penetrates to the dermis, triggers oxidative damage via reactive oxygen species (ROS) and thus indirectly harms DNA.25 UVA also activates MMPs and might alter mitochondrial DNA inside fibroblasts.1 Photodamaged skin demonstrates dermal elastosis, formed after overactive MMPs degrade collagen and elastin and irradiated fibroblasts, synthesize defective elastotic material.26
Proposed pathogenetic mechanisms in dermatoporosis are summarized in Table 2.
Evaluation
Clinical features of dermatoporosis. The initial signs of skin fragility emerge around 60 years of age and become well-established around 70 to 90 years of age.1 These signs could possibly be overlooked as inconsequential by the examining clinician and might be discussed in passing by the patient. Characteristic findings include atrophic skin, senile purpura, and white pseudoscars (Figure 1).
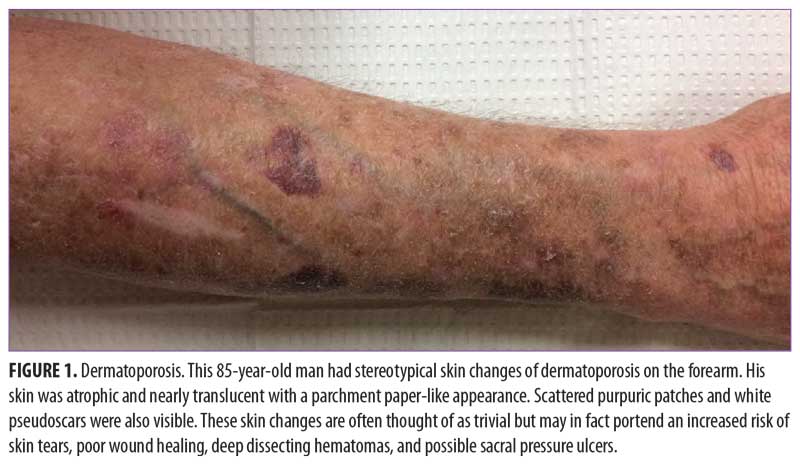
The thin skin of dermatoporosis may be nearly translucent, revealing a prominence of the underlying veins and tendons. It is frequently found on the sun-exposed area of the extremities, most commonly on the extensor forearms, dorsal hands, and the lower legs. Sometimes thinning skin develops on the presternal chest and scalp.22 Ultrasonography of atrophic skin shows a reduced thickness of the epidermis and dermis (about 0.7–0.8mm) in comparison with normal skin (about 1.4–1.5mm).1
Senile purpura, also called solar or actinic purpura, was first described by Bateman in 1818 after he discovered that violaceous patches on the forearms of elderly patients corresponded with extravasated erythrocytes in the dermis.27 The condition involves non-blanchable red to purple patches that resolve over 1 to 3 weeks in the manner of typical bruises, leaving residual brownish-yellow discoloration secondary to hemosiderin deposition. Often, senile purpura seems to arise spontaneously, as patients might not recall a history of trauma. A recent immunohistochemical study on microvasculature in senile purpura found increased thickness of basement membrane (collagen IV) around the dermal vessels of patients with senile purpura in comparison with those of healthy young controls.28 The authors hypothesized that increased vascular permeability, rather than mechanical rupture, led to a leakage of erythrocytes into the dermis and the deposition of collagen IV around the vessels as reparative phenomenon.
White pseudoscars are the third morphological feature of dermatoporotic skin. They are classically described as having a stellate configuration, but might also be linear or plaque-like.1 They are thought to represent stretching and fracture of dermal collagen fibers without macroscopic disruption of the overlying epidermis.
Complications of dermatoporosis. Linear skin tears and lacerations might occur after minimal trauma in patients with dermatoporosis. A Danish study of 140 nursing home residents aged 65 years or older found that residents with ecchymoses were more than five times more likely to have skin tears or have a history of skin tears than residents without ecchymoses.7
Delayed wound healing is a common problem in senescent skin, especially on the lower extremities (i.e., below the knee). While a detailed explanation of pathogenesis of delayed wound healing in the elderly population is beyond the scope of this article, dermatoporosis is an important risk factor for the development of chronic wounds, as keratinocytes and fibroblasts lack proliferative capacity in this setting and matrix metalloproteinases (MMPs) might be overexpressed.1,29
Deep dissecting hematomas (DDH) are a complication of dermatoporosis that usually occur on the lower legs after extensive bleeding into the potential space between skin and subcutaneous fat and along myofascia. The initial differential diagnosis includes cellulitis, as it is unilateral and manifests as an erythematous, edematous, warm leg. Fever and other signs of infection are absent. Eventually, skin necrosis may occur superficial to the DDH. Immediate surgical evacuation and debridement of the necrotic tissue is the treatment of choice. Anticoagulation and the use of systemic corticosteroids are risk factors.1 One case series from Switzerland described 34 patients with DDH, with females being up to five times more likely to be affected than males.30 All patients underwent deep incision or surgical debridement, with 17 (50%) requiring split-thickness skin grafting and seven (21%) requiring vacuum-assisted closure therapy. The mean hospital stay was 24 days with a mean cost of $32,000 per patient.
A recent case series from Japan reports three patients with sacral pressure ulcers who also demonstrated dermatoporosis of the forearms.31 They postulate that the presence of dermatoporosis of the forearms may confer a higher risk of pressure ulcer development.
Dermatoporosis in post-menopausal women may be linked to a risk of osteoporosis and osteoporotic fractures. Many decades ago, publications linked thin, transparent skin with brittle bones.32,33
Self-diagnosis tool. Published in 2017, Saurat et al developed the Index Dermatoporosis Assessment (IDA), a self-diagnosis tool validated in French individuals aged 65 years or older.3 The IDA is a 14-item questionnaire with positive and negative predictive values of 0.78 and 0.81, respectively.
Prevention
Sun protection measures, including topical application of broad-spectrum sunscreen, wearing long sleeves and pants, avoiding the midday sun, and participating in shade-seeking behavior should be encouraged. Purpura and skin lacerations can be reduced by avoiding furniture with sharp edges. Tibia protectors can be inserted into stockings to protect against DDH.30
Emollients can be recommended for epidermal barrier repair. Moisturizer application and padded bed rails have been associated with fewer skin tears among elderly inpatients.7,34 A preventive skin protocol was employed in one study that examined nosocomial skin tears among 209 patients in a long-term care facility.35 The protocol included nurse training, skin sleeves, padded side rails, gentle skin cleansers, and daily use of skin moisturizer. Fifteen months post-intervention, the number of skin tears decreased from 18.7 per month to 8.73 per month (P<.001).
Therapy
A literature-based approach to the treatment of dermatoporosis in patients aged 65 years or older is summarized in Table 3.
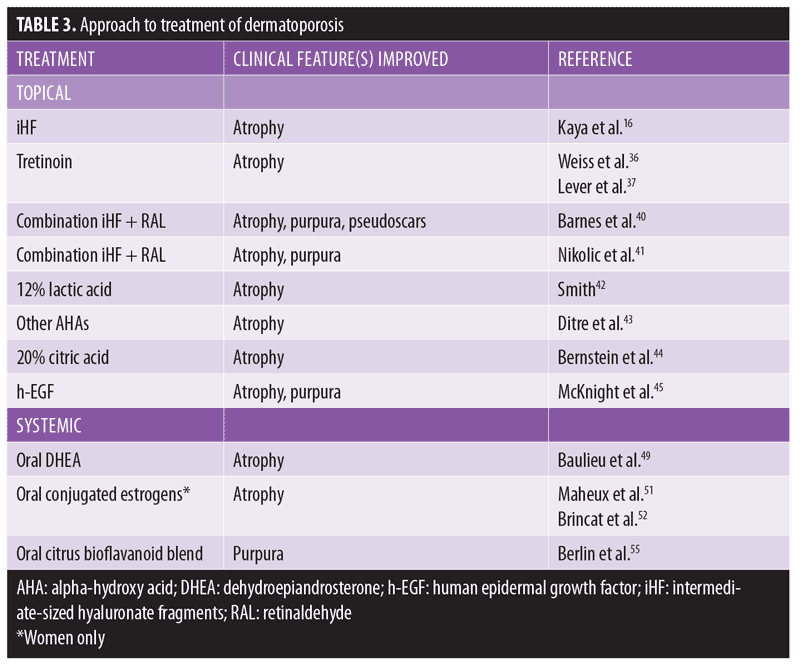
Topical approach. Topical retinoids are a mainstay in reversing thin skin. Multiple studies report improvement of skin atrophy after topical tretinoin was applied to the forearms in photoaged skin of older adults.36,37 Topical retinoids have been shown to stimulate epidermal hyperplasia, possibly by inducing HB-EGF.38,39
As discussed, topical iHF might benefit fragile, atrophic skin.1 Topical application of iHF 1% on the forearms of patients with dermatoporosis for one month led to significant reduction of purpura and an improvement in atrophy.16 Echographic imaging of these patients depicted increased skin thickness, with severely dermatoporotic patients showing the most improvement. Topical iHF with retinaldehyde might demonstrate a more pronounced synergistic effect on keratinocyte proliferation versus either solution alone.40,41
Alpha-hydroxy acid (AHA) products might combat dermatoporosis. In one study, 42 subjects applied either 5% or 12% lactic acid twice daily for three months. At the conclusion of treatment, the group using 12% lactic acid noted increased epidermal and dermal firmness, thickness, and smoothness. Studies examining other AHAs have shown similar efficacy, improving skin atrophy from 16.3 percent to 25 percent.43,44
McKnight and colleagues45 investigated the efficacy of topical human epidermal growth factor (h-EGF) in the treatment of senile purpura. Six subjects applied h-EGF topically twice daily for six weeks. Purpuric lesions decreased from a mean of 15 to 2.3. Additionally, ultrasound examination of the skin showed a trend toward increased thickness, from 0.876mm to 1.071mm.
A single randomized, control trial examined topical dehydroepiandrosterone (DHEA) 1% cream twice daily for four months for the treatment of atrophic skin of women.46 Investigators found it counteracted the papery appearance and epidermal atrophy associated with postmenopausal skin aging.
Systemic approach. With regards to general nutrition, older adults should be cognizant of ingesting adequate protein. Low protein intake is associated with decreased immune function, poorer healing, and increased skin fragility.47 Traditionally, recommended daily protein intake is 0.8mg/kg. However, adults older than 70 years of age should consume 1.0g/kg per day, with higher amounts necessary to aid in healing wounds or replace normal losses. Animal protein (i.e., meat, poultry, and seafood) is the dietary source of protein with the greatest biological value.48 One author considers eggs the most cost-effective and valuable source of protein in terms of amino acid profile.47
There is scant literature regarding systemic therapy with dehydroepiandrosterone (DHEA), a weak androgen produced by the adrenal glands, in the treatment of thin skin. Ballieu and colleagues49 showed increased skin hydration, enhanced sebogenesis, and reduced skin atrophy after one year of oral DHEA 50mg daily in a randomized, placebo-controlled trial of 280 men and women aged 60 to 79 years. Oral DHEA supplementation for skin tear prophylaxis needs more research.50
In postmenopausal women, estrogen replacement therapy is well-established in the reversal of cutaneous atrophy. A randomized, double-blind, placebo-controlled study by Maheux and colleagues51 demonstrated a significant increase in skin thickness in postmenopausal women taking conjugated estrogens. Other studies have corroborated this finding.52–54
A single, placebo-controlled, double-blind study evaluated the efficacy of a citrus bioflavanoid blend in the treatment of senile purpura.55 Sixty-seven subjects with senile purpura were randomized to receive the proprietary oral supplement or placebo twice daily for six weeks. The group supplemented with the oral citrus bioflavanoid blend experienced a 50-percent reduction in purpuric lesions compared to the placebo group.
Other. One author recommended a novel method of skin closure for aging or fragile skin.56 After cleansing but before excision, the surgeon advised applying a polyethylene film with acrylate adhesive over the surgical site. The lesion is then excised and margins are approximated, with the suture needle passing through both the film and skin. The adhesive film may remain in place for two or more weeks until suture removal. This technique might bolster wound edges and minimize risk of dehiscence in elderly patients.
Conclusion
While cutaneous failure in the acute setting is well-known as toxic epidermal necrolysis, dermatologic literature has historically left physicians unequipped to discuss skin failure secondary to chronic decline in function. Through the concept of dermatoporosis, skin fragility of the elderly has been highlighted as a unique clinical syndrome, and further research should be undertaken to address its prevention and reduce its sequelae.
References
- Kaya G, Saurat JH. Dermatoporosis: a chronic cutaneous insufficiency/fragility syndrome. clinicopathological features, mechanisms, prevention and potential treatments. 2007;215(4):284–294.
- Mengeaud V, Dautezac-Vieu C, Josse G, et al. Prevalence of dermatoporosis in elderly French hospital in-patients: a cross-sectional study. Br J Dermatol. 2012;166(2):442–443.
- Saurat JH, Mengeaud V, Georgescu V, et al. A simple self-diagnosis tool to assess the prevalence of dermatoporosis in France. J Eur Acad Dermatol Venereol. 2017;31(8):1380–1386.
- Reszke R, Pelka D, Walasek A, et al. Skin disorders in elderly patients. Int J Dermatol. 2015;54(9):e332–e338.
- Carville K, Lewin G. Caring in the community: a wound prevalence study. Prim Inten. 1998;6:54–62.
- Everett S, Powell T. Skin tears—the underestimated wound. Prim Inten.1994;2(1):8–30.
- Skiveren J, Wahlers B, Bermark S. Prevalence of skin tears in the extremities among elderly residents at a nursing home in Denmark. J Wound Care. 2017;26(Sup2):S32–S36.
- Sanada H, Nakagami G, Koyano Y, et al. Incidence of skin tears in the extremities among elderly patients at a long-term medical facility in Japan: a prospective cohort study. Geriatr Gerontol Int. 2015;15(8):1058–1063.
- Colby SL, Ortman JM. The baby boom cohort in the United States: 2012 to 2060. United States Census Bureau. 2014;5:1–16.
- Gerber PA, Buhren BA, Schrumpf H, et al. Mechanisms of skin aging induced by EGFR inhibitors. Support Care Cancer. 2016;24(10):4241–4248.
- Whitmore SE, Levine MA. Risk factors for reduced skin thickness and bone density: possible clues regarding pathophysiology, prevention, and treatment. J Am Acad Dermatol. 1998;38(2 Pt 1):248–255.
- Malone ML, Rozario N, Gavinskin M, Goodwin J. The epidemiology of skin tears in the institutionalized elderly. J Am Geriatr Soc. 1991;39(6):591–595.
- McGough-Csarny J, Kopac C. Skin tears in institutionalized elderly: an epidemiological study. Ostomy Wound Manage. 1998;44(3A Suppl): S14–S25.
- Koyano Y, Nakagami G, Iizaka S, et al. Skin property can predict the development of skin tears among elderly patients: a prospective cohort study. Int Wound J. 2017;14(4):691–697.
- Kaya G, Rodriguez I, Jorcano JL, et al. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 1997;11(8):996–1007.
- Kaya G, Tran C, Sorg O, et al. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PloS Med. 2006;3(12):e493.
- Kaya G, Grand D, Hotz R, et al. Upregulation of CD44 and hyaluronate synthases by topical retinoids in mouse skin. J Invest Dermatol. 2005;124(1):284–287.
- Calikoglu E, Sorg O, Tran C, et al. UVA and UVB decrease expression of CD44 and hyaluronate in mouse epidermis which is counteracted by topical retinoids. Photochem Photobiol. 2006;82(5): 1342–1347.
- Barnes L, Ino F, Jaunin F, Saurat JH, Kaya G. Inhibition of putative hyalurosome platform in keratinocytes as a mechanism for corticosteroid-induced epidermal atrophy. J Invest Dermatol. 2013;133(4):1017–1026.
- Kaya G, Tran C, Sorg O, et al. Topical hyaluronate fragments prevent corticosteroid-induced skin atrophy in mouse without interfering with anti-inflammatory effect. J Invest Dermatol. 2007;127:S55.
- Schoepe S, Schäcke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006;15(6):406–420.
- Kaya G. New therapeutic targets in dermatoporosis. J Nutr Health Aging. 2012;16(4):285–288.
- Hornebeck W. Down-regulation of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol. 2003;51(10):569–573.
- Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571(1–2):3–17.
- Kvam E, Tyrell RM. Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. 1997;18(12):2379–2384.
- Sellheyer D. Pathogenesis of solar elastosis: synthesis or degradation?. J Cutan Pathol. 2003;30(2):123–127.
- Bateman T. A Practical Synopsis of Cutaneous Diseases. London, England: Longman, Hurst, Reese, and Brown; 1818: 118–119.
- Borroni RG, Grassi S, Concardi M, et al. Involvement of dermal microvascular basement membrane in senile purpura: quantitative immunohistochemical study. J Eur Acad Dermatol Venereol. 2016;30(10):e63–e65.
- Bolognia JL. Dermatologic and cosmetic concerns of the older woman. Clin Geriatr Med. 1993;9(1):209–229.
- Kaya G, Jacobs F, Prins C, et al. Deep dissecting hematoma: an emerging severe complication of dermatoporosis. Arch Dermatol. 2008;144(10):1303–1308.
- Kurashige Y, Minemuta T, Nagatani T. Three cases of sacral pressure ulcers presenting primary dermatoporosis on the forearms. Case Reports Dermatol. 2013;5(1):73–78.
- Albright F, Bloomberg E, Smith PH. Postmenopausal osteoporosis. Trans Assoc Am Physicians. 1940;55:298–305.
- McConkey B, Fraser GR, Bligh AS, et al. Transparent skin and osteoporosis. 1963;I:693–695.
- Carville K, Leslie G, Osseiran-Moisson R, et al. The effectiveness of twice-daily skin-moisturising regimen for reducing the incidence of skin tears. Int Wound J. 2014;11(4):446–453.
- Bank D, Nix D. Preventing skin tears in a nursing and rehabilitation center: an interdisciplinary effort. Ost Manage. 2006;52(9):38–40.
- Weiss JS, Ellis CN, Headington JT, et al. Treatment of photodamaged facial skin with topical tretinoin. J Am Med Assoc. 1988;259:527–532.
- Lever L, Kumar P, Marks R, et al. Topical retinoic acid in treatment of solar damage. Br J Dermatol. 1990;122(1):91–98.
- Didierjean L, Carraux P, Grand D, et al. Topical retinaldehyde increases skin content of retinoic acid and exerts biologic activity in mouse skin. J Invest Dermatol. 1996;107(5):714–719.
- Saurat JH, Didierjean L, Masgrau E, et al. Topical retinaldehyde on human skin: biologic effects and tolerance. J Invest Dermatol. 1994;103(6):770–774.
- Barnes L, Tran C, Sorg O, et al. Synergistic effect of hyaluronate fragments in retinaldehyde-induced skin hyperplasia which is a Cd44-dependent phenomenon. PLoS One. 2010;5(12):e14372.
- Nikolic DS, Ziori C, Kostaki M, et al. Hyalurosome gene regulation and dose-dependent restoration of skin atrophy by retinaldehyde and defined-size hyaluronate fragments in dermatoporosis. 2014;229(2):110–115.
- Smith WP. Epidermal and dermal effects of topical lactic acid. J Am Acad Dermatol.1996;35(3 Pt 1):388–391.
- Ditre CM, Griffin TD, Murphy GF, et al. Effects of alpha-hydroxy acids on photoaged skin: a pilot clinical, histologic, and ultrastructural study. J Am Acad Dermatol. 1996;34(2 Pt 1):187–195.
- Bernstein EF, Underhill CB, Lakkakorpi J, et al. Citric acid increases viable epidermal thickness and glycosaminoglycan content of sun-damaged skin. Dermatol Surg. 1997;23(8):689–694.
- McKnight B, Seidel R, Moy R. Topical human epidermal growth factor in the treatment of senile purpura and the prevention of dermatoporosis. J Drugs Dermatol. 2015;14(10):1147–1150.
- Nouveau S, Bastien P, Baldo F, et al. Effects of topical DHEA on aging skin: a pilot study. 2008;59(2):174–181.
- Chernoff R. Protein and older adults. J Amer Coll Nutr. 2004;23(6):627S–630S.
- Food and Nutrition Board, National Research Counsel. Recommended Dietary Allowances. 10th ed. Washington, DC: National Academy Press; 1989.
- Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge study to a sociobiomedical issue. Proc Natl Acad Sci USA. 2000;97(8):4279–4284.
- Daniell HW. Potential prevention by oral DHEA of superficial tears in elderly atrophic skin. J Steroid Biochem Mol Biol. 2017;171:155–156.
- Maheux R, Naud F, Rioux M, et al. A randomized, double-blind, placebo-controlled study on the effect of conjugated estrogens on skin thickness. Am J Obstet Gynecol. 1994;170(2):642–649.
- Brincat M, Moniz CJ, Studd JW, et al. Long-term effects of the menopause and sex hormones on skin thickness. Br J Obstet Gynaecol. 1985;92(3):256–259.
- Rauramo L, Punnonen R. [Effect of oral estrogen treatment with estriol succinate on the skin of castrated women]. Z Haut Geschlechtskr. 1969;44(13):4463–470. Article in German.
- Punnonen R. On the effect of castration and peroral estrogen therapy on the skin. Acta Obstet Gynecol Scand. 1971;9 Suppl 9:32.
- Berlin JM, Eisenberg DP, Berlin MB, et al. A randomized, placebo-controlled, double-blind study to evaluate the efficacy of a citrus bioflavanoid blend in the treatment of senile purpura. J Drugs Dermatol. 2011;10(7):718–722.
- Lipnik MJ. A novel method of skin closure for aging or fragile skin. 2015;96(4):260–262.

