 J Clin Aesthet Dermatol. 2018;11(11):20–24
J Clin Aesthet Dermatol. 2018;11(11):20–24
by Celine Couteau, PhD; Eva Paparis; and Laurence Coiffard, PhD
Drs. Couteau and Coiffard and Ms. Paparis are with the Faculty of Pharmacy at the University of Nantes in Nantes, France.
FUNDING: No funding was provided.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
Abstract. Background. In patients undergoing chemotherapy, the application of nail polish and/or a colorless base coat is recommended in order to prevent the adverse effects that can occur on the nails throughout treatment.
Objective.In this study, the photoprotective effects of different clear nail polishes were examined.
Methods.Twelve commercially available, colorless nail products were tested in vitro. Two layers of the product were applied, as recommended to patients, on polymethyl methacrylate (PMMA) plates. The efficacy indices in the ultraviolet (UV) B sun protection factor (SPF) and UVA regions were then determined with an integrated sphere spectophotometer. Water resistance was also evaluated.
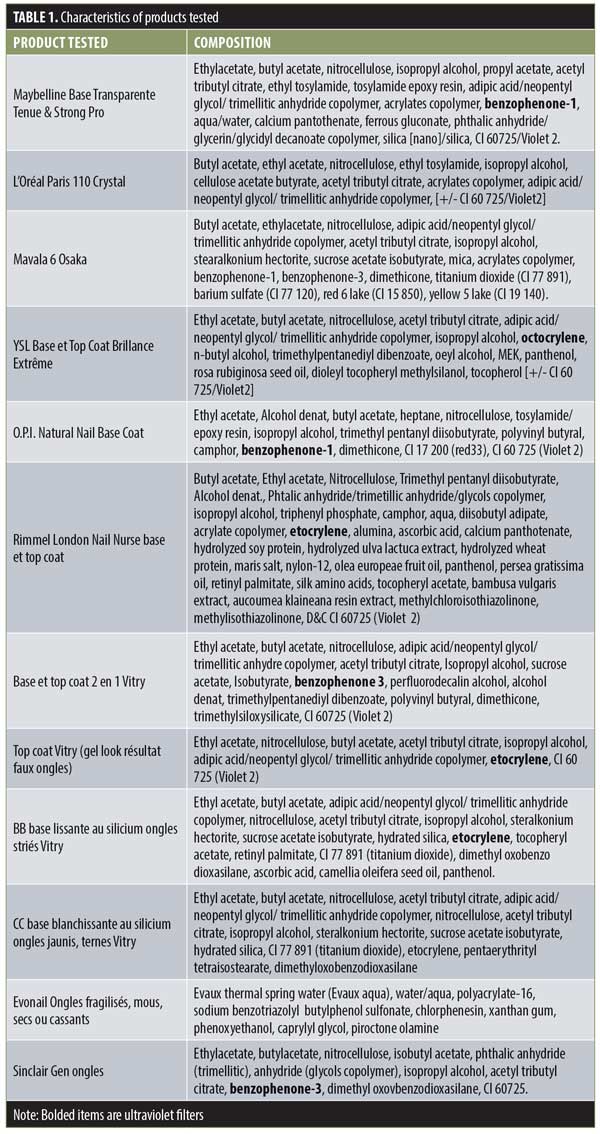
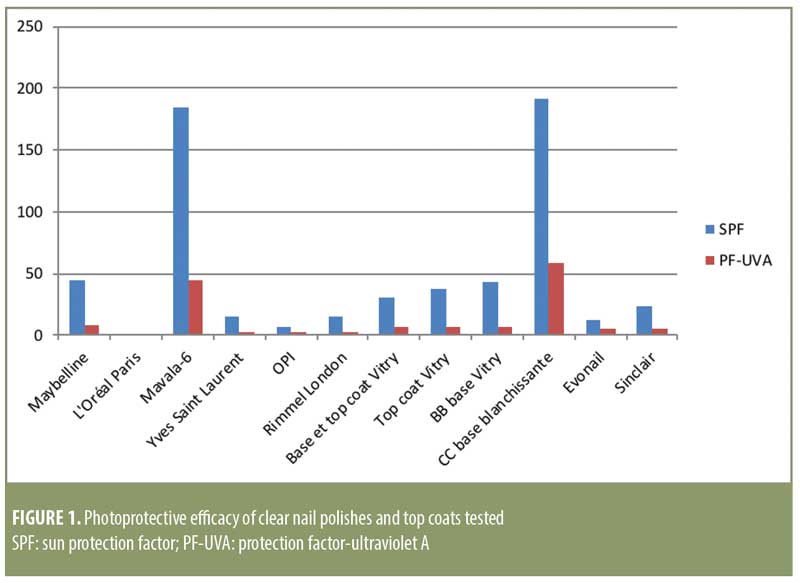
Results. Certain colorless nail polishes, such as Mavala 6 Osaka or CC bleaching base with silicium for yellow nails (Vitry), can provide a significant level of photoprotection, with SPF values of above 150. All of the products tested, with the exception of the aqueous gel, are water resistant.
Conclusion. Colorless nail polish applied in two layers provides UV protection for the nails in the UVB and UVA ranges. The nature of the vehicle influences the photoprotective efficiency and the water resistance.
Keywords: Colorless nail polish, efficacy, prevention, water resistance
Introduction
Adverse effects concerning the nails are common in patients with cancer who are undergoing chemotherapy. In cases where cytotoxic drugs are used, these adverse effects are observed mainly in the nail bed, whereas targeted therapies cause damage on the perionychium.1 Although the application of nail polish is not documented, in many cancer treatment centers, patients receiving treatment for cancer apply 1 to 2 coats of nail polish throughout the treatment period and for 5 to 6 weeks after the end of treatment. Once-daily application of filmogenic solution during the course of chemotherapy and for at least 2 to 3 months thereafter is an alternative to nail polish for reducing ungual or periungual damage.2–4 In a recent study, we assessed the efficiency of approximately 50 nail polishes of various colors in protecting against ultraviolet (UV) B sun protection factor (SPF) and UVA rays.5 At a practical level, the person undergoing treatment does not always wish to use colored nail polish, especially if the patient is male. In the study presented here, we evaluate the photoprotective effects of colorless nail polish formulas.
Materials and Methods
We included 12 commercially available products (Table 1), one of which is an aqueous gel. The products tested came from different distribution channels, including pharmacy (e.g., Evonail, Sinclair, Vitry), hypermarkets and supermarkets (e.g., L’Oréal Paris, Maybelline), perfumeries (e.g., OPI, Rimmel London, Yves Saint Laurent), and beauty salons (e.g., Mavala).
Two nail polish layers, according to the protocol recommended to patients, were applied over the entire surface (25cm²) of polymethyl methacrylate (PMMA) plates (Europlast, Aubervilliers, France) using the dispensing device remover from the bottle. Three plates were prepared for each product and nine measures were performed on each plate.5 Transmission measurements between 290nm and 400nm or 320nm and 400nm were carried out using a spectrophotometer equipped with an integrating sphere (UV Transmittance Analyzer UV1000S; Labsphere, North Sutton, New Hampshire). The calculations for either range used the following mathematical formula:
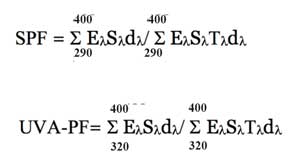
E-lambda is the International Commission on Illumination (CIE) erythemal spectral effectiveness, S-lambda is the solar spectral irradiance, T-lambda is the spectral transmittance of the sample at wavelength lambda, d-lambda is the wavelength increment (1 nm).6–8 To measure water resistance, the plates were immersed in a polycarbonate bath (Cuve IKA® Werke EH4.1; Grosseron, St. Herblain, France) equipped with an immersion heater (thermoplongeur Yellow line Basic ET; Grosseron, St. Herblain, France) filled with distilled water or salt water, at a rate of two successive baths of 20 minutes with each punctuated by a drying period. This system allowed us to maintain the water temperature at 29?±2°C with the possibility of creating a moderate agitation (pumping head of 5L/min?1). SPF and UVA-PF were then measured again using the same protocol as before.9
Results and Discussion
In terms of composition, all products tested are solutions of nitrocellulose in a mixture of ethyl acetate and butyl acetate, with the exception of Evonail®, which is an aqueous gel.
Nitrocellulose was chosen because it demonstrates an optimal drying time. Moreover, due to the presence of nitrocellulose, the film deposited on the nail is partially heat resistant, has the desired level of shine and hardness, and demonstrates resistance to abrasion.10,11 Nitrocellulose can be partially substituted by cellulose acetate butyrate (like in the L’Oréal Paris product).
In order for the deposited film to adhere to the nail, various types of secondary resins are added (e.g., aryl sulfonamide-formaldehyde or aryl sulfonamide-epoxy resins, polyester resins, alkyd type resins, polyurethane resins, polyester-polyurethane resins, polyether-polyurethane resins, and vinyl and/or acrylic resins). These secondary resins are used alone or in a mixture.11
Solvents and diluents represent the volatile part of the formula of a nail polish. They are used to disperse the film-forming components and the rest of the nonvolatile elements, including the coloring raw materials, and create a homogeneous mixture. One ingredient used in the Mavala, Yves Saint Laurent (YSL), and Vitry products is adipic acid/neopentyl glycol/trimellitic anhydride copolymer. Phthalic anhydride/trimellitic anhydride/glycols copolymer, present in the Rimmel and Sinclair products, phthalic anhydride/glycerin/glycidyl decanoate copolymer, used in the Maybelline product, and acrylates copolymer, used in the Mavala and Rimmel products, are also used as secondary filmogenic resins.
It is this category of ingredients that poses the most problems. Such is indeed the case for tosylamide/formaldehyde resin, which causes allergic reactions to polishes.12?20 Tosylamide/formaldehyde resin is absent from all of the products we studied.
Another ingredient known to cause allergic reactions are epoxy resins, used in the Maybelline and OPI products (tosylamide/epoxy resin).21 Tosylamide or 4-toluenesulfonamide is a substitute for toluene and is found in the ethyl tosylamide ingredient of the Maybelline and L’Oréal Paris products. Phthalic anhydride/trimellitic anhydride/glycols copolymer, a flammable ingredient found in the Rimmel and Sinclair products, is used as a plastifying and filmogenic agent. Cases of allergy to phthalic anhydride/trimellitic anhydride/glycols copolymer have been reported.22?25
Another category of ingredients used in nail polish is plastifiers. As their name suggests, plastifiers are designed to regulate the flexibility of the film without weakening its physical resistance. Historically, phtalates were used for this purpose.11 Currently, citrates (e.g., tributyl acetylcitrate, which is the plastifier most frequently used as a substitute for dibutyl phthalate) and phosphates are more frequently found.11 Benzoates such as trimethylpentanediyl dibenzoate, found in the Vitry and YSL products, and various esters, such as sucrose acetate isobutyrate (found in the Mavala and Vitry products), trimethyl pentanyl diisobutyrate, and isosorbide dicaprylate/caprate are also found. The oldest plastifier, which is used in the OPI product we studied, is camphor.
Solvents occupy an important place in the formula, as they enable regulation of the speed at which a product dries. Consequently, they must be eliminated as totally and quickly as possible. The relative proportions of different solvents are important. Indeed, the composition of the mixture of solvents chosen must vary as little as possible throughout the drying time. Currently, solvents and diluents are chosen from among esters and alcohols.11 Ethyl acetate and butyl acetate, as we have seen previously, are the esters that are frequently found.11 Isopropylic alcohol is the most frequently used alcohol, but isobutylic alcohol, ethylic alcohol, and, less frequently, oleic alcohol (found in the YSL product) are also seen. Aromatic hydrocarbons such as toluene are all but eliminated. Incidentally, we did not find any aromatic hydrocarbons in the products that we tested.
In this base, a pigment called 1-hydroxy-4-(p-toluidino) anthraquinone, better known as D&C violet No. 2 (CI 60725), classed within the anthraquinone dyes, is put into suspension. In order to ensure the homogeneity of the suspension, a derivative of hectorite or bentonite is often used, as are stearalkonium hectorite (found in the Mavala and Vitry products), stearalkonium bentonite, or both.
UV filters were found in seven out of 12 of the formulas studied. UVB filters can thus be found with a narrow spectrum such as derivatives of ?,?-diphenylacrylate. These are octocrylene, which is found in the YSL product, and etocrylene, which is found in the Rimmel and Vitry products. Broad-spectrum filters belonging to the benzophenone family, such as benzophenone-1 in the Maybelline product and benzophenone-3 (i.e., oxybenzone) found in the Vitry and Sainclair products, ensure UVB and UVA protection. In the Mavala product, a combination of these two benzophenones can be found. These are molecules that, for many years, have been known for their sensitizing properties.26,27
Because the composition of base coats and polishes is not favorable for the development of microorganisms, they usually do not contain preservatives in their formulas. However, among the products we tested, one preservative was found. The Rimmel product contains methylchloroisothiazolinone combined with methylisothiazolinone. This mixture has been known since the 1980s to cause allergic reactions.28 Thus, it would be prudent to make sure that this ingredient is not present in the chosen product for patients undergoing chemotherapy.
Bases are not all equivalent in terms of photoprotection (Figure 1). Among the products we studied, the Mavala base and the CC whitening base demonstrated the most photoprotective effects in the UVB domain, with SPF values of more than 180. These values, although high, are nevertheless much lower than those seen in colored nail polishes.28,29 Because there is no classification system specific to polishes, we must refer to the classification of sun protection products. From this, we can say that these product bases provide a high level of protection, as the determined by an SPF value 60 or higher. This photoprotective activity is due to benzophenone-1 (found in the Mavala product), benzophenone-3, and etocrylene (found in the Vitry product). Physicians recommending nail products containing benzophenones to patients undergoing chemotherapy should be careful, as these filters are known for their allergenic and even photoallergenic properties.27
The Evonail® product provides a mediocre level of protection (Figure 1). This is linked to the galenic form of the product and to the aqueous character of the excipient.
Concerning water resistance, all of the products tested turned out to be efficient, with results between 84 and 100 percent, except for the Evonail® product, which did not qualify as water-resistant (Table 2). This term only applies to products that have a calculated value greater than 50 percent, according the Guidelines for Evaluating Sun Product Water Resistance.9 The results obtained here can once again be explained by the formulation of the products. Indeed, 11 out of the 12 products tested are organic solutions, which are almost impossible to remove with water. Furthermore, in practice, removing a base coat or a nail polish is done by dissolving the product with an acetone base. Alternatively, an ethyl acetate base can be used when the product being removed does not contain acetone. These products cannot be removed with water, and the film deposited on the nail will be more resistant to handwashing.
The compostition of the Evonail® product is different from the other products tested. It is an aqueous gel; the gelling agent used is xanthan gum. Such a formula cannot resist being immersed in a bath of water and the percentage of water resistance is lower than 50 percent. Therefore, we cannot classify this product as water resistant.
Conclusion
Our results support applying two colorless products on the nails of people undergoing treatments that cause increased photosensitivity of the nails. Mavala 6 Osaka and Vitry CC bleaching base with silicium for yellow nails are the best of the tested products. The galenic form of the product is important, as only the organic products tested are water resistant and continue to provide protection to the nails after the person has washed their hands.
References
- Robert, Sibaud V, Mateus C, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16(4):e181–e189.
- Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56(3):460–465.
- Galimont-Collen AFS, Vos LE, Lavrijsen APM, et al. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer. 2007;43(5):845–851.
- Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract. 2009;15(3):143–155.
- Couteau C, Paparis E, Coiffard L. Comparison of different nail polish in terms of photoprotective efficacy. Interest of their use as supportive care in patients with cancer. Bull Cancer. 2016;103
(7–8):612–621. - Sayre RM, Agin PP, LeVee GJ, et al. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol. 1979; 29(3):559–566.
- Couteau C, Pommier M, Paparis E, et al. Study of the efficacy of 18 sun filters authorized in European Union tested in vitro. Pharmazie. 2007;62(6):449–452.
- Couteau C, El-Boury S, Paparis E, et al. In vitro UV-A protection factor (PF-UVA) of organic and inorganic sunscreens. Pharm Dev Technol. 2009;14(4): 369–372.
- Choquenet B, Couteau C, Paparis E, et al. Development of an in vitro test to determine the water-resistance of sunscreens. Pharmazie. 2008;63(7):525–527.
- Koch W, Phillips HC, Wint R. Nitrocellulose lacquers. Ind Eng Chem. 1946;38(5):518–521.
- Couteau C, Coiffard L. La formulation cosmétique à l’usage des professionnels et des amateurs. Les éditions Le Moniteur des Pharmacies, collection Pro Officina; 2014.
- Staines KS, Felix DH, Forsyth A. Desquamative gingivitis, sole manifestation of tosylamide/formaldehyde resin allergy. Contact Dermatitis. 1998;39(2):90.
- Vilaplana J, Romaguera C. Contact dermatitis from tosylamide/formaldehyde resin with photosensitivity. Contact Dermatitis. 2000;42(5):311–312.
- Duarte I, Lazzarini R, Kobata CM. Contact dermatitis in adolescents. Am J Contact Dermat. 2003;14(4):200–202.
- Orton DI, Wilkinson JD. Cosmetic allergy: incidence, diagnosis, and management. Am J Clin Dermatol. 2004;5(5):327–337.
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatatis. 2016;55(5):280–285.
- Jacob SE, Stechschulte SA. Tosylamide/formaldehyde resin allergy—a consideration in the atopic toddler. Contact Dermatitis. 2008;58(5) 312–313.
- Yokota M, Thong HY, Hoffman CA, Maibach HI. Allergic contact dermatitis caused by tosylamide formaldehyde resin in nail varnish: an old allergen that has not disappeared. Contact Dermatitis. 2007;57(4):277.
- Ozkaya E, Mirzoyeva L. Tosylamide/formaldehyde resin allergy in a young boy exposure from bitter nail varnish used against nail biting. Contact Dermatitis. 2009;60(3):171–172.
- Stechschulte SA, Avashia N, Jacob SE. Tosylamide formaldehyde resin. Dermatitis. 2008;19(3): E18–E19.
- Fischer AA. Chap. 27 Plastics, Adhesives and synthetic resins. In: Rietschel RL. Fisher’s Contact Dermatitis. Philadelphia, PA: Williams & Wilkins; 2008.
- Moffitt DL, Sansom JE. Allergic contact dermatitis from phthalic anhydride/trimellitic anhydride/glycols copolymer in nail varnish. Contact Dermatitis. 2002;46(4):236.
- Gach JE, Stone NM, Finch TM. A series of four cases of allergic contact dermatitis to phthalic anhydride/trimellitic anhydride/glycols copolymer in nail varnish. Contact Dermatitis. 2005;53(1):63–64.
- Quartier S, Garmyn M, Becart S, et al. Allergic contact dermatitis to copolymers in cosmetics—case report and review of the literature. Contact Dermatitis. 2006;55(5):257–267.
- Nassif AS, Le Coz CJ, Collet E. A rare nail polish allergen: phthalic anhydride, trimellitic anhydride and glycols copolymer. Contact Dermatitis. 2007;56(3):172–173.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70(4):193–204.
- Hanson JL, Warshaw EM. Sensitivity to multiple benzophenone sunscreen agents. Dermatitis. 2015;26(4):192–194.
- Rastogi SC. Kathon CG and cosmetic products. Contact Dermatitis. 1990;22(3):155–160.
- Couteau C, Sebille-Rivain V, Jourdan E, et al. Impact of socio-aesthetics as supportive care in a large, multi-specialty hospital. J Dermatological Res. 2017;2(1):96–102.

