 J Clin Aesthet Dermatol. 2021;14(8):57–60.
J Clin Aesthet Dermatol. 2021;14(8):57–60.
by Nuntida Salakshna, MD; Wilai Thanasarnaksorn, MD;and Thanan Supasiri, MD
Dr. Thanasarnaksorn is with the Samitivej Esthetics Institute, Samitivej Sukhumvit Hospital, and the Division of Dermatology, Department of Medicine, Faculty of Medicine at Ramathibodi Hospital of Mahidol University in Bangkok, Thailand. Dr. Salakshna is with the Samitivej Esthetics Institute, Samitivej Chinatown Hospital in Bangkok, Thailand and the Science Division at Mahidol University International College in Nakhon Pathom, Thailand. Dr. Supasiri is with the Life Center, Samitivej Sukhumvit Hospital, and the Department of Preventive and Social Medicine, Faculty of Medicine at Chulalongkorn University in Bangkok, Thailand.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Botulinum toxin type A (BTxA) is used for cosmetic procedures, but its use for nasal dorsum augmentation has, to our knowledge, never been studied.
Objective. Here, we describe a method for using BTxA injection for nasal dorsum augmentation.
Methods. This was a pilot study. Participants aged 20 to 60 years were recruited and injected with BTxA in the upper nasal area with either Xeomin® (Merz Pharmaceuticals GmbH, Frankfurt, Germany) or Dysport® (Galderma Laboratories, Fort Worth, Texas). The primary outcome measured was the upper nasal area’s volume change from baseline, calculated using a Quantificare camera (San Francisco, California). The volumizing effect was subjectively graded by two blinded dermatologists and participants, and pain scores and adverse events were recorded.
Results. Fourteen participants, including two men and 12 women, aged 35.78±9.16 years were recruited. Overall, the volume of the upper nasal area increased after BTxA injection (p<0.001). The volume increase immediately and, at one week after injection, presented a statistically significant difference from baseline, with median (interquartile range) volume differences of 0.095mL (0.010–0.205; p<0.001) and 0.095mL (0.0475–0.155; p<0.001), respectively. Two blinded dermatologists and all participants observed volumizing of the nasal dorsum. The effect appeared to last for one month.
Conclusion. This study suggests that injecting BTxA in the upper nasal area is a convenient, minimally invasive technique with minimal side effects for nasal dorsum augmentation.
Keywords: Nasal dorsum augmentation, botulinum toxin, injection
Nasal dorsum augmentation is one of the most common aesthetic procedures, especially in Asian patients, due to their commonly low nasal bridge.1 The two most common procedures for dorsal augmentation are surgical rhinoplasty and filler rhinoplasty. However, there are many procedural complications that can occur following surgical rhinoplasty, such as asymmetry, infection, numbness, and extrusion of prosthetic implants.2 Conversely, filler rhinoplasty is noninvasive3; however, there are serious potential vascular complications, such as vision loss and skin necrosis.2–5
Botulinum toxin A (BTxA) reduces imperfections due to muscle hypertonicity.6 BTxA injection is a minimally invasive procedure with few complications that has been used to correct the nasal profile for plunging nasal tip and nasal ala,6 but, to our knowledge, has not yet been studied for nasal dorsum augmentation. We aim to use BTxA injections to lift the nasal dorsum. The technique illustrated in this study is a potential alternative to surgical intervention or filler injection.
Methods
A quasi-experimental pilot study was performed to determine the clinical effects and safety of BTxA for nasal dorsum augmentation. We recruited participants aged 20 to 60 years with no prior aesthetic procedures in the nose or glabella area. Participants were excluded if they had any underlying neuromuscular diseases. Participants were injected with BTxA, either with Xeomin® (incobotulinum toxin; Merz Pharmaceuticals GmbH, Frankfurt, Germany) or Dysport® (abobotulinum toxin A; Galderma Laboratories, Fort Worth, Texas). Xeomin® (50U) was diluted with saline solution to 1U BTxA per 0.025mL. Dysport® (500U) was diluted with saline solution to 1U BTxA per 0.015mL.8 A cooling pack was applied to the nasal dorsum before injection, which used a 1-mL syringe and 30-gauge needle. BTxA was injected in tiny aliquots on the midline and lateral portions along the upper nasal dorsum, targeted to the superficial layer of the procerus muscle with the needle directed at 15 degrees from the dorsum (Figure 1). The total amount of BTxA injected was 4U (Xeomin®) or 7U (Dysport®).
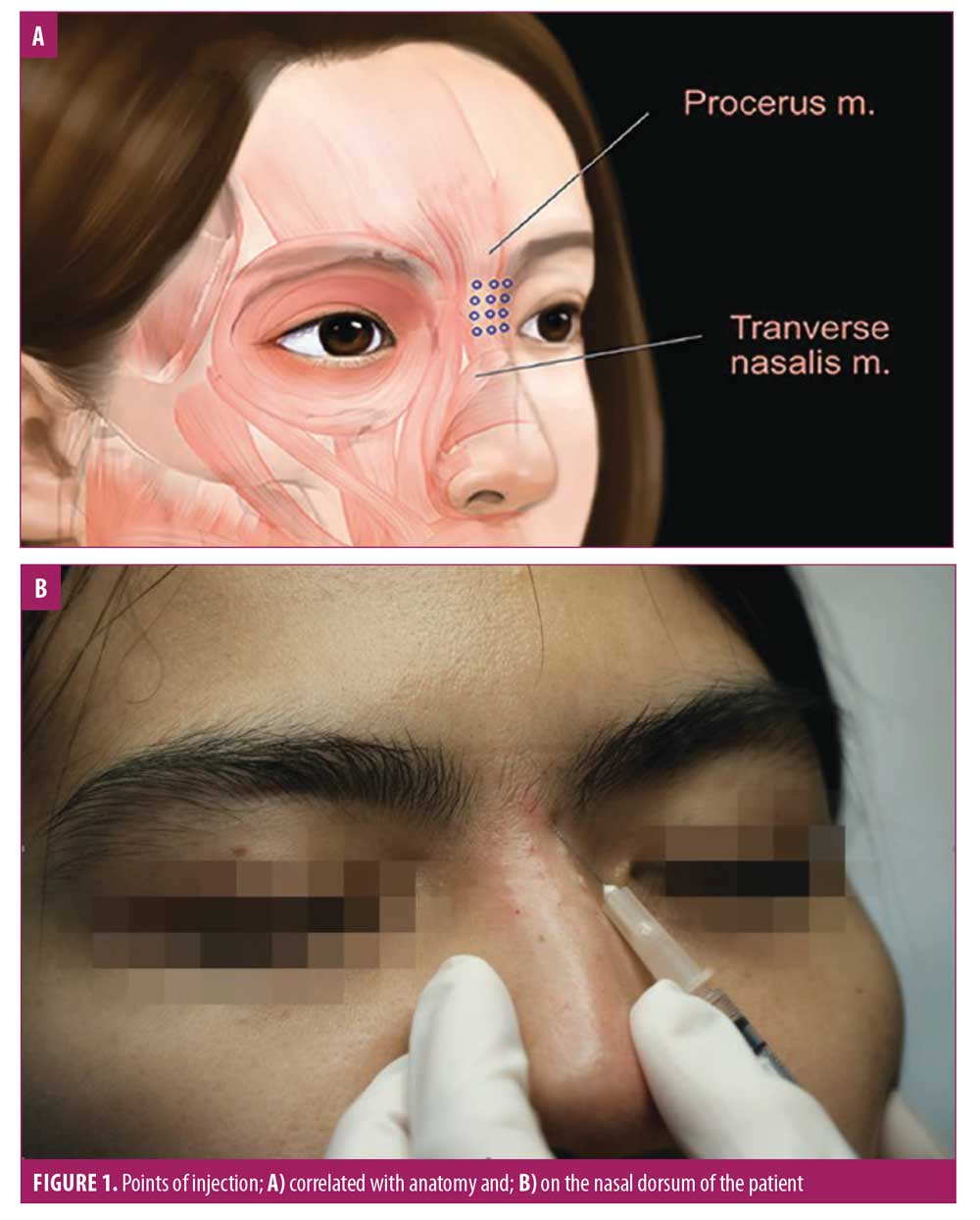
Participants were assessed at the baseline immediately after the BTxA injection and at one week, two weeks, one month, and two months after the treatment. All participants were photographed in anteroposterior and 45 degrees positions from both the right and left sides using a Quantificare camera (San Francisco, California). Photographs were also taken using a digital single-lens reflex camera in anteroposterior and 90-degree positions from both the right and left sides. The primary outcome was the volume change from baseline, calculated using the Quantificare camera. The nasal augmentation was also subjectively graded by two blinded dermatologists using the photographs and by participants using a quartile grading scale (0=no improvement, 1=1%–25% improvement, 2=26%–50% improvement, 3=51%–75% improvement, and 4=76%–100% improvement). Participants rated pain by using a visual analog scale (0–10 points). Side effects were recorded immediately after the procedure and at every study visit.
Baseline characteristics and data at each study visit were analyzed by descriptive statistics. The main outcome (i.e., volume difference in the nasal dorsum from baseline, was analyzed with the Friedman test, performed using the Statistical Package for the Social Sciences version 25.0 (IBM Corporation, Armonk, New York) and STATA/IC 15.1 (StataCorp LLC, College Station, Texas).
Results
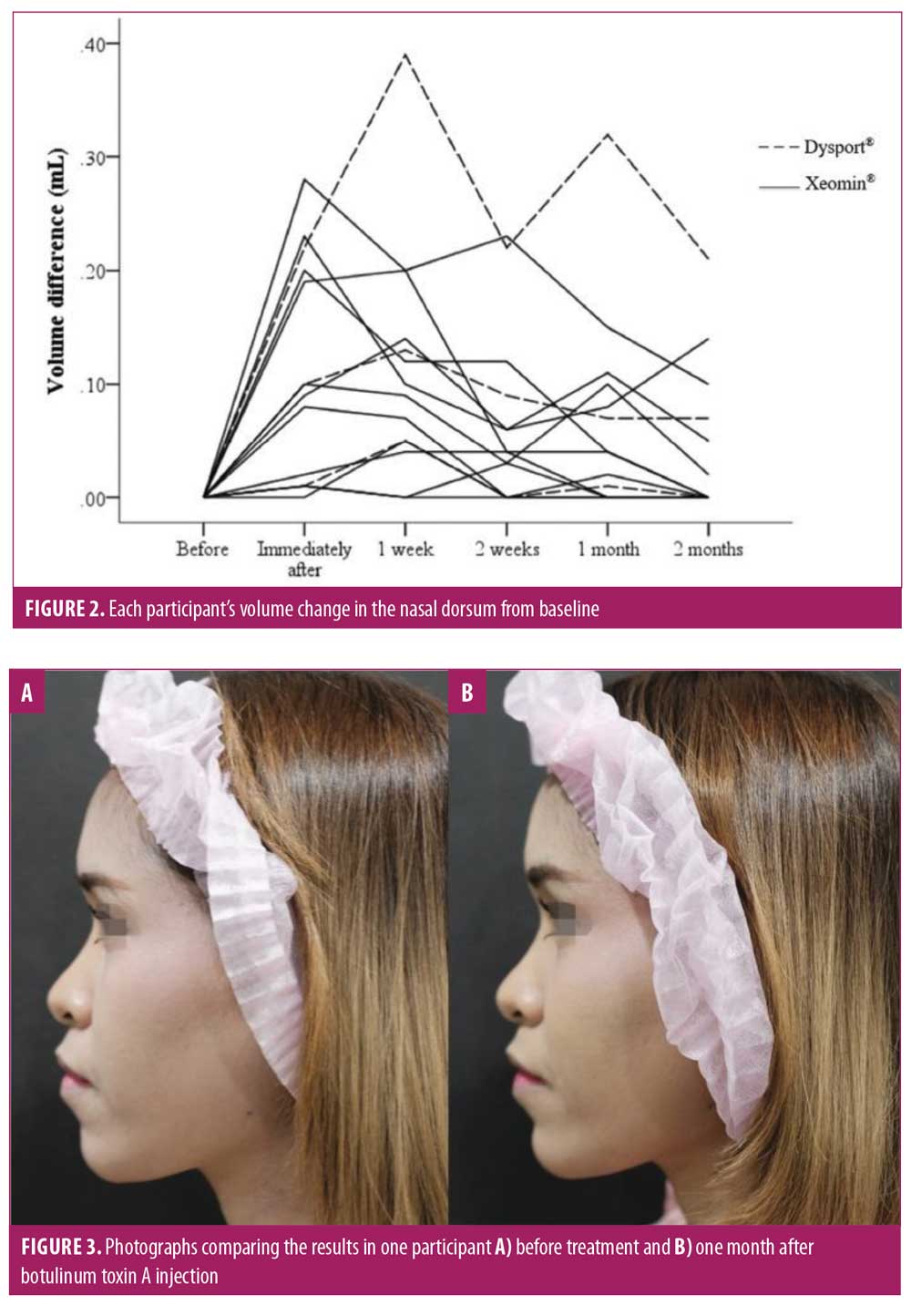
Fourteen participants, including two men and 12 women, aged 35.78±9.16 years were recruited for this pilot study. Eleven participants were injected with Xeomin® and three participants were injected with Dysport®. Each participant’s volume differences in the nasal dorsum from baseline is shown in Figure 2 and summarized in Table 1. The nasal volume increased significantly immediately and at one week after treatment, with median (interquartile range) volume differences of 0.10mL (0.01–0.21; p<0.001) and 0.10mL (0.05–0.16; p<0.001), respectively. The volume of the nasal dorsum remained increased after one month, with a median (interquartile range) volume difference of 0.04mL (0–0.10), although this result was not statistically significant (p=0.14). After two months, eight participants showed no change from baseline, but six participants still saw some effect. The Friedman test for overall nasal volume difference showed statistical significance with a p-value of less than 0.001. Two blinded dermatologists observed volumizing of the nasal dorsum in all participants, with improvement ranging from 51 percent to 100 percent from baseline during the follow-up period, consistent with the participants’ grading (Table 1). None of the participants experienced any significant adverse effects. The pain was tolerated by all participants, with only a mild to moderate stinging sensation reported during injection, scored as 2.92±1.14.
Discussion
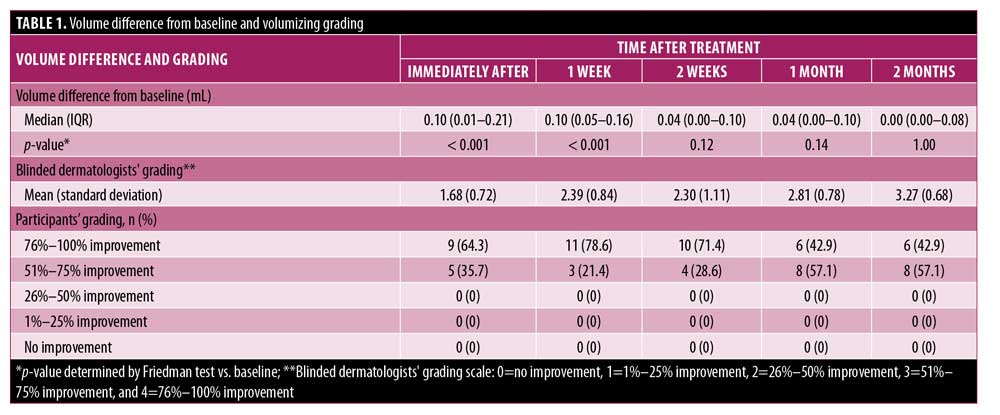
Our findings suggest that injecting BTxA had a volumizing effect on all participants’ nasal dorsa. The immediate result was statistically significantly increased until one week after treatment. Even though the effect at one month was not statistically significant, this could be due to our small sample size; thus, it is possible that the effect could last for at least one month. Although we measured the nasal volume increase, we focused on raising the nasal dorsum in Asian patients with mostly low nasal bridges. All participants reported being satisfied with their results for at least two months, consistent with the scores reported by the two blinded dermatologists.
We propose a method of using BTxA for nasal dorsum augmentation. It could be an alternative to surgical intervention or filler injection due to the appreciable results and minimal adverse events following BTxA injection. Although the effects of one injection only last for about 1 to 2 months, BTxA injection has well-documented rules that allow for reproducible results over time without major side effects.6
BTxA injection has been used for aesthetic purposes for many decades because it relaxes the muscles.1,7 In the nasal area, BTxA’s nasal dorsum augmentation effect could be explained by triggering relaxing of the procerus muscle, which can be either a nasal elevator or a depressor, depending on various associated anatomical relationships.9 The superficial layer of the procerus consists of a middle part and two lateral parts, and it extends to the transverse part of the nasalis, indicating that the procerus can assist in depressing the nose.9 Thus, relaxing the superficial layer of the procerus with BTxA can cause nasal dorsum elevation. Meanwhile, the lateral part of the superficial layer of the procerus extends to the lateral part of the nose, which can also be a target point of injection to highlight the lateral part of the nose.
Limitations. Our study had some limitations. The volume of the nasal dorsum is very small, so a precision of volume measurement was difficult to achieve. The concentration of BTxA used in this study was based on previous experience. Further study is required to discern suitable concentrations and dosage. Since this was a pilot study with a small sample size, a larger study with a longer follow-up period might offer additional insight and verification.
Conclusion
This study demonstrated the ability to temporarily augment the nasal dorsum by BTxA injection. This approach is an alternative option to surgery or filler rhinoplasty and is minimally invasive, has minimal side effects, and can be conveniently done.
Acknowledgment
We acknowledge Dr. Kiattawee Thanasawad, who inspired this injection technique, and Professor Dr. Chulaluk Komoltri, Faculty of Medicine Siriraj Hospital, for statistical advice.
References
- Redaelli A. Medical rhinoplasty with hyaluronic acid and botulinum toxin A: a very simple and quite effective technique. J Cosmet Dermatol. 2008;7(3):210–220.
- Liew S, Scamp T, de Maio M, et al. Efficacy and safety of a hyaluronic acid filler to correct aesthetically detracting or deficient features of the Asian nose: a prospective, open-label, long-term study. Aesthet Surg J. 2016;36(7):760–772.
- Singh S. Practical tips and techniques for injection rhinoplasty. J Cutan Aesthet Surg. 2019;12(1):60–62.
- Robati RM, Moeineddin F, Almasi-Nasrabadi M. The risk of skin necrosis following hyaluronic acid filler injection in patients eith a history of cosmetic rhinoplasty. Aesthet Surg J. 2018;38(8):883–888.
- Thanasarnaksorn W, Cotofana S, Rudolph C, et al. Severe vision loss caused by cosmetic filler augmentation: case series with review of cause and therapy. J Cosmet Dermatol. 2018;17(5):712–718.
- Redaelli A, Limardo P. Minimally invasive procedures for nasal aesthetics. J Cutan Aesthet Surg. 2012;5(2):115–120.
- Wanitphakdeedecha R, Ungaksornpairote C, Kaewkes A, et al. The comparison between intradermal injection of abobotulinumtoxinA and normal saline for face-lifting: a split-face randomized controlled trial. J Cosmet Dermatol. 2016;15(4):452–457.
- Sirithanabadeekul P, Lapsomboonsiri S, Rungjang A, et al. Split face comparison between common concentration vs double dilution of intradermal abobotulinum toxin type A (Dysport) injection for facial lifting in Asians. J Cosmet Dermatol. 2018;17(3): 355–360.
- Hur MS. Anatomical relationships of the procerus with the nasal ala and the nasal muscles: transverse part of the nasalis and levator labii superioris alaeque nasi. Surg Radiol Anat. 2017;39(8):865–869.
- Chang SP, Tsai HH, Chen WY, et al. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47(12):1287–1294.

